Phage Display Approaches for the Isolation of Monoclonal Antibodies Against Dengue Virus Envelope Domain III from Human and Mouse Derived Libraries
Abstract
:1. Introduction
2. Results
2.2. Biopanning the HX02 Library with DENV Virus Particles
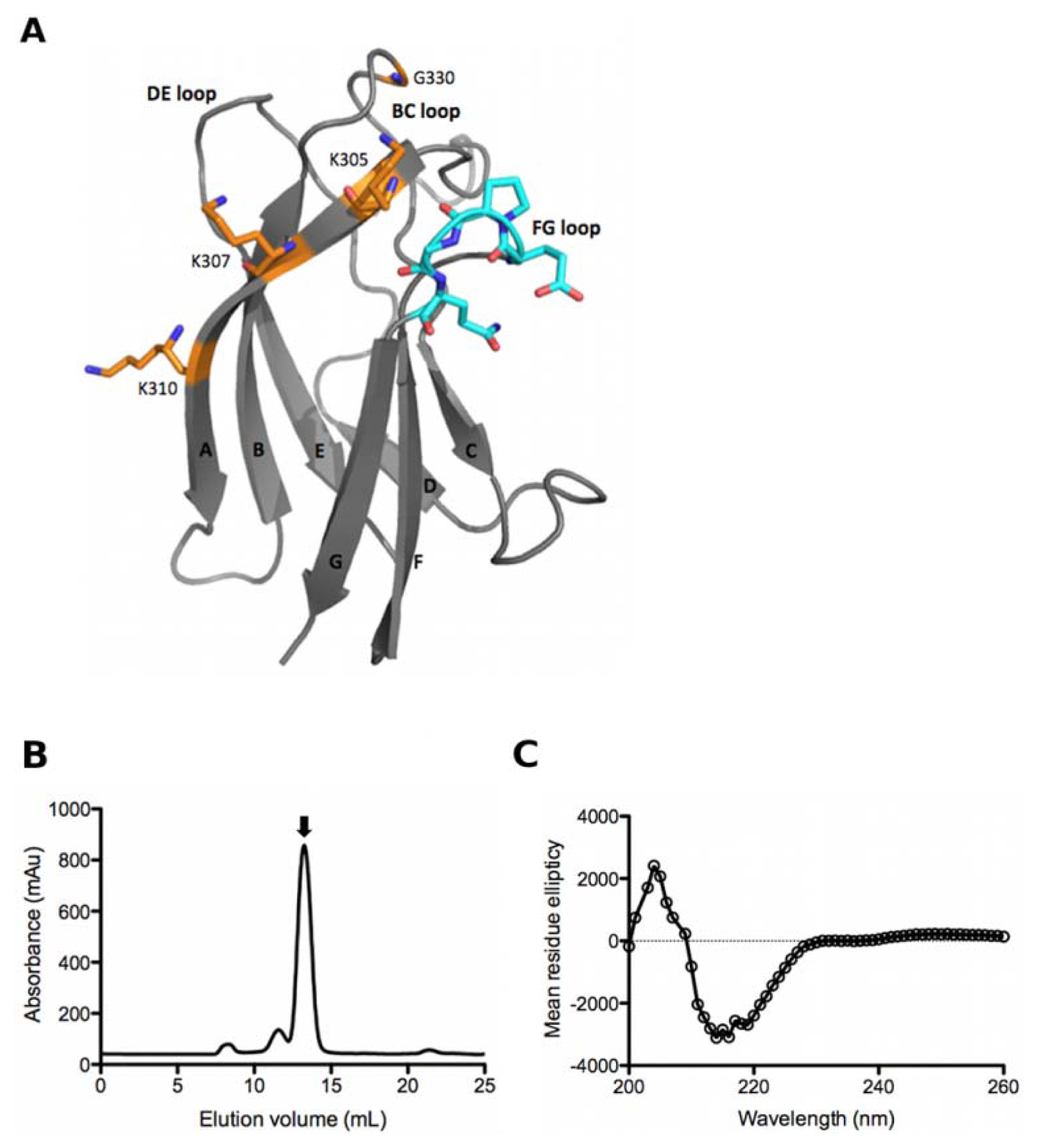
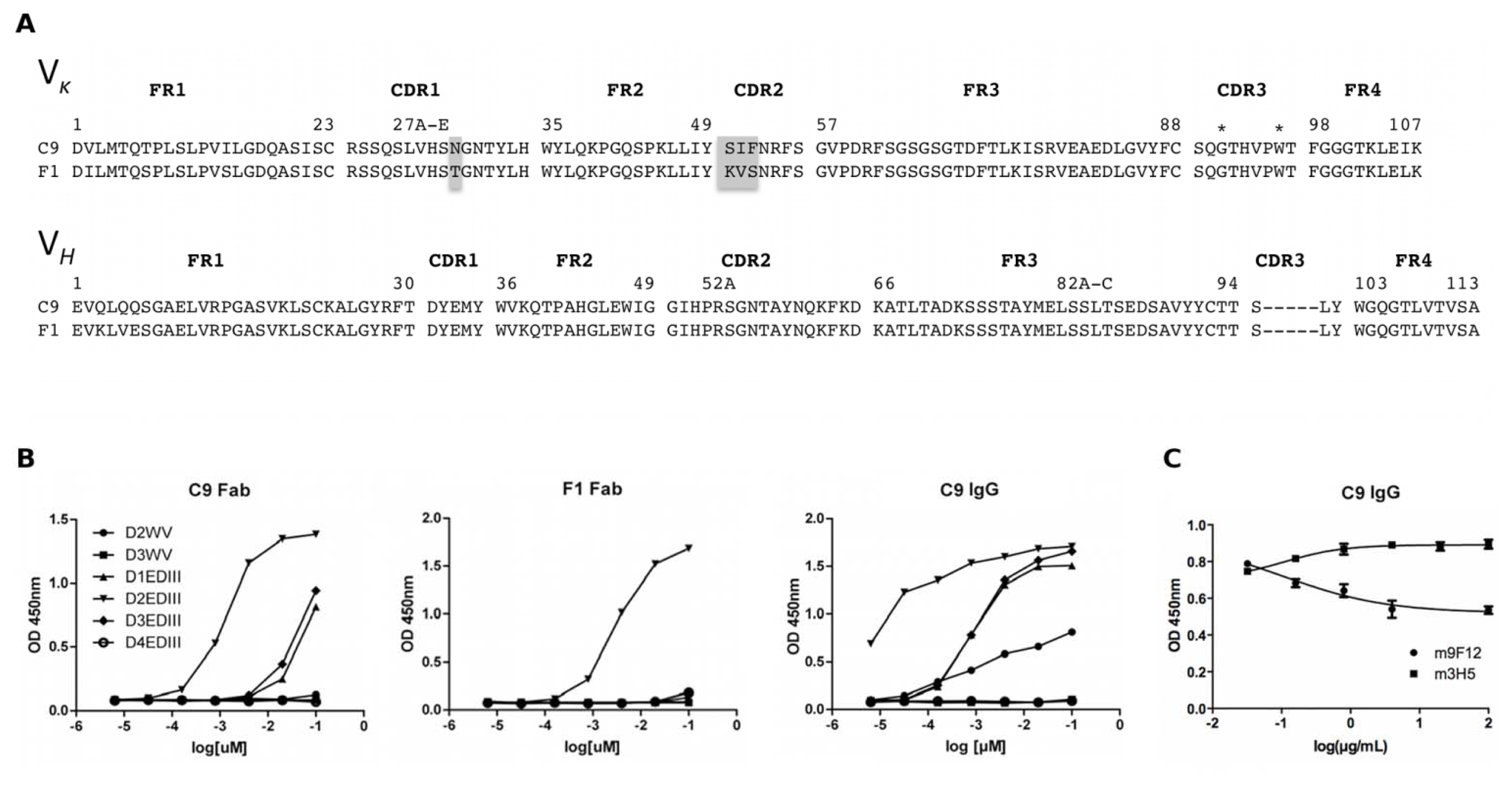
2.3. Characterisation of the Unique Anti-DENV Antibodies
2.4. Generation and Biopanning with the Chimeric Library
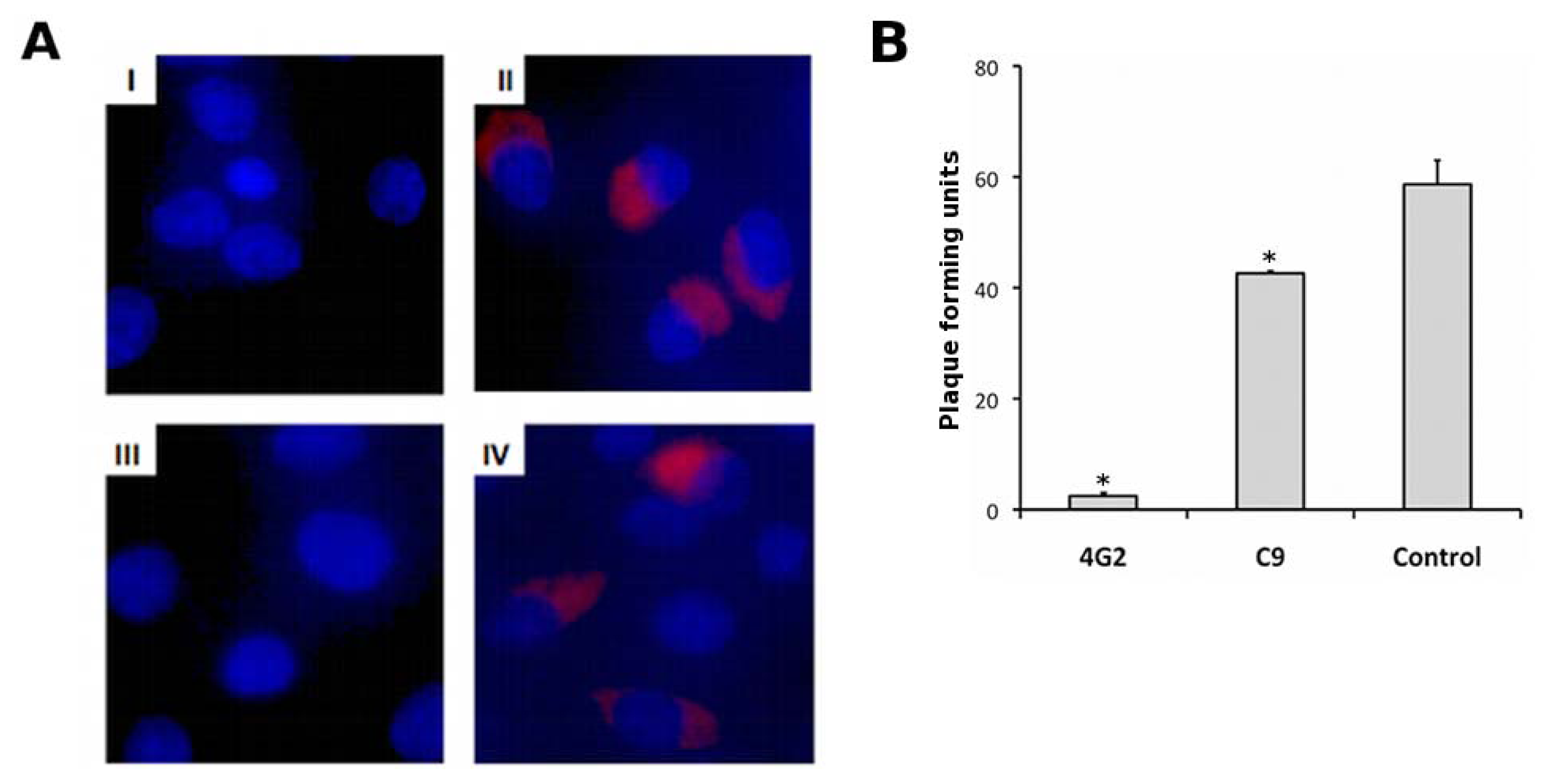
2.5. Characterisation of Anti-EDIII Fab and IgG
2.6. Saturation Mutagenesis of C9 Light Chain
3. Discussion
4. Materials and Methods
4.1. Antigen Preparation
4.2. HX02 Fab-Phage Library Biopanning
4.3. Construction and Biopanning of the Chimeric Fab-Phage Library
4.4. Phage Clone Screening
4.5. Expression and Purification of Fab and IgG
4.6. Elisa and Western Blot with Fab and IgG
4.7. Affinity Maturation of C9 light Chain by Phage Display
4.8. DENV Immunostaining and Neutralization Assays
5. Conclusions
Acknowledgments
References
- Lindenbach, B.D.; Thiel, H.J.; Rice, C.M. Flaviviridae: the viruses and their replication. In Fields Virology, 5th ed; Lippincott William: Philadelphia, PA, USA, 2007; pp. 1101–1151. [Google Scholar]
- Halstead, S.B. Dengue. Lancet 2007, 370, 1644–1652. [Google Scholar]
- Fink, J.; Gu, F.; Vasudevan, S.G. Role of T cells, cytokines and antibody in dengue fever and dengue haemorrhagic fever. Rev. Med. Virol 2006, 16, 263–275. [Google Scholar]
- Falconar, A.K. Identification of an epitope on the dengue virus membrane (M) protein defined by cross-protective monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Arch. Virol 1999, 144, 2313–2330. [Google Scholar]
