Antiproliferative and Proapoptotic Activities of Methanolic Extracts from Ligustrum vulgare L. as an Individual Treatment and in Combination with Palladium Complex
Abstract
:1. Introduction
2. Results and Discussion
2.1. Time- and Dose-Dependent Effects of L. vulgare Extracts on Cell Proliferation
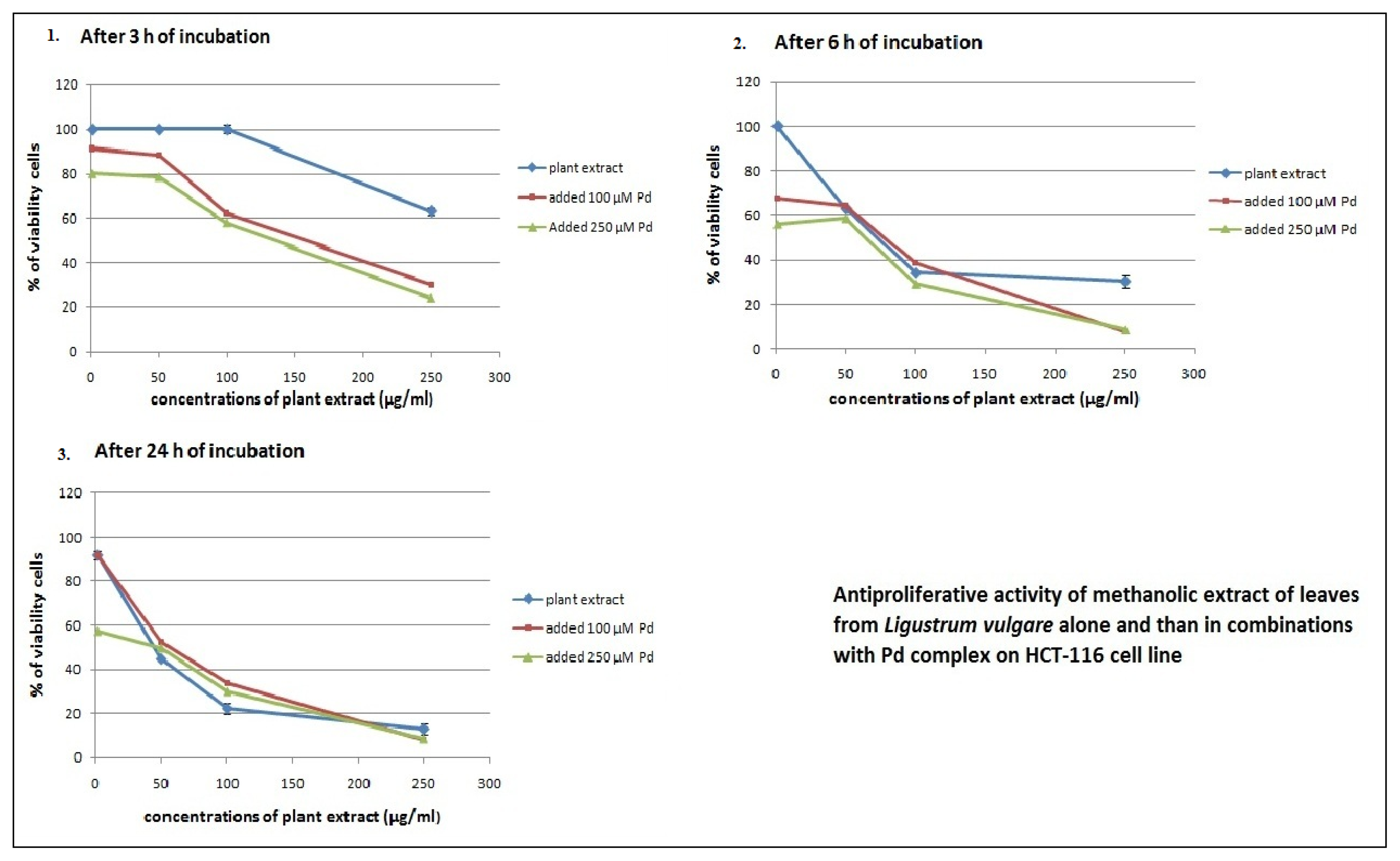
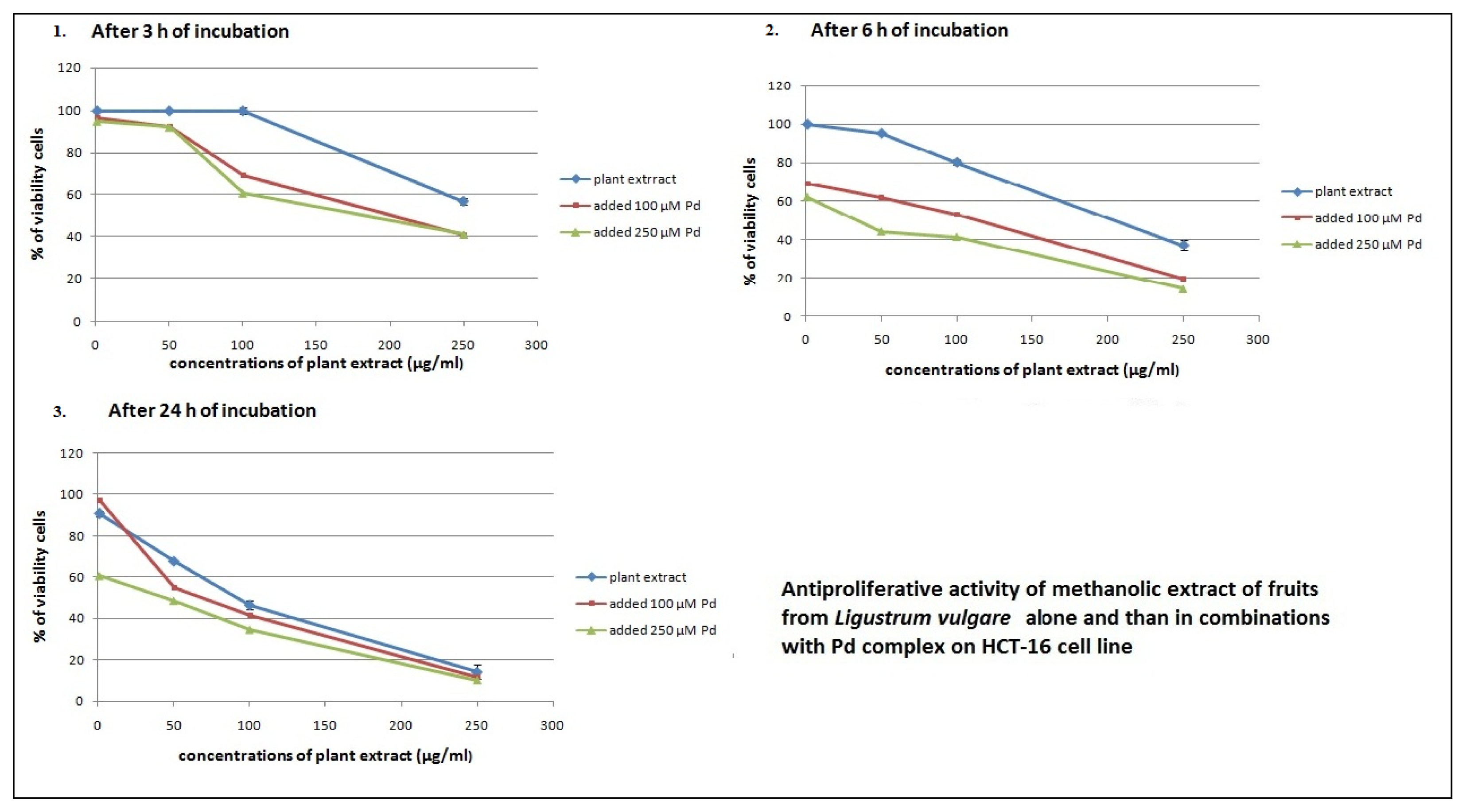
2.2. Antiproliferative Effects of L. vulgare Extracts in co-Treatment with Pd(apox) Complex
3. Experimental Section
3.1. Chemicals
3.2. Preparation of [Pd(apox)]
3.3. Preparation of Plant Extracts
3.4. Cell Preparation, Culturing and Treatments
3.5. Cell Viability Assay (MTT Assay)
3.6. Fluorescence Microscopic Analysis of Cell Death
3.7. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Jovanović, B. Ligustrum. In Flora of Republic of Serbia, 5th ed; Josifović, M., Ed.; Serbian Academy of Science and Arts: Belgrade, Serbia, 1973; pp. 456–457. [Google Scholar]
- Pieroni, A.; Pachaly, P.; Huang, Y.; van Poel, B.; Vlietinck, A.J. Studies on anti-complementary activity of extracts and isolated flavones from Ligustrum vulgare and Phillyrea latifolia leaves (Oleaceae). J. Ethnopharmacol 2000, 70, 213–217. [Google Scholar]
- Baróniková, S.; Nagy, M.; Grančai, D. Changes in immunomodulatory activity of human mononuclear cells after cultivation with leaf decoctions from the genus Ligustrum L. Phytother. Res 1999, 13, 692–695. [Google Scholar]
- Yim, T.K.; Wu, W.K.; Pak, W.F.; Ko, K.M. Hepatoprotective action in an oleanolic acid-enriched extracts of Ligustrum lucidum fruits is mediated through an enhancement on hepatic glutathione regeneration capacity in mice. Phytother. Res 2001, 15, 589–592. [Google Scholar]
