The Effect of Toll-Like Receptor 4 in the Aqueous Humor of Endotoxin-Induced Uveitis
Abstract
:1. Introduction
2. Results
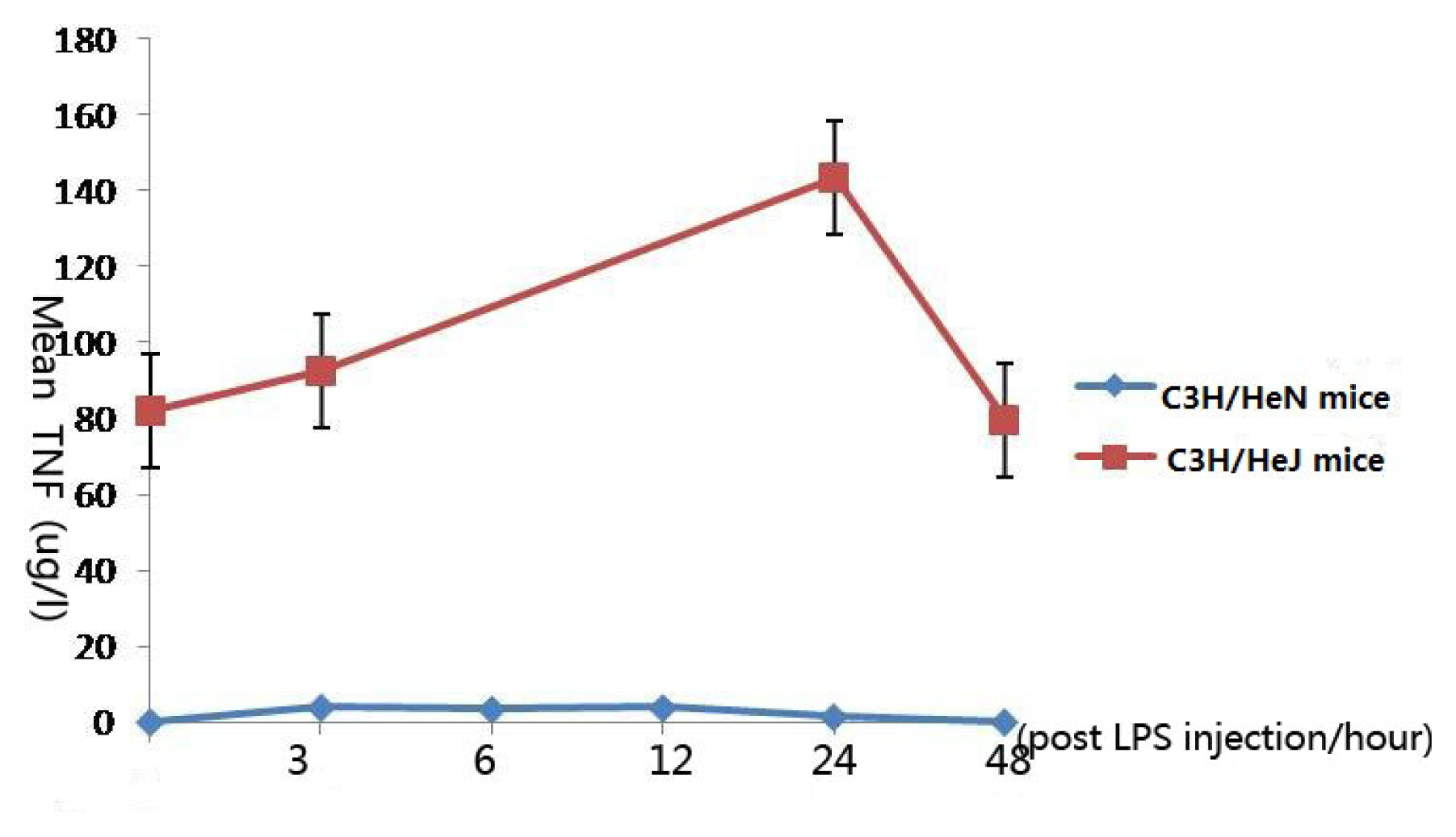
2.1. Concentration of Tumor Necrosis Factor-α in Aqueous Humor
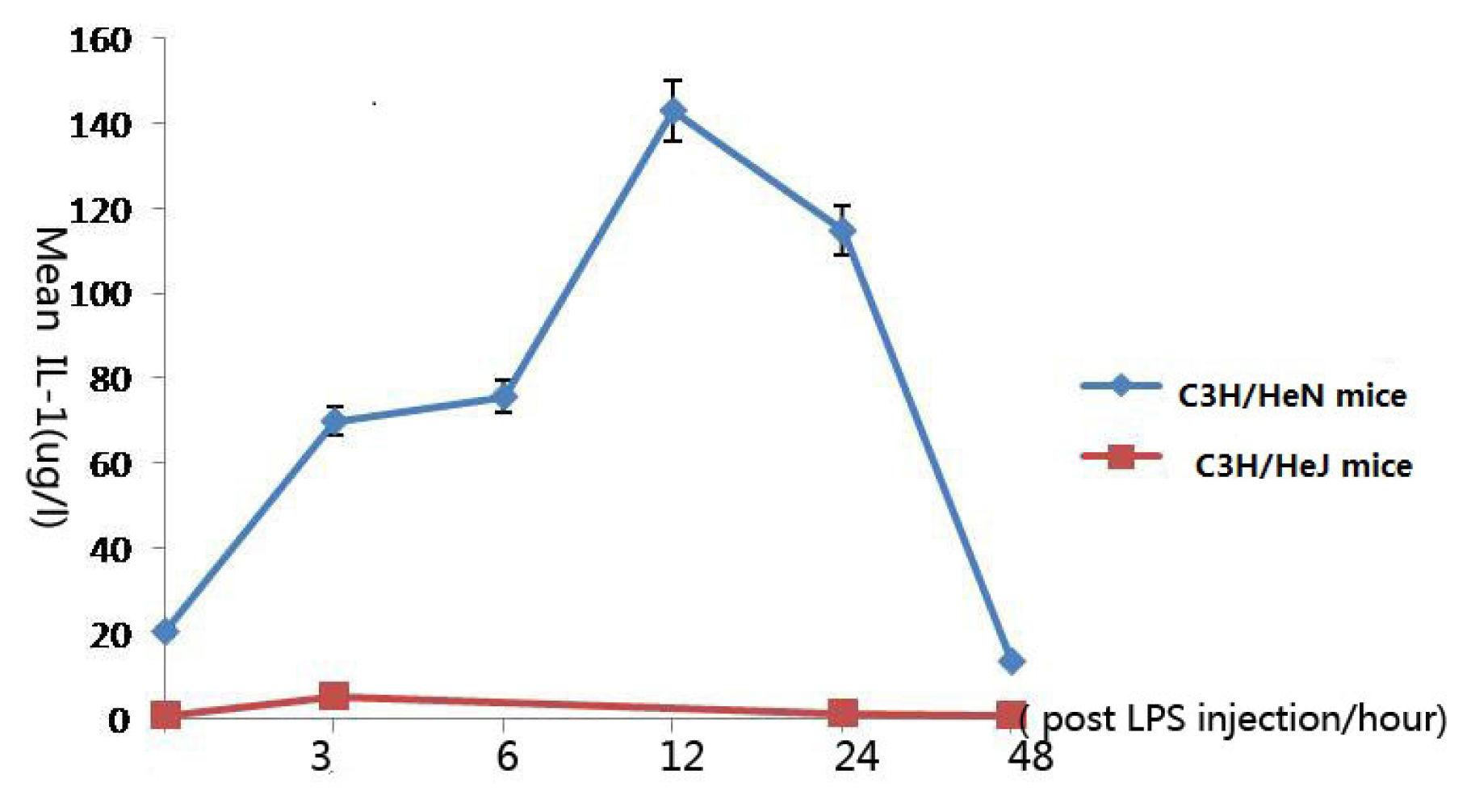
2.2. Concentration of Interleukin-1 in Aqueous Humor
2.3. Concentration of Interleukin-6 in Aqueous Humor
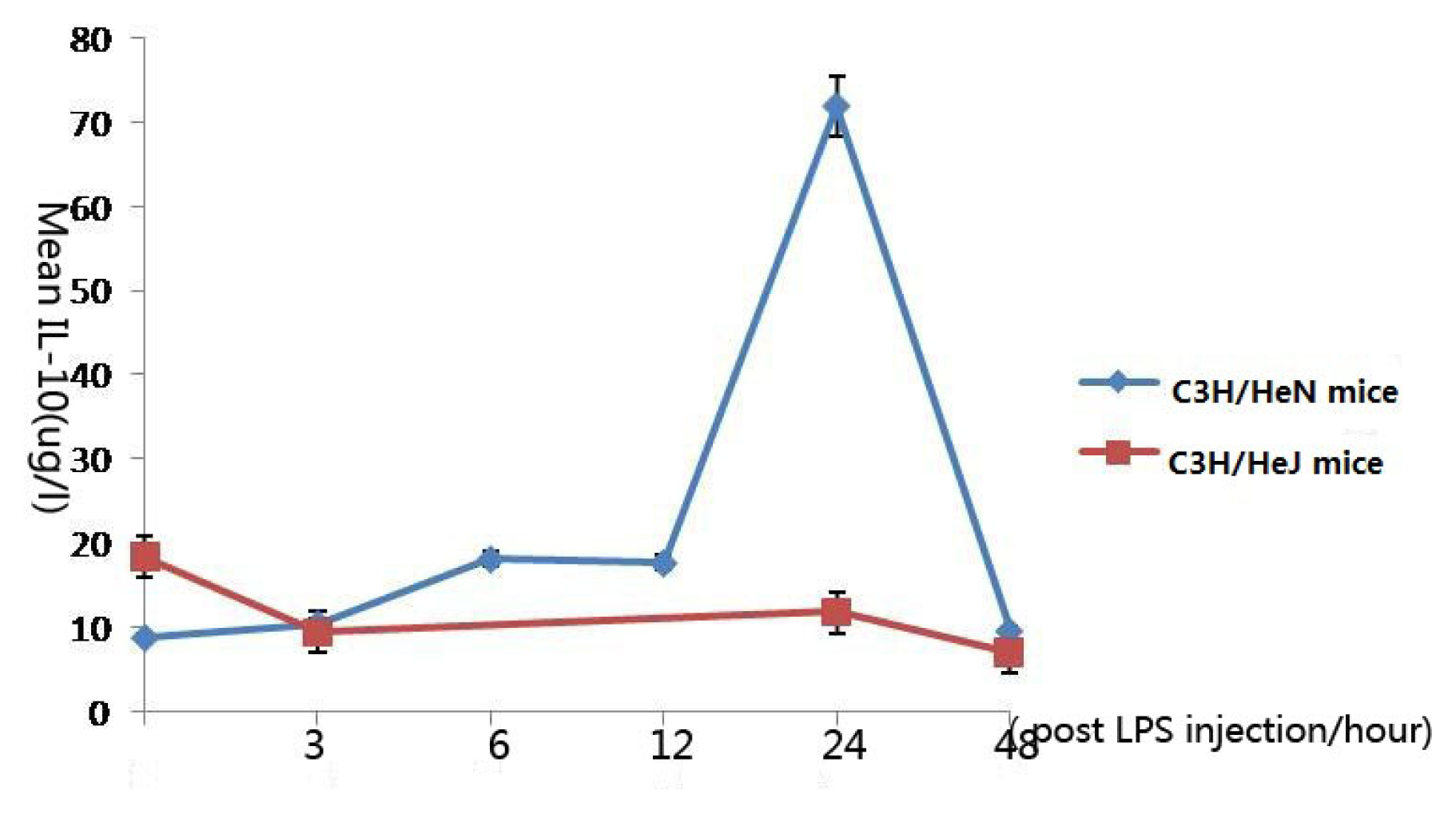
2.4. Concentration of Interleukin-10 in Aqueous Humor
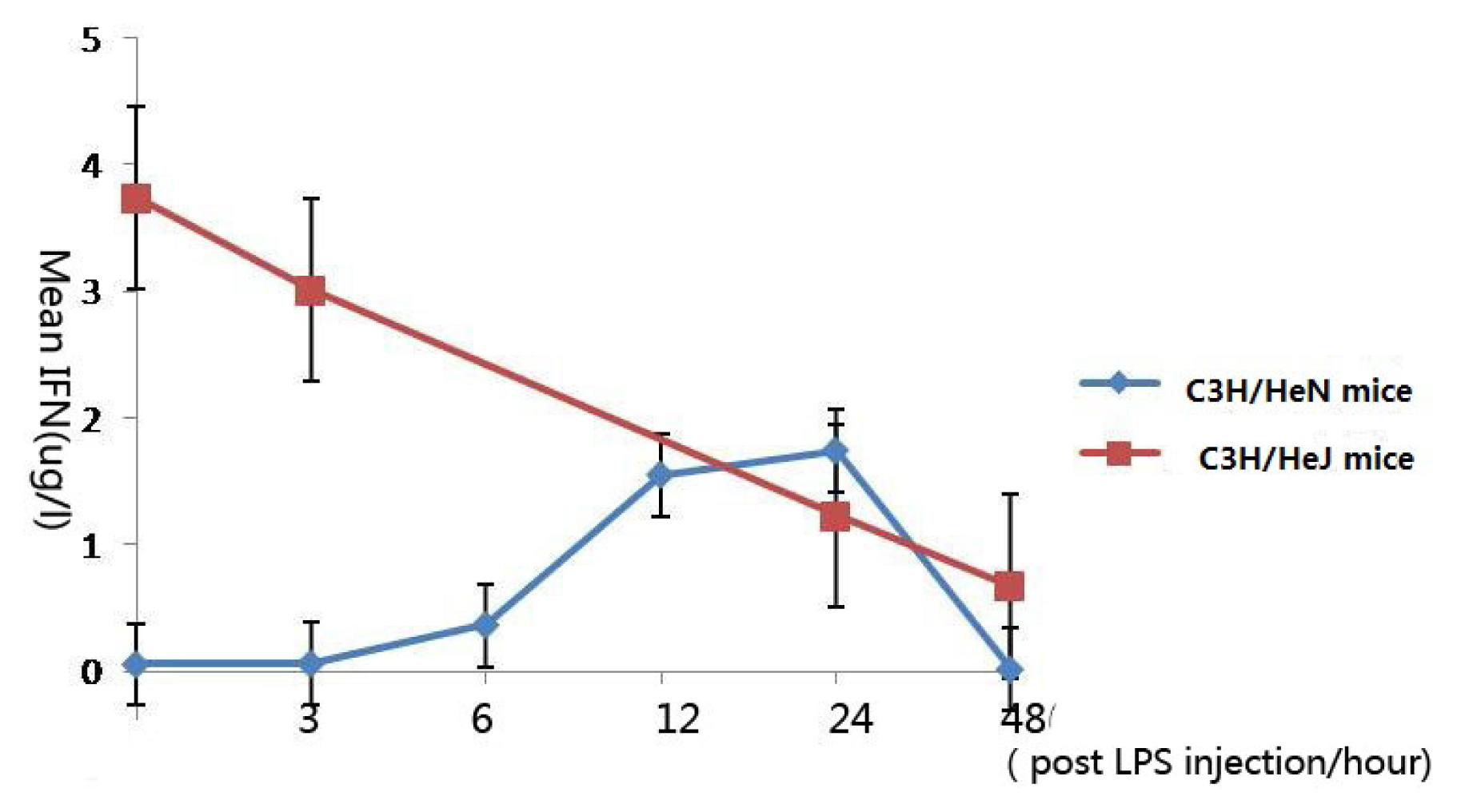
2.5. Concentration of Interferon-γ in Aqueous Humor
3. Discussion
4. Experimental Section
4.1. LPS
4.2. Animals
4.3. Apparatus Used in Cytometric Bead Array
4.4. Sample Collection
4.5. Generation of Standard Curves
4.6. Sample Preparation and Analysis
4.7. Statistics
5. Conclusions
Acknowledgments
References
- Kleemann, R.; Zadelaar, S.; Kooistra, T. Cytokines and atherosclerosis: acomprehensive review of studies in mice. Cardiovasc. Res 2008, 79, 360–376. [Google Scholar]
- Chang, J.H.; McCluskey, P.J.; Wakefield, D. Acute anterior uveitis and HLA-B27. Surv. Ophthalmol 2005, 50, 364–388. [Google Scholar]
