Injury-Induced Accumulation of Glial Cell Line-Derived Neurotrophic Factor in the Rostral Part of the Injured Rat Spinal Cord
Abstract
:1. Introduction
2. Results
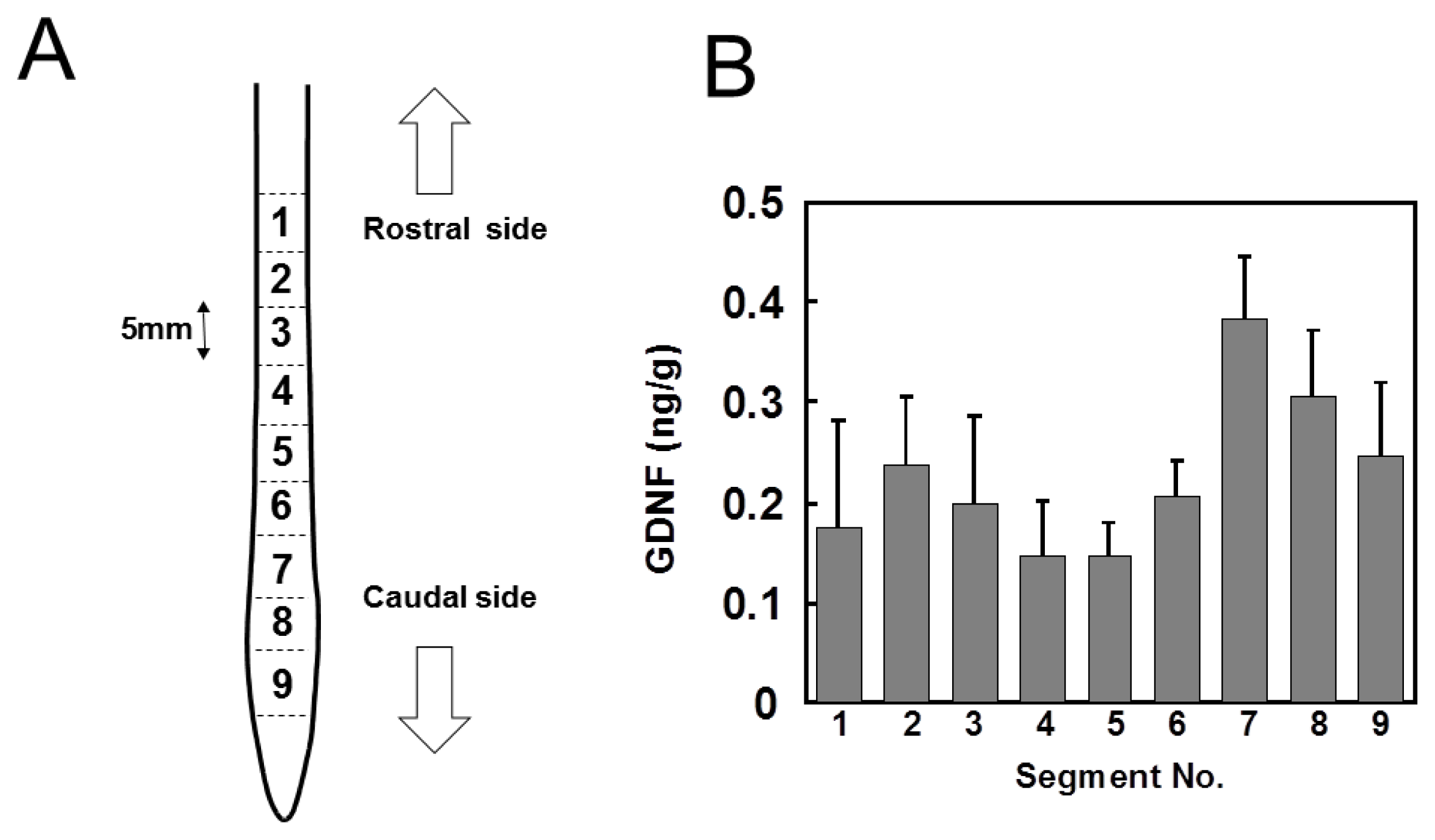
2.1. Distribution of GDNF in Intact Spinal Cord
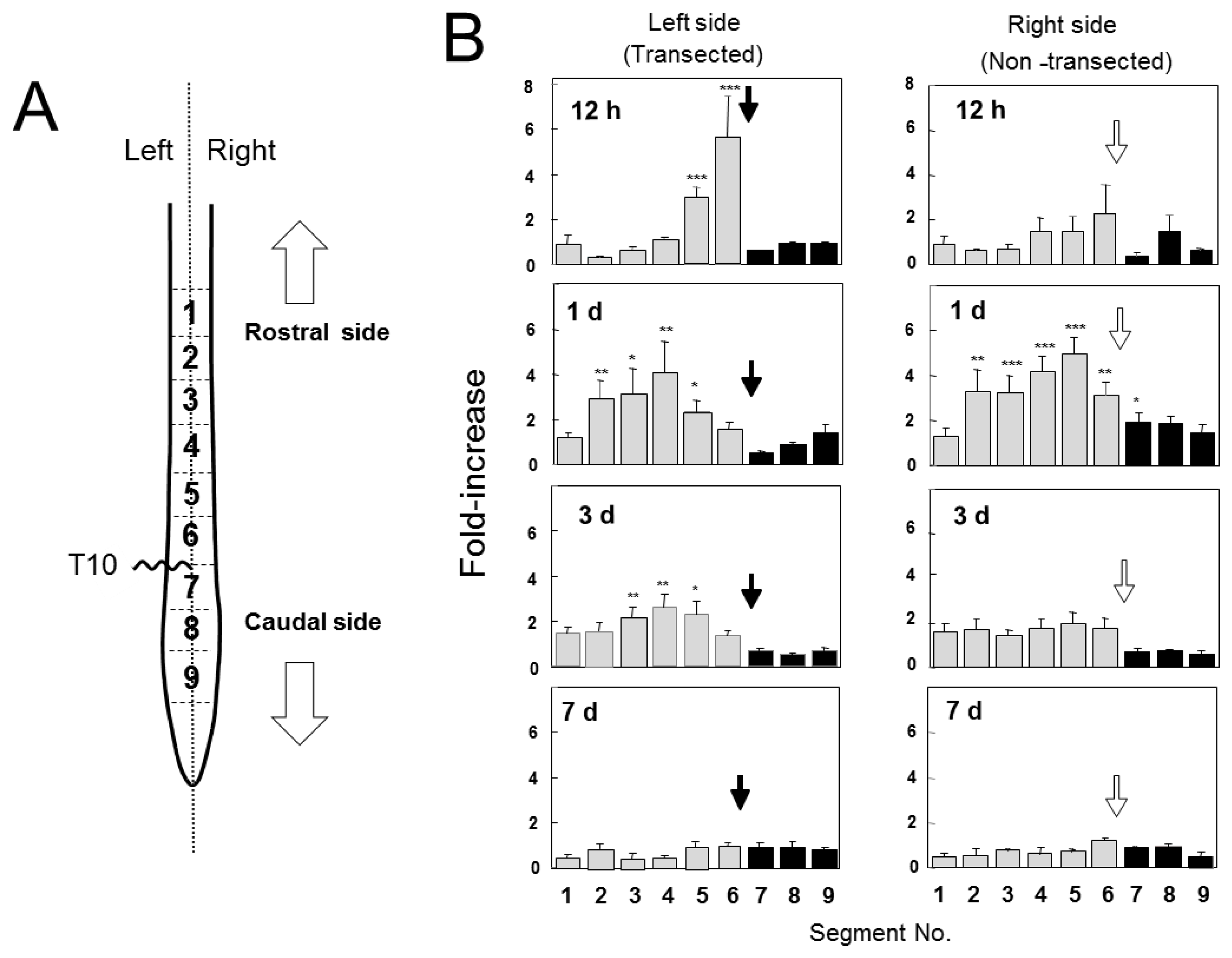
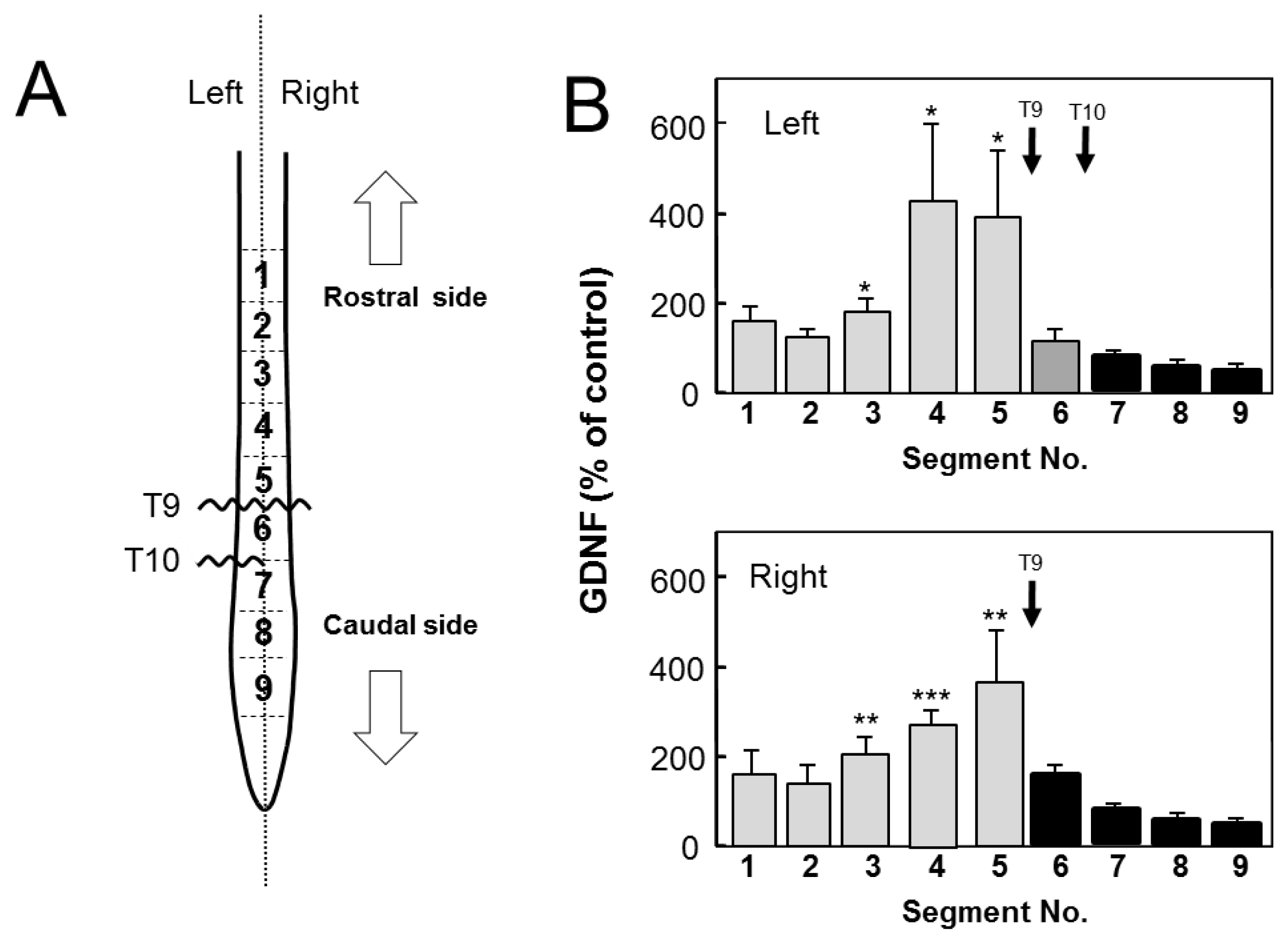
2.2. Changes in GDNF Protein Content after SCI
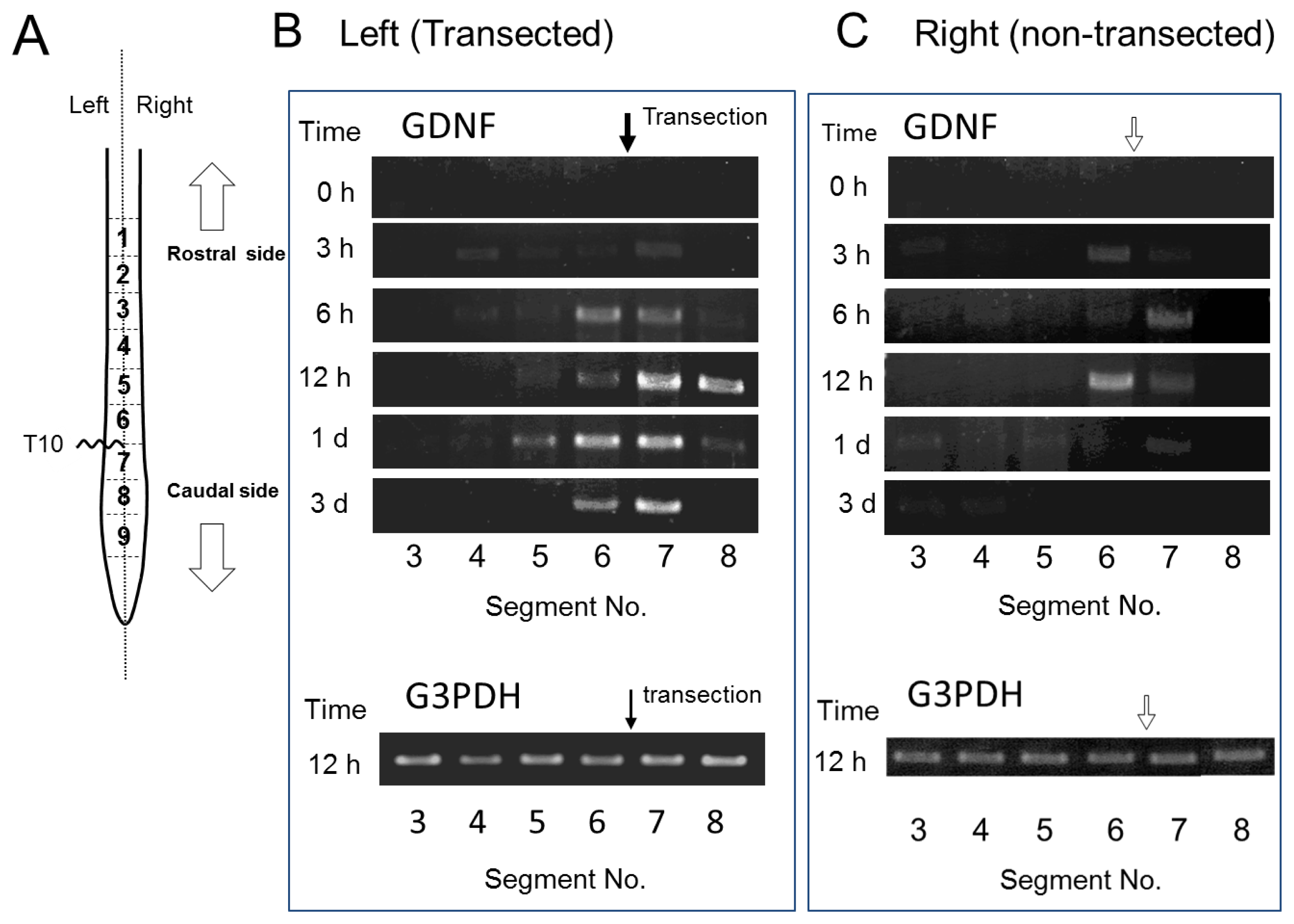
2.3. GDNF mRNA Expression after Spinal Cord Injury
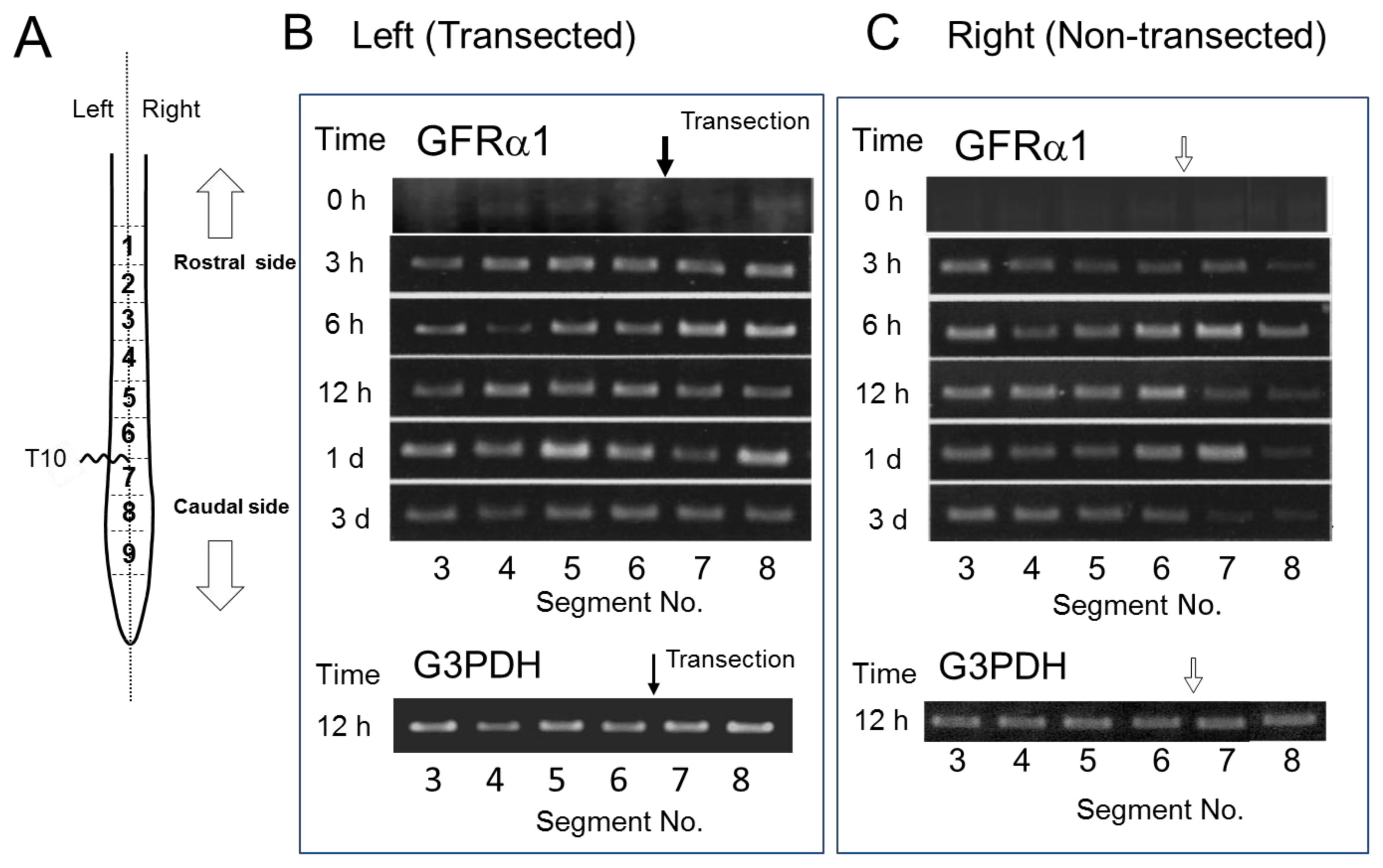
2.4. GFRα1 mRNA Expression after Spinal Cord Injury
2.5. GDNF Immunoreactivity in Intact and Injured Spinal Cord
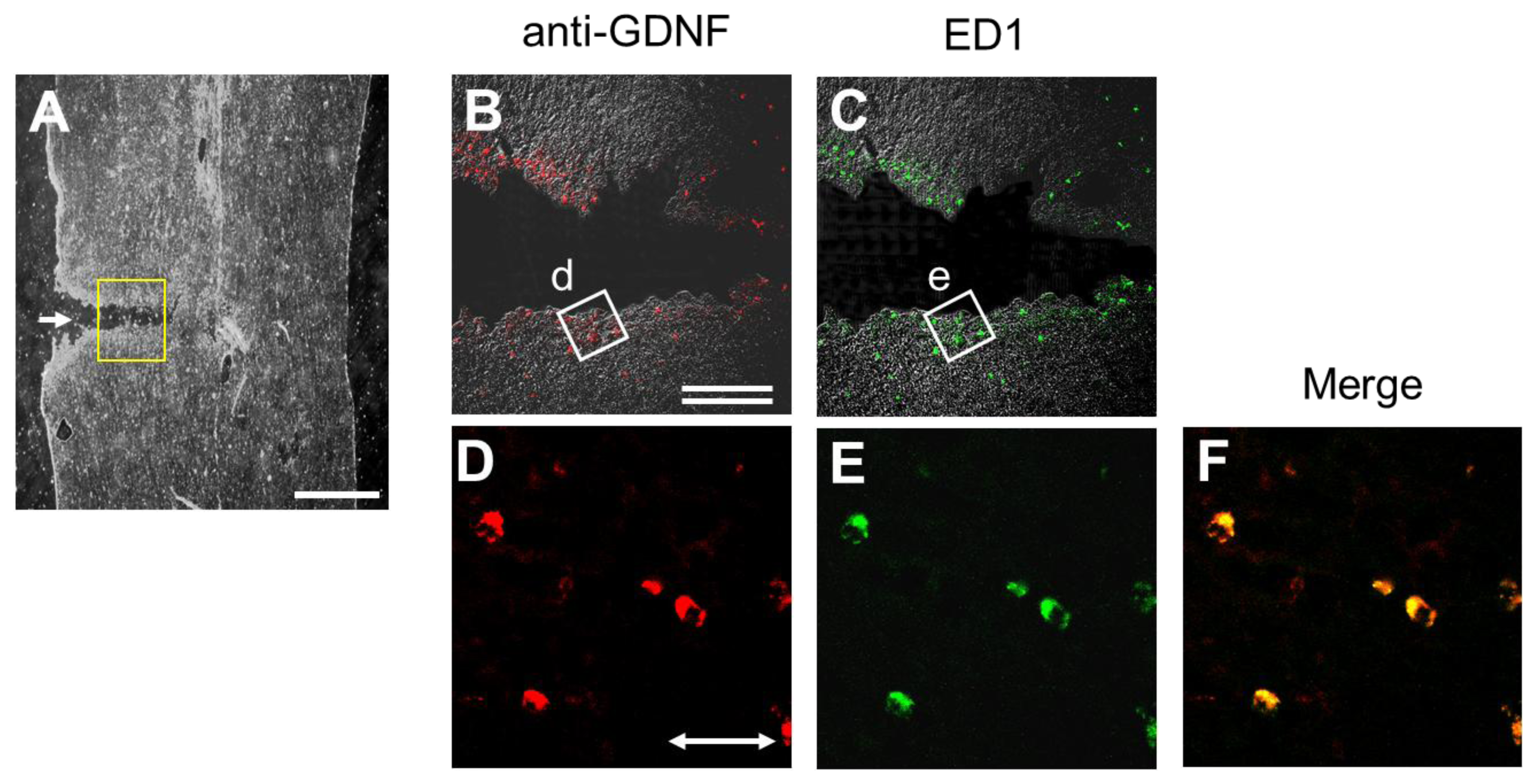
2.6. Injury-Induced GDNF Synthesis in Immune Cells
3. Discussion
4. Experimental Section
4.1. Animals and Surgery
4.2. Preparation of Tissue Samples
4.3. Measurement of GDNF Content by Enzyme Immunoassay
4.4. Immunohistochemical Procedures
4.5. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.6. Statistical Analyses
5. Conclusions
Acknowledgements
References
- Widenfalk, J.; Lundstromer, K.; Jubran, M.; Brene, S.; Olson, L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J. Neurosci 2001, 21, 3457–3475. [Google Scholar]
- Hashimoto, M.; Nitta, A.; Fukumitsu, H.; Nomoto, H.; Shen, L.; Furukawa, S. Inflammation-induced GDNF improves locomotor function after spinal cord injury. Neuroreport 2005, 16, 99–102. [Google Scholar]