- Jantová, S.; Nagy, M.; Ružeková, L.; Grančai, D. Antibacterial activity of plant extracts from the families Fabaceae, Oleaceae, Philadelphaceae, Rosaceae and Staphyleaceae. Phytother. Res 2000, 14, 601–603. [Google Scholar]
- Andrade-Cetto, A.; Henrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol 2005, 99, 325–348. [Google Scholar]
- Sersen, F.; Mucaji, P.; Grancai, D.; Nagy, M. Antioxidative properties of methanol extracts from leaves and fruits of Ligustrum vulgare L. Acta Facult. Pharm. Univ. Comenianae 2005, 52, 204–209. [Google Scholar]
- Jantova, S.; Nagy, M.; Ruzekova, L.; Grancai, D. Cytotoxic activity of plant extracts from the families Fabaceae, Oleaceae, Philadelphaceae, Rosaceae and Staphyleaceae. Phytother. Res 2001, 15, 22–25. [Google Scholar]
- He, Z.D.; Lau, K.M.; But, P.P.; Jiang, R.W.; Dong, H.; Ma, S.C.; Fung, K.P.; Ye, W.C.; Sun, H.D. Antioxidative glycosides from the leaves of Ligustrum robustum. J. Nat. Prod 2003, 66, 851–854. [Google Scholar]
- Cragg, M.G.; Newman, D.J. Plants as a source of anticancer agents. J. Ethopharmacol 2005, 100, 72–79. [Google Scholar]
- Reddy, L.; Odhav, B.; Bhoola, K. Natural products for cancer prevention: global perspective. Pharmacol. Ther 2003, 99, 1–13. [Google Scholar]
- Houghton, P. The role of plants in traditional medicine and current therapy. J. Alt. Comple. Med 1995, 1, 131–143. [Google Scholar]
- Lippert, B. Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug; VHCA & WileynVCH: Zurich, Switzerland, 1999. [Google Scholar]