- Falconar, A.K. Antibody responses are generated to immunodominant ELK/KLE-type motifs on the nonstructural-1 glycoprotein during live dengue virus infections in mice and humans: implications for diagnosis, pathogenesis, and vaccine design. Clin. Vaccine Immunol 2007, 14, 493–504. [Google Scholar]
- Huang, K.J.; Yang, Y.C.; Lin, Y.S.; Huang, J.H.; Liu, H.S.; Yeh, T.M.; Chen, S.H.; Liu, C.C.; Lei, H.Y. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J. Immunol 2006, 176, 2825–2832. [Google Scholar]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 2003, 100, 6986–6991. [Google Scholar]
- Chin, J.F.; Chu, J.J.; Ng, M.L. The envelope glycoprotein domain III of dengue virus serotypes 1 and 2 inhibit virus entry. Microbes Infect 2007, 9, 1–6. [Google Scholar]
- Bhardwaj, S.; Holbrook, M.; Shope, R.E.; Barrett, A.D.; Watowich, S.J. Biophysical characterization and vector-specific antagonist activity of domain III of the tick-borne flavivirus envelope protein. J. Virol 2001, 75, 4002–4007. [Google Scholar]
- Volk, D.E.; Beasley, D.W.; Kallick, D.A.; Holbrook, M.R.; Barrett, A.D.; Gorenstein, D.G. Solution structure and antibody binding studies of the envelope protein domain III from the New York strain of West Nile virus. J. Biol. Chem 2004, 279, 38755–38761. [Google Scholar]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature 2004, 427, 313–319. [Google Scholar]
- Gromowski, G.D.; Barrett, N.D.; Barrett, A.D.T. Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus. J. Virol 2008, 82, 8828–8837. [Google Scholar]
- Shrestha, B.; Brien, J.D.; Sukupolvi-Petty, S.; Austin, S.K.; Edeling, M.A.; Kim, T.; O’Brien, K.M.; Nelson, C.A.; Johnson, S.; Fremont, D.H.; Diamond, M.S. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog 2010, 6, e1000823. [Google Scholar]