- Hashimoto, M.; Ito, T.; Fukumitsu, H.; Nomoto, H.; Furukawa, Y.; Furukawa, S. Stimulation of production of glial cell line-derived neurotrophic factor and nitric oxide by lipopolysaccharide with different dose-responsiveness in cultured rat macrophages. Biomed. Res 2005, 26, 223–229. [Google Scholar]
- Fawcett, J.W.; Asher, R.A. The glial scar and central nervous system repair. Brain Res. Bull 1999, 49, 377–391. [Google Scholar]
- Franzen, R.; Schoenen, J.; Leprince, P.; Joosten, E.; Moonen, G.; Martin, D. Effects of macrophage transplantation in the injured adult rat spinal cord: a combined immunocytochemical and biochemical study. J. Neurosci. Res 1998, 51, 316–327. [Google Scholar]
- Schnell, L.; Schneider, R.; Kolbeck, R.; Barde, Y.A.; Schwab, M.E. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature 1994, 367, 170–173. [Google Scholar]
- Grill, R.; Murai, K.; Blesch, A.; Gage, F.H.; Tuszynski, M.H. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J. Neurosci 1997, 17, 5560–5572. [Google Scholar]
- Menei, P.; Montero-Menei, C.; Whittemore, S.R.; Bunge, R.P.; Bunge, M.B. Schwann cellsgenetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur. J. Neurosci 1998, 10, 607–621. [Google Scholar]
- Zhou, H.L.; Yang, H.J.; Li, Y.M.; Wang, Y.; Yan, L.; Guo, X.L.; Ba, Y.C.; Liu, S.; Wang, T.H. Changes in Glial cell line-derived neurotrophic factor expression in the rostral and caudal stumps of the transected adult rat spinal cord. Neurochem. Res 2008, 33, 927–937. [Google Scholar]