- Kosmider, B.; Wyszynska, K.; Janik-Spiechowicz, E.; Osiecka, R.; Zyner, E.; Ochocki, J.; Ciesielska, E.; Wasowicz, W. Evaluation of the genotoxicity of cis-bis(3-aminoflavone) dichloroplatinum(II) in comparison with cis-DDP. Mutat. Res 2004, 558, 93–110. [Google Scholar]
- Stanković, M.; Ćurčić, M; Žižić, J.; Topuzović, M.; Solujić, S.; Marković, S. Teucrium plant species as natural sources of novel anticancer compounds: antiproliferative, proapoptotic and antioxidant properties. Int. J. Mol. Sci. 2011, 12, 4190–4205. [Google Scholar]
- Kampa, M.; Nifli, A.P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharmacol 2007, 159, 79–113. [Google Scholar]
- Galati, G.; Teng, S.; Moridani, M.Y.; Chan, T.S.; O’Brien, P.J. Cancer chemoprevention and apoptosis mechanism induced by diatary polyphenolics. Drug Metabol. Drug Interact 2000, 17, 311–349. [Google Scholar]
- Oh, H.; Livingston, R.; Smith, K.; Abrishamian-Garcia, L. Comparative study of the time dependency of cell death assays. MIT Undergranduate Res. J 2004, 11, 53–62. [Google Scholar]
- Darzynkiewicz, Z.; Traganos, F.; Wu, J.M.; Chen, S. Chinese herbal mixture PC SPES in treatment of prostate cancer (review). Int. J. Oncol 2000, 17, 729–736. [Google Scholar]
- Nagy, M.; Sersen, F. Free radical scavenging activity of different extracts and some constituents from the leaves of Ligustrum vulgare and L. delavayanum. Fitoterapia 2006, 77, 395–397. [Google Scholar]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicininduced congestive heart failure. Ann. Intern. Med 1979, 91, 710–717. [Google Scholar]
- Raghavan, D.; Koczwara, B.; Javle, M. Evolving strategies of cytotoxic chemotherapy for advanced prostate cancer. Eur. J. Cancer 1997, 33, 566–574. [Google Scholar]