- Sukupolvi-Petty, S.; Austin, S.K.; Engle, M.; Brien, J.D.; Dowd, K.A.; Williams, K.L.; Johnson, S.; Rico-Hesse, R.; Harris, E.; Pierson, T.C.; Fremont, D.H.; Diamond, M.S. Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. J. Virol 2010, 84, 9227–9239. [Google Scholar]
- Sukupolvi-Petty, S.; Austin, S.K.; Purtha, W.E.; Oliphant, T.; Nybakken, G.E.; Schlesinger, J.J.; Roehrig, J.T.; Gromowski, G.D.; Barrett, A.D.; Fremont, D.H.; Diamond, M.S. Type- and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J. Virol 2007, 81, 12816–12826. [Google Scholar]
- Wahala, W.M.P.B.; Kraus, A.A.; Haymore, L.B.; Accavitti-Loper, M.A.; de Silva, A.M. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology 2009, 392, 103–113. [Google Scholar]
- Crill, W.D.; Hughes, H.R.; Delorey, M.J.; Chang, G.-J.J. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One 2009, 4, e4991. [Google Scholar]
- Midgley, C.M.; Bajwa-Joseph, M.; Vasanawathana, S.; Limpitikul, W.; Wills, B.; Flanagan, A.; Waiyaiya, E.; Tran, H.B.; Cowper, A.E.; Chotiyarnwon, P.; Grimes, J.M.; Yoksan, S.; Malasit, P.; Simmons, C.P.; Mongkolsapaya, J.; Screaton, G.R. An in-depth analysis of original antigenic sin in dengue virus infection. J. Virol 2011, 85, 410–421. [Google Scholar]
- Beltramello, M.; Williams, K.L.; Simmons, C.P.; Macagno, A.; Simonelli, L.; Quyen, N.T.; Sukupolvi-Petty, S.; Navarro-Sanchez, E.; Young, P.R.; de Silva, A.M.; Rey, F.A.; Varani, L.; Whitehead, S.S.; Diamond, M.S.; Harris, E.; Lanzavecchia, A.; Sallusto, F. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 2010, 8, 271–283. [Google Scholar]
- Rajamanonmani, R.; Nkenfou, C.; Clancy, P.; Yau, Y.H.; Shochat, S.G.; Sukupolvi-Petty, S.; Schul, W.; Diamond, M.S.; Vasudevan, S.G.; Lescar, J. On a mouse monoclonal antibody that neutralizes all four dengue virus serotypes. J. Gen. Virol 2009, 90, 799–809. [Google Scholar]