- Frisén, J.; Fried, K.; Sjögren, A.M.; Risling, M. Growth of ascending spinal axons in CNS scar tissue. Int. J. Dev. Neurosci 1993, 11, 461–475. [Google Scholar]
- Yamada, Y.; Shimizu, K.; Nitta, A.; Soumiya, H.; Fukumitsu, H.; Furukawa, S. Axonal regrowth downregulates the synthesis of glial cell line-derived neurotrophic factor in the lesioned rat sciatic nerve. Neurosci. Lett 2004, 364, 11–15. [Google Scholar]
- Hirakawa, A.; Shimizu, K.; Fukumitsu, H.; Furukawa, S. Pyrroloquinoline quinone attenuates iNOS gene expression in the injured spinal cord. Biochem. Biophys. Res. Commun 2009, 378, 308–312. [Google Scholar]
- Hirakawa, A.; Shimizu, K.; Fukumitsu, H.; Soumiya, H.; Iinuma, M.; Furukawa, S. 2-Decenoic acid ethyl ester, a derivative of unsaturated medium-chain fatty acids, facilitates functional recovery of locomotor activity after spinal cord injury. Neuroscience 2010, 171, 1377–1385. [Google Scholar]
- Schmidt, H.; Rathjen, F.G. DiI-labeling of DRG neurons to study axonal branching in a whole mount preparation of mouse embryonic spinal cord. J. Vis. Exp 2011, 58. [Google Scholar] [CrossRef]
- Hammarberg, H.; Piehl, F.; Risling, M.; Cullheim, S. Differential regulation of trophic factor receptor mRNAs in spinal motoneurons after sciatic nerve transection and ventral root avulsion in the rat. J. Comp. Neurol 2000, 426, 587–601. [Google Scholar]