- Hortobagyi, G.N. Progress in systemic chemotherapy of primary breast cancer: an overview. J. Natl. Cancer Inst. Monogr 2001, 30, 72–79. [Google Scholar]
- Tyagi, A.K.; Singh, R.P.; Agarwal, C.; Chan, D.C.; Agarwal, R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-Marrest, and apoptosis. Clin. Cancer Res 2002, 8, 3512–3519. [Google Scholar]
- Cetin, R.; Devrim, E.; Kilicoglu, B.; Avci, A.; Candir, O.; Durak, I. Cisplatin impairs antioxidant system and causes oxidation in rat kidney tissues: Possible protective roles of natural antioxidant foods. J. Appl. Toxicol 2006, 1, 42–46. [Google Scholar]
- Keter, F.K. Pyrazole and pyrazolyl palladium(II) and platinum(II) complexes: synthesis and in vitro evaluation as anticancer agents. M.S. Thesis, University of the Western Cape, Cape Town, South Africa, November 2004. [Google Scholar]
- Ferguson, P.; Kurowska, E.; Freman, D.; Chambers, A.; Koropatnick, D. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell line. J. Natur 2004, 134, 1529–1535. [Google Scholar]
- Robi, T.; Bishayee, A. Terpenoids and brest cancer chemoprevention. Brest cancer Res. Treat 2009, 115, 223–239. [Google Scholar]
- Zhang, K.; Chew, M.; Wong, K.P. Modulation of cisplatin cytotoxcity and cisplatin-induced DNA crosslink in HepG2 cells by regulation of glutatione-related mechanisms. Mol. Pharmacol 2001, 59, 837–843. [Google Scholar]
- Suzuki, T.; Nishio, K.; Tanabe, S. The MRP family anticancer drug metabolism. Curr. Drug Metab 2001, 2, 368–377. [Google Scholar]
- Journauh, Y.; Sletten, J.; Kahn, O. Tunable interaction in p-Oxamido Copper(II) Binuclear Complexes. Inorg. Chem 1985, 24, 4063–4069. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth 1983, 65, 55–63. [Google Scholar]