- Krebber, A.; Bornhauser, S.; Burmester, J.; Honegger, A.; Willuda, J.; Bosshard, H.R.; Plückthun, A. Reliable cloning of functional antibody variable domains from hybridomas and spleen cell repertoires employing a reengineered phage display system. J. Immunol. Methods 1997, 201, 35–55. [Google Scholar]
- Azzazy, H.M.E.; Highsmith, W.E. Phage display technology: clinical applications and recent innovations. Clin. Biochem 2002, 35, 425–445. [Google Scholar]
- Xu, J.L.; Davis, M.M. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity 2000, 13, 37–45. [Google Scholar]
- Brochet, X.; Lefranc, M.P.; Giudicelli, V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucl. Acids Res 2008, 36, W503–W508. [Google Scholar]
- Barbas, C.F.; Burton, D.R.; Scott, J.K.; Silverman, G.J. Phage Display, A Laboratory Manual; Cold Spring Harbor Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Abhinandan, K.R.; Martin, A.C. Analysis and improvements to Kabat and structurally correct numbering of antibody variable domains. Mol. Immunol 2008, 45, 3832–3839. [Google Scholar]
- Rader, C.; Popkov, M.; Neves, J.A.; Barbas, C.F. Integrin alpha(v)beta3 targeted therapy for Kaposi’s sarcoma with an in vitro evolved antibody. FASEB J 2002, 16, 2000–2002. [Google Scholar]
- Hofer, T.; Tangkeangsirisin, W.; Kennedy, M.G.; Mage, R.G.; Raiker, S.J.; Venkatesh, K.; Lee, H.; Giger, R.J.; Rader, C. Chimeric rabbit/human Fab and IgG specific for members of the Nogo-66 receptor family selected for species cross-reactivity with an improved phage display vector. J. Immunol. Methods 2007, 318, 75–87. [Google Scholar]
- Hiramatsu, K.; Tadano, M.; Men, R.; Lai, C.J. Mutational analysis of a neutralization epitope on the dengue type 2 virus (DEN2) envelope protein: monoclonal antibody resistant DEN2/DEN4 chimeras exhibit reduced mouse neurovirulence. Virology 1996, 224, 437–445. [Google Scholar]