- Chang, J.H.; McCluskey, P.J.; Wakefield, D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br. J. Ophthalmol 2006, 90, 103–108. [Google Scholar]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol 2003, 1, 335–376. [Google Scholar]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol 2005, 17, 1–14. [Google Scholar]
- Schnare, M.; Barton, G.M.; Holt, A.C.; Takeda, K.; Akira, S.; Medzhitov, R. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol 2001, 2, 947–950. [Google Scholar]
- Chen, W.; Hu, X.; Zhao, L.; Li, S.; Lu, H. Expression of toll-like receptor 4 in uvea-resident tissue macrophages during endotoxin-induced uveitis. Mol. Vis 2009, 15, 619–628. [Google Scholar]
- Wang, J.; Lu, H.; Hum, X. Nuclear factor translocation and acute anterior uveitis. Mol. Vis. 2011, 17, 170–176. [Google Scholar]
- Chang, J.H.; McCluskey, P.; Wakefield, D. Expression of toll-like receptor 4 and its associated lipopolysaccharide receptor complex by resident antigen-presenting cells in the human uvea. Invest. Ophthalmol. Vis. Sci 2004, 45, 1871–1878. [Google Scholar]
- Xu, Y.; Chen, W.; Lu, H. The expression of cytokines in the aqueous humor and serum during endotoxin-induced uveitis in C3H/HeN mice. Mol. Vis 2010, 16, 1689–1695. [Google Scholar]
- Li, S.; Lu, H.; Hu, X.; Chen, W.; Xu, Y.; Wang, J. Expression of TLR4-MyD88 and NF-KB in the iris during endotoxin-induced uveitis. Mediators Inflamm 2010. [Google Scholar] [CrossRef]
- Zarember, K.A.; Godowski, P.J. Tissue expression of human toll like receptors and differential regulation of toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol 2002, 168, 554–561. [Google Scholar]
- Mita, Y.; Dobashi, K.; Endou, K.; Kawata, T.; Shimizu, Y.; Nakazawa, T.; Mori, M. Toll-like receptor 4 surface expression on human monocytes and B cells is modulated by IL-2 and IL-4. Immunol. Lett 2002, 81, 71–75. [Google Scholar]
- Pasterkamp, G.; Versteeg, D.; de Kleijn, D.P. Immune regulatory cells: circulating biomarker factories in cardiovascular disease. Clin. Sci. (Lond) 2008, 115, 129–131. [Google Scholar]