- Rind, H.B.; von Bartheld, C.S. Anterograde axonal transport of internalized GDNF in sensory and motor neurons. Neuroreport 2002, 13, 659–664. [Google Scholar]
- Garcès, A.; Haase, G.; Airaksinen, M.S.; Livet, J.; Filippi, P.; deLapeyrière, O. GFRα1 is required for development of distinct subpopulations of motoneuron. J. Neurosci 2000, 20, 4992–5000. [Google Scholar]
- Oppenheim, R.W.; Houenou, L.J.; Parsadanian, A.S.; Prevette, D.; Snider, W.D.; Shen, L. Glial cell line-derived neurotrophic factor and developing mammalian motoneurons: Regulation of programmed cell death among motoneuron subtypes. J. Neurosci 2000, 20, 5001–5011. [Google Scholar]
- Gould, T.W.; Yonemura, S.; Oppenheim, R.W.; Ohmori, S.; Enomoto, H. The neurotrophic effects of glial cell line-derived neurotrophic factor on spinal motoneurons are restricted to fusimotor subtypes. J. Neurosci 2008, 28, 2131–2146. [Google Scholar]
- Cacalano, G.; Fariñas, I.; Wang, L.C.; Hagler, K.; Forgie, A.; Moore, M.; Armanini, M.; Phillips, H.; Ryan, A.M.; Reichardt, L.F.; et al. GFRα1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron 1998, 21, 53–62. [Google Scholar]
- Airaksinen, M.S.; Saarma, M. The GDNF family: Signalling, biological functions and therapeutic value. Nat. Rev. Neurosci 2002, 3, 383–394. [Google Scholar]