- Nielsen, U.B.; Marks, J.D. Affinity Maturation of Phage Antibodies. In Phage Display: a Practical Approach; Clackson, T., Lowman, H.B., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 289–315. [Google Scholar]
- Schier, R.; Bye, J.; Apell, G.; McCall, A.; Adams, G.P.; Malmqvist, M.; Weiner, L.M.; Marks, J.D. Isolation of high-affinity monomeric human anti-c-erbB-2 single chain Fv using affinity-driven selection. J. Mol. Biol 1996, 255, 28–43. [Google Scholar]
- Bradbury, A.R.M.; Marks, J.D. Antibodies from phage antibody libraries. J. Immunol. Methods 2004, 290, 29–49. [Google Scholar]
- Moreland, N.J.; Tay, M.Y.; Lim, E.; Paradkar, P.N.; Doan, D.N.; Yau, Y.H.; Shochat, S.G.; Vasudevan, S.G. High affinity human antibody fragments to dengue virus non-structural protein 3. PLoS Negl. Trop. Dis 2010, 4, e881. [Google Scholar]
- Moreland, N.J.; Tay, M.Y.; Lim, E.; Rathore, A.P.; Lim, A.P.; Hanson, B.J.; Vasudevan, S.G. Monoclonal antibodies against dengue NS2B and NS3 proteins for the study of protein interactions in the flaviviral replication complex. J. Virol. Methods 2011, 179, 97–103. [Google Scholar]
- Lim, A.P.C.; Chan, C.E.Z.; Wong, S.K.K.; Chan, A.H.Y.; Ooi, E.E.; Hanson, B.J. Neutralizing human monoclonal antibody against H5N1 influenza HA selected from a Fab-phage display library. Virol. J 2008, 5, 130. [Google Scholar]
- Gould, L.H.; Sui, J.; Foellmer, H.; Oliphant, T.; Wang, T.; Ledizet, M.; Murakami, A.; Noonan, K.; Lambeth, C.; Kar, K.; Anderson, J.F.; de Silva, A.M.; Diamond, M.S.; Koski, R.A.; Marasco, W.A.; Fikrig, E. Protective and therapeutic capacity of human single-chain Fv-Fc fusion proteins against West Nile virus. J. Virol 2005, 79, 14606–14613. [Google Scholar]