- Leon, C.G.; Tory, R.; Jia, J.; Sivak, O.; Wasan, K.M. Discovery and development of toll-like receptor 4 (TLR4) antagonists: a new paradigm for treating sepsis and other diseases. Pharm. Res 2008, 25, 1751–1761. [Google Scholar]
- Pérez-Guijo, V.; Santos-Lacomba, M.; Sanchez-Hernandez, M. Tumor necrosis factor-alpha levels in aqueous humor and serum from patients with uveitis: the involvement of HLAB27. Curr. Med. Res. Opin 2004, 20, 155–157. [Google Scholar]
- Santos Lacomba, M.; Marcos-Martin, C.; Gallardo-Galera, J.M. Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic. Res 2001, 33, 251–255. [Google Scholar]
- Schreiber, T.; Ehlers, S.; Heitmann, L.; Rausch, A.; Mages, J.; Murray, P.J. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J. Immunol 2009, 183, 1301–1312. [Google Scholar]
- Lightman, S. Uveitis: what do we know and how does it help? Clin. Experiment Ophthalmol 2001, 29, 48–51. [Google Scholar]
- Sugita, S.; Takase, H.; Taguchi, C.; Mochizuki, M. The role of soluble TNF receptros for TNF-α in uveitis. Invest. Ophthalmol. Vis. Sci 2007, 48, 3246–3252. [Google Scholar]