- Whitehead, J.; Keller-Peck, C.R.; Kucera, J. Glial cell-line derived neurotrophic factor-dependent fusimotor neuron survival during development. Mech. Dev 2005, 122, 27–41. [Google Scholar]
- Haase, G.; Dessaud, E.; Garcès, A.; de Bovis, B.; Birling, M.; Filippi, P. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron 2002, 35, 893–905. [Google Scholar]
- Livet, J.; Sigrist, M.; Stroebel, S.; de Paola, V.; Price, S.R.; Henderson, C.E.; Jessell, T.M.; Arber, S. ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron 2002, 35, 877–892. [Google Scholar]
- Kramer, E.R.; Knott, L.; Su, F.; Dessaud, E.; Krull, C.E.; Helmbacher, F. Cooperation between GDNF/Ret and ephrinA/EphA4 signals for motor-axon pathway selection in the limb. Neuron 2006, 50, 35–47. [Google Scholar]
- Nosrat, C.A.; Tomac, A.; Lindqvist, E.; Lindskog, S.; Humpel, C.; Strömberg, I. Cellular expression of GDNF mRNA suggests multiple functions inside and outside the nervous system. Cell Tissue Res 1996, 286, 191–207. [Google Scholar]
- Peng, C.; Aron, L.; Klein, R.; Li, M.; Wurst, W.; Prakash, N.; Le, W. Pitx3 Is a Critical Mediator of GDNF-Induced BDNF Expression in Nigrostriatal Dopaminergic Neurons. J. Neurosci 2011, 31, 12802–12815. [Google Scholar]
- Pochon, N.A.; Menoud, A.; Tseng, J.L.; Zurn, A.D.; Aebischer, P. Neuronal GDNF expression in the adult rat nervous system identified by in situ hybridization. Eur. J. Neurosci 1997, 9, 463–471. [Google Scholar]