- Baskić, D.; Popović, S.; Ristić, P.; Arsenijević, N.N. Analysis of cyclohexamide-induced apoptosis in human leukocytes: Fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol. Int 2006, 30, 924–932. [Google Scholar]
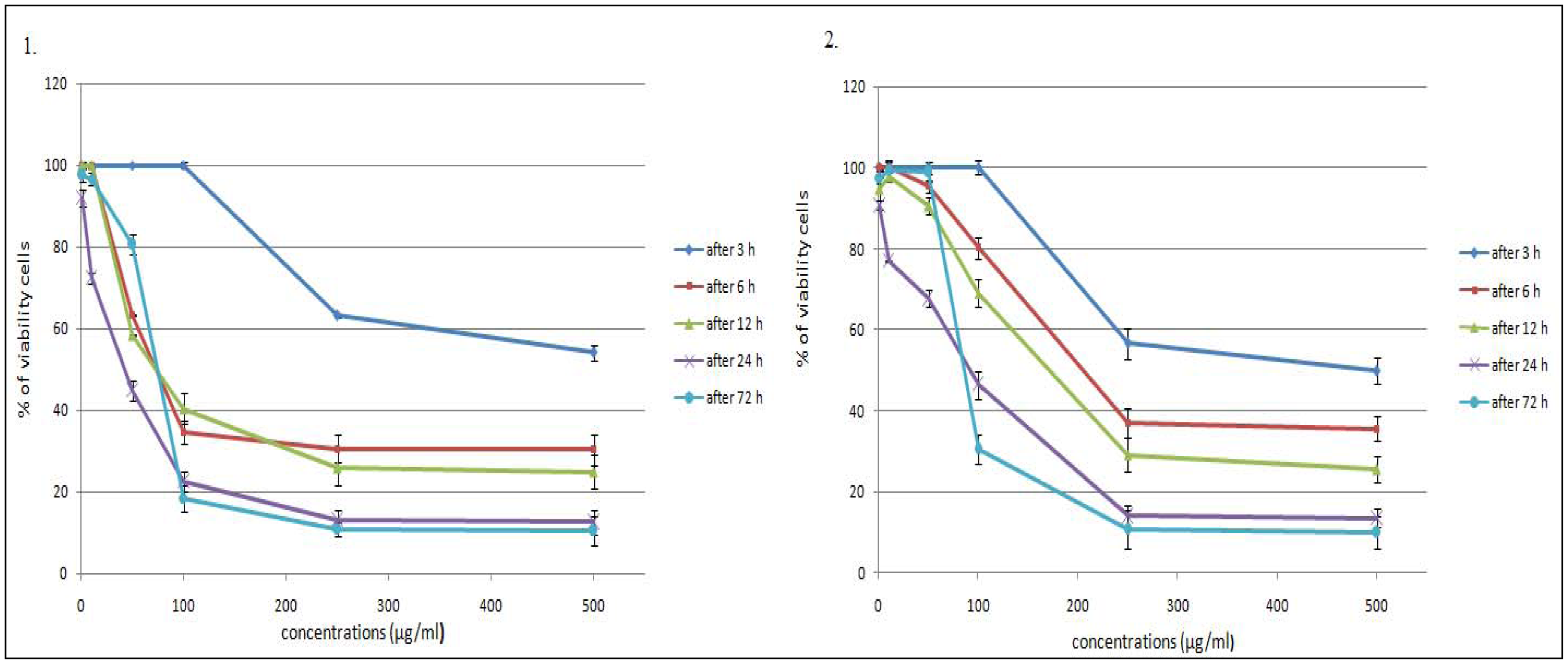

| Methanolic extracts of L. vulgare | After 3 h | After 6 h | After 12 h | After 24 h | After 72 h |
|---|---|---|---|---|---|
| leaf | > 500 | 159.5 ± 1.92 | 147.8 ± 7.29 | 28.2 ± 0.76 | 64.6 ± 3.84 |
| fruit | > 500 | 325.9 ± 4.51 | 240.8 ± 3.64 | 47.4 ± 3.54 | 108.0 ± 5.32 |
| VC | EA | LA | N | ||
|---|---|---|---|---|---|
| 3 h | untreated cells | 96.5 ± 0.19 | 3.4 ± 0.26 | 0.1 ± 0.13 | |
| leaf extract | 83.3 ± 1.37 | 16.4 ± 1.34 | 0.2 ± 0.09 | 0.1 ± 0.08 | |
| fruit extract | 80.0 ± 4.14 | 16.1 ± 4.48 | 3.1 ± 0.23 | 0.7 ± 0.1 | |
| 6 h | untreated cells | 87.3 ± 7.09 | 12.7 ± 0.28 | 0.1 ± 0.105 | − |
| leaf extract | 74.4 ± 3.53 | 25.2 ± 3.59 | 0.4 ± 0.027 | − | |
| fruit extract | 77.0 ± 6.11 | 22.9 ± 6.10 | − | − | |
| 12 h | untreated cells | 90.9 ± 0.08 | 8.7 ± 0.215 | 0.4 ± 0.29 | − |
| leaf extract | 56.1 ± 2.08 | 41.3 ± 2.48 | 2.5 ± 1.57 | 0.1 ± 0.07 | |
| fruit extract | 73.6 ± 4.78 | 25.9 ± 4.71 | 0.2 ± 0.17 | 0.1 ± 0.06 | |
| 24 h | untreated cells | 96.3 ± 0.38 | 2.2 ± 0.10 | 1.4 ± 0.29 | − |
| leaf extract | 52.5 ± 2.10 | 45.7 ± 2.47 | 1.9 ± 0.36 | − | |
| fruit extract | 53.2 ± 3.85 | 45.6 ± 4.52 | 1.2 ±0.66 | − | |
| 72 h | untreated cells | 97.1 ± 3.58 | 2.9 ± 2.15 | − | − |
| leaf extract | 68.3 ± 2.59 | 29.4 ± 1.87 | 2.3 ± 0.35 | − | |
| fruit extract | 71.9 ± 1.13 | 21.8 ± 0.98 | 1.2 ± 1.01 | 3.1 ± 0.15 |
| Measured after 24 h | Measured after 72 h | ||
|---|---|---|---|