- Throsby, M.; Geuijen, C.; Goudsmit, J.; Bakker, A.Q.; Korimbocus, J.; Kramer, R.A.; Clijsters-van der Horst, M.; de Jong, M.; Jongeneelen, M.; Thijsse, S.; Smit, R.; Visser, T.J.; Bijl, N.; Marissen, W.E.; Loeb, M.; Kelvin, D.J.; Preiser, W.; ter Meulen, J.; de Kruif, J. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile Virus. J. Virol 2006, 80, 6982–6992. [Google Scholar]
- de Alwis, R.; Beltramello, M.; Messer, W.B.; Sukupolvi-Petty, S.; Wahala, W.M.; Kraus, A.; Olivarez, N.P.; Pham, Q.; Brien, J.D.; Tsai, W.Y.; Wang, W.K.; Halstead, S.; Kliks, S.; Diamond, M.S.; Baric, R.; Lanzavecchia, A.; Sallusto, F.; de Silva, A.M. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl. Trop. Dis 2011, 5, e1188. [Google Scholar]
- Pierson, T.C.; Diamond, M.S. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Expert Rev. Mol. Med 2008, 10, e12. [Google Scholar]
- de Haard, H.J.; van Neer, N.; Reurs, A.; Hufton, S.E.; Roovers, R.C.; Henderikx, P.; de Bruïne, A.P.; Arends, J.W.; Hoogenboom, H.R. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J. Biol. Chem 1999, 274, 18218–18230. [Google Scholar]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007, 43, S25–S30. [Google Scholar]
- Hudson, P.J.; Souriau, C. Engineered antibodies. Nat. Med 2003, 9, 129–134. [Google Scholar]
- Parren, P.W.; Burton, D.R. The antiviral activity of antibodies in vitro and in vivo. Adv. Immunol 2001, 77, 195–262. [Google Scholar]

| Procedure | Strategy 1 | Strategy 2 | Strategy 3 | Strategy 4 |
|---|---|---|---|---|
| Antigen immobilisation | DENV1 EDIII Immunotube | DENV1 EDIII Immunoplate | Biotinylated EDIII (DENV1 and DENV2) Streptavidin resin | DENV1 whole virus Immunoplate |
| Antigen concentration | 50 μg/mL (Pan1) 25 μg/mL, (Pan2&3) | 10 μg/mL (Pan1–4) 1 μg/mL (Pan5&6) | 100 nM (Pan1&2) 50 nM (Pan3–6) | 20 μg/mL (Pan1&2) 10 μg/mL (Pan3&4) |