- Golden, J.P.; Baloh, R.H.; Kotzbauer, P.T.; Lampe, P.A.; Osborne, P.A.; Milbrandt, J.; Johnson, E.M., Jr. Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J. Comp. Neurol 1998, 398, 139–150. [Google Scholar]
- Kawamoto, Y.; Nakamura, S.; Matsuo, A.; Akiguchi, I.; Shibasaki, H. Immunohistochemical localization of glial cell line-derived neurotrophic factor in the human central nervous system. Neuroscience 2000, 100, 701–712. [Google Scholar]
- Katoh-Semba, R.; Tsuzuki, M.; Miyazaki, N.; Yoshida, A.; Nakajima, H.; Nakagawa, C.; Kitajima, S.; Matsuda, M. Distribution and immunohistochemical localization of GDNF protein in selected neural and non-neural tissues of rats during development and changes in unilateral 6-hydroxydopamine lesions. Neurosci. Res 2007, 59, 277–287. [Google Scholar]
- Tokumine, J.; Kakinohana, O.; Cizkova, D.; Smith, D.W.; Marsala, M. Changes in spinal GDNF, BDNF, and NT-3 expression after transient spinal cord ischemia in the rat. J. Neurosci. Res 2003, 74, 552–561. [Google Scholar]
- Tokumine, J.; Sugahara, K.; Kakinohana, O.; Marsala, M. The spinal GDNF level is increased after transient spinal cord ischemia in the rat. Acta Neurochir. Suppl 2003, 86, 231–234. [Google Scholar]