- Trittibach, P.; Barker, S.E.; Broderick, C.A.; Natkunarajah, M.; Duran, Y.; Robbie, S.J.; Bainbridge, J.W.; Smith, A.J.; Sarra, G.M.; Dick, A.D.; Ali, R.R. Lentiviral-vector-mediated expression of murine IL-1 receptor antagonist or IL-10 reduces the severity of endotoxin-induced uveitis. Gene. Ther 2008, 15, 1478–1488. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, J.; Lu, H.; Xu, Y.; Hu, X.; Chen, W.; Li, S. The Effect of Toll-Like Receptor 4 in the Aqueous Humor of Endotoxin-Induced Uveitis. Int. J. Mol. Sci. 2012, 13, 2110-2118. https://doi.org/10.3390/ijms13022110
Wang J, Lu H, Xu Y, Hu X, Chen W, Li S. The Effect of Toll-Like Receptor 4 in the Aqueous Humor of Endotoxin-Induced Uveitis. International Journal of Molecular Sciences. 2012; 13(2):2110-2118. https://doi.org/10.3390/ijms13022110
Chicago/Turabian StyleWang, Jing, Hong Lu, Yingzhi Xu, Xiaofeng Hu, Wei Chen, and Shang Li. 2012. "The Effect of Toll-Like Receptor 4 in the Aqueous Humor of Endotoxin-Induced Uveitis" International Journal of Molecular Sciences 13, no. 2: 2110-2118. https://doi.org/10.3390/ijms13022110