| combination of drugs | Pd(apox) complex added after 3 h of cell incubation with plant extract | Pd(apox) complex added after 6 h of cell incubation with plant extract | Pd(apox) complex added after 24 h of cell incubation with plant extract |
| Leaf extract and 100 μM Pd | 269.5 ± 2.89 | 13.8 ± 1.21 | 27.1 ± 1.47 |
| Leaf extract and 250 μM Pd | 121.1 ± 3.25 | 5.5 ± 0.75 | 4.8 ± 0.65 |
| Fruit extract and 100 μM Pd | 347.9 ± 4.63 | 33.5 ± 1.56 | 145.4 ± 1.26 |
| Fruit extract and 250 μM Pd | 422.2 ± 5.41 | 8.1 ± 1.01 | 6.0 ± 0.25 |
| Time of cell counting | VC | EA | LA | N | ||
|---|---|---|---|---|---|---|
| after 24 h | control, untreated cells | 96.3 ± 0.38 | 2.2 ± 0.10 | 1.4 ± 0.29 | - | |
| after 72 h | 97.1 ± 3.58 | 2.9 ± 2.15 | - | - | ||
| 24 h after treatment | 100 μM Pd(apox) complex | 98.1 ± 0.89 | 1.9 ± 0.28 | - | - | |
| 250 μM Pd(apox) complex | 96.6 ± 3.81 | 3.4 ± 1.09 | - | - | ||
| Pd(apox) complex | combination of drugs | |||||
| 24 h after initial treatment with plant extracts | after 3 h | leaf extract and 100 μM Pd | 74.1 ± 0.93 | 25.7 ± 0.47 | 0.2 ± 0.29 | - |
| leaf extract and 250 μM Pd | 62.3 ± 6.35 | 36.5 ± 4.39 | 1.1 ± 0.39 | - | ||
| fruit extract and 100 μM Pd | 79.1 ± 0.77 | 20.7 ± 1.24 | 0.2 ± 0.74 | - | ||
| fruit extract and 250 μM Pd | 68.9 ± 4.05 | 31.1 ± 1.87 | - | - | ||
| after 6 h | leaf extract and 100 μM Pd | 44.9 ± 4.76 | 41.1 ± 0.79 | 13.7 ± 1.25 | 0.2 ± 2.56 | |
| leaf extract and 250 μM Pd | 32.1 ± 2.68 | 45.2 ± 5.05 | 20.8 ± 1.74 | 1.8 ± 3.21 | ||
| fruit extract and 100 μM Pd | 59.1 ± 1.57 | 32.7 ± 0.41 | 8.2 ± 1.13 | - | ||
| fruit extract and 250 μM Pd | 50.1 ± 2.91 | 39.7 ± 2.31 | 10.1 ± 2.01 | - | ||