| Rounds of panning | Three rounds | Six rounds | Six rounds | Four rounds |
| E. coli strain | TG1 | XL-1 Blue | TG1 | XL-1 Blue |
| Clone screening | 190 (Pan3) | 95 (Pan4), 95 (Pan5), 95 (Pan6) | 95 (Pan3), 285 (Pan5), 190 (Pan6) | 384 (Pan4) |
| DENV positive clones | Nil | Nil | Nil | 188/384 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moreland, N.J.; Susanto, P.; Lim, E.; Tay, M.Y.F.; Rajamanonmani, R.; Hanson, B.J.; Vasudevan, S.G. Phage Display Approaches for the Isolation of Monoclonal Antibodies Against Dengue Virus Envelope Domain III from Human and Mouse Derived Libraries. Int. J. Mol. Sci. 2012, 13, 2618-2635. https://doi.org/10.3390/ijms13032618
Moreland NJ, Susanto P, Lim E, Tay MYF, Rajamanonmani R, Hanson BJ, Vasudevan SG. Phage Display Approaches for the Isolation of Monoclonal Antibodies Against Dengue Virus Envelope Domain III from Human and Mouse Derived Libraries. International Journal of Molecular Sciences. 2012; 13(3):2618-2635. https://doi.org/10.3390/ijms13032618
Chicago/Turabian StyleMoreland, Nicole J., Patricia Susanto, Elfin Lim, Moon Y. F. Tay, Ravikumar Rajamanonmani, Brendon J. Hanson, and Subhash G. Vasudevan. 2012. "Phage Display Approaches for the Isolation of Monoclonal Antibodies Against Dengue Virus Envelope Domain III from Human and Mouse Derived Libraries" International Journal of Molecular Sciences 13, no. 3: 2618-2635. https://doi.org/10.3390/ijms13032618
APA StyleMoreland, N. J., Susanto, P., Lim, E., Tay, M. Y. F., Rajamanonmani, R., Hanson, B. J., & Vasudevan, S. G. (2012). Phage Display Approaches for the Isolation of Monoclonal Antibodies Against Dengue Virus Envelope Domain III from Human and Mouse Derived Libraries. International Journal of Molecular Sciences, 13(3), 2618-2635. https://doi.org/10.3390/ijms13032618