- Kokaia, Z.; Airaksinen, M.S.; Nanobashvili, A.; Larsson, E. GDNF family ligands and receptors are differentially regulated after brain insults in the rat. Eur. J. Neurosci 1999, 11, 1202–1216. [Google Scholar]
- Foust, K.D.; Flotte, T.R.; Reier, P.J.; Mandel, R.J. Recombinant adeno-associated virus-mediated global anterograde delivery of glial cell line-derived neurotrophic factor to the spinal cord: Comparison of rubrospinal and corticospinal tracts in the rat. Hum. Gene. Ther 2008, 19, 71–82. [Google Scholar]
- Lu, Y.Y.; Wang, L.J.; Muramatsu, S.; Ikeguchi, K.; Fujimoto, K.; Okada, T.; Mizukami, H.; Matsushita, T.; Hanazono, Y.; Kume, A.; et al. Intramuscular injection of AAV-GDNF results in sustained expression of transgenic GDNF, and its delivery to spinal motoneurons by retrograde transport. Neurosci. Res 2003, 45, 33–40. [Google Scholar]
- Heumann, R.; Korsching, S.; Bandtlow, C.; Thoenen, H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J. Cell Biol 1987, 104, 1623–1631. [Google Scholar]
- Meyer, M.; Matsuoka, I.; Wetmore, C.; Olson, L.; Thoenen, H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J. Cell Biol 1992, 119, 45–54. [Google Scholar]
- Sendtner, M.; Stöckli, K.A.; Thoenen, H. Synthesis and localization of ciliary neurotrophic factor in the sciatic nerve of the adult rat after lesion and during regeneration. J. Cell Biol 1992, 118, 139–148. [Google Scholar]