| 72 h after initial treatment with plant extracts | after 24 h | leaf extract and 100 μM Pd | 37.1 ± 1.58 | 26.7 ± 1.87 | 34.3 ± 1.36 | 1.8 ± 0.97 |
| leaf extract and 250 μM Pd | 30.5 ± 2.02 | 29.5 ± 2.14 | 37.9 ± 1.91 | 2.1 ± 1.67 | ||
| fruit extract and 100 μM Pd | 40.8 ± 2.14 | 30.6 ± 1.57 | 25.9 ± 1.36 | 2.6 ± 1.07 | ||
| fruit extract and 250 μM Pd | 42.9 ± 1.64 | 31.3 ± 1.42 | 24.8 ± 1.42 | 1.0 ± 1.34 | ||
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ćurčić, M.G.; Stanković, M.S.; Mrkalić, E.M.; Matović, Z.D.; Banković, D.D.; Cvetković, D.M.; Đačić, D.S.; Marković, S.D. Antiproliferative and Proapoptotic Activities of Methanolic Extracts from Ligustrum vulgare L. as an Individual Treatment and in Combination with Palladium Complex. Int. J. Mol. Sci. 2012, 13, 2521-2534. https://doi.org/10.3390/ijms13022521
Ćurčić MG, Stanković MS, Mrkalić EM, Matović ZD, Banković DD, Cvetković DM, Đačić DS, Marković SD. Antiproliferative and Proapoptotic Activities of Methanolic Extracts from Ligustrum vulgare L. as an Individual Treatment and in Combination with Palladium Complex. International Journal of Molecular Sciences. 2012; 13(2):2521-2534. https://doi.org/10.3390/ijms13022521
Chicago/Turabian StyleĆurčić, Milena G., Milan S. Stanković, Emina M. Mrkalić, Zoran D. Matović, Dragić D. Banković, Danijela M. Cvetković, Dragana S. Đačić, and Snežana D. Marković. 2012. "Antiproliferative and Proapoptotic Activities of Methanolic Extracts from Ligustrum vulgare L. as an Individual Treatment and in Combination with Palladium Complex" International Journal of Molecular Sciences 13, no. 2: 2521-2534. https://doi.org/10.3390/ijms13022521