- Nitta, A.; Ohmiya, M.; Jin-nouchi, T.; Sometani, A.; Asami, T.; Kinukawa, H.; Fukumitsu, H.; Nomoto, H.; Furukawa, S. Endogenous neurotrophin-3 is retrogradely transported in the rat sciatic nerve. Neuroscience 1999, 88, 679–685. [Google Scholar]
- Koelsch, A.; Feng, Y.; Fink, D.J.; Mata, M. Transgene-mediated GDNF expression enhances synaptic connectivity and GABA transmission to improve functional outcome after spinal cord contusion. J. Neurochem 2010, 113, 143–152. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hara, T.; Fukumitsu, H.; Soumiya, H.; Furukawa, Y.; Furukawa, S. Injury-Induced Accumulation of Glial Cell Line-Derived Neurotrophic Factor in the Rostral Part of the Injured Rat Spinal Cord. Int. J. Mol. Sci. 2012, 13, 13484-13500. https://doi.org/10.3390/ijms131013484
Hara T, Fukumitsu H, Soumiya H, Furukawa Y, Furukawa S. Injury-Induced Accumulation of Glial Cell Line-Derived Neurotrophic Factor in the Rostral Part of the Injured Rat Spinal Cord. International Journal of Molecular Sciences. 2012; 13(10):13484-13500. https://doi.org/10.3390/ijms131013484
Chicago/Turabian StyleHara, Takuya, Hidefumi Fukumitsu, Hitomi Soumiya, Yoshiko Furukawa, and Shoei Furukawa. 2012. "Injury-Induced Accumulation of Glial Cell Line-Derived Neurotrophic Factor in the Rostral Part of the Injured Rat Spinal Cord" International Journal of Molecular Sciences 13, no. 10: 13484-13500. https://doi.org/10.3390/ijms131013484




