Inhibition of Enzyme Activity of Rhipicephalus (Boophilus) microplus Triosephosphate Isomerase and BME26 Cell Growth by Monoclonal Antibodies
Abstract
:1. Introduction
2. Results
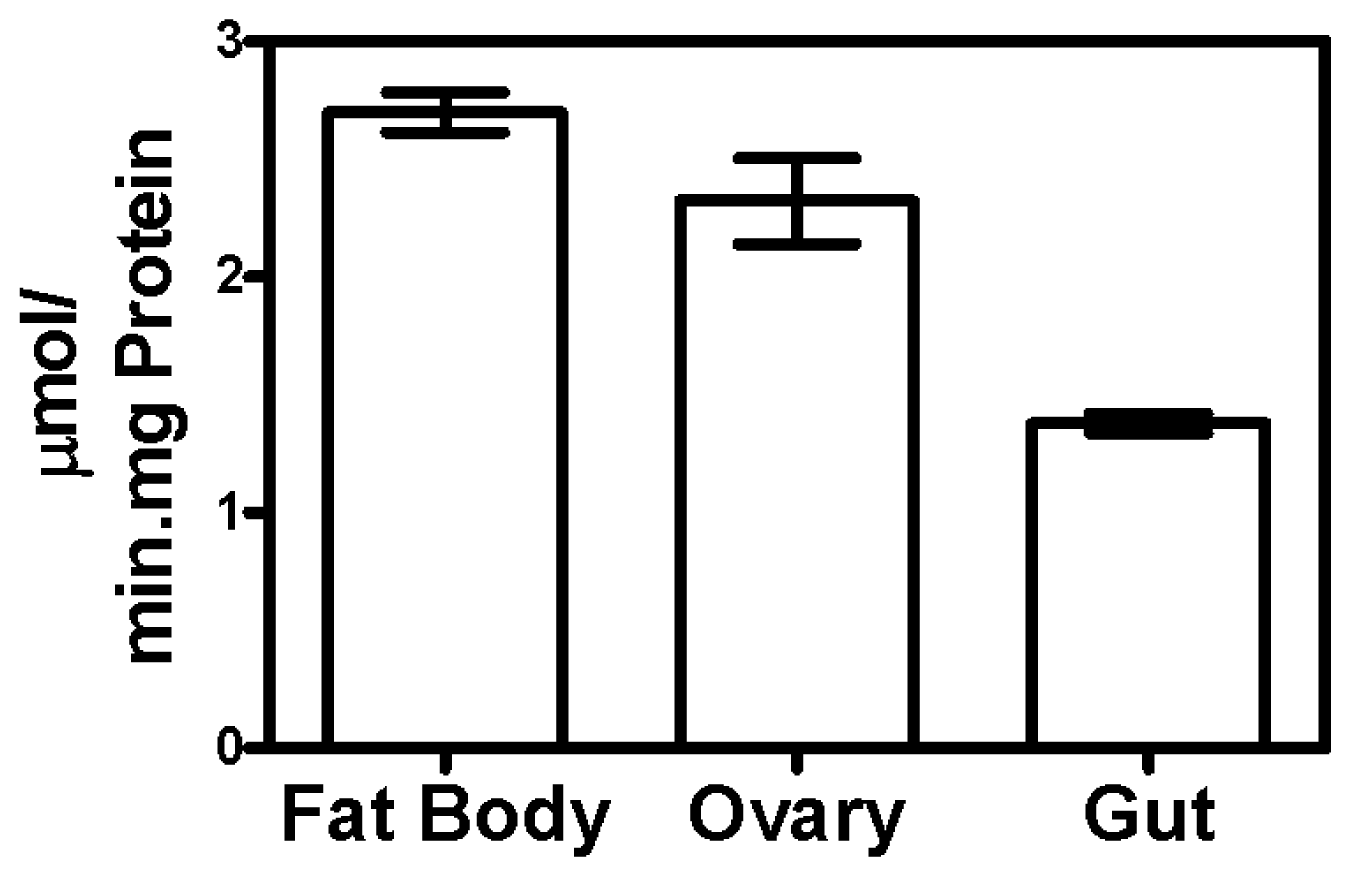
2.1. Triosephosphate Isomerase Activity in Different Tissues
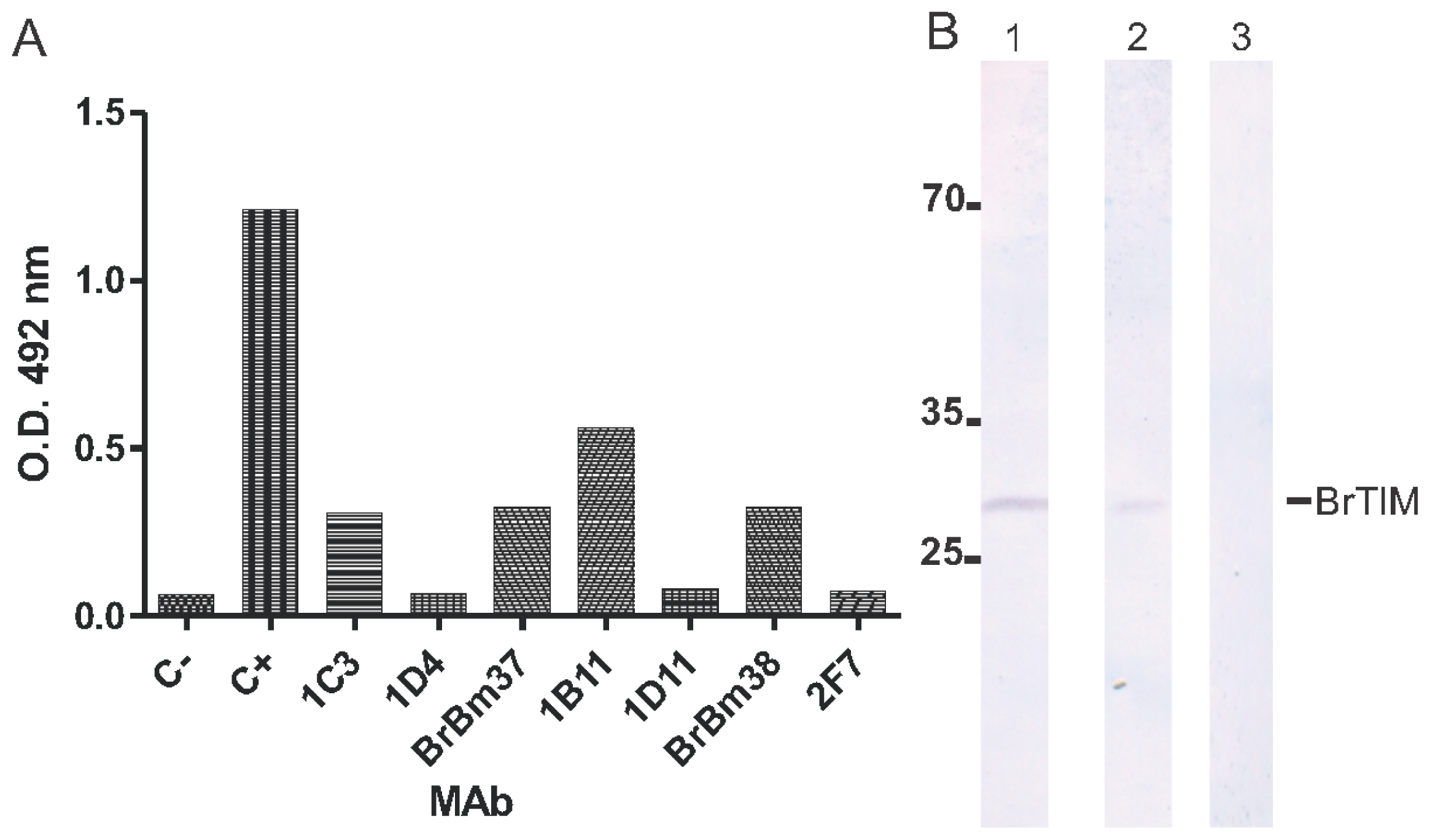
2.2. Monoclonal Antibodies
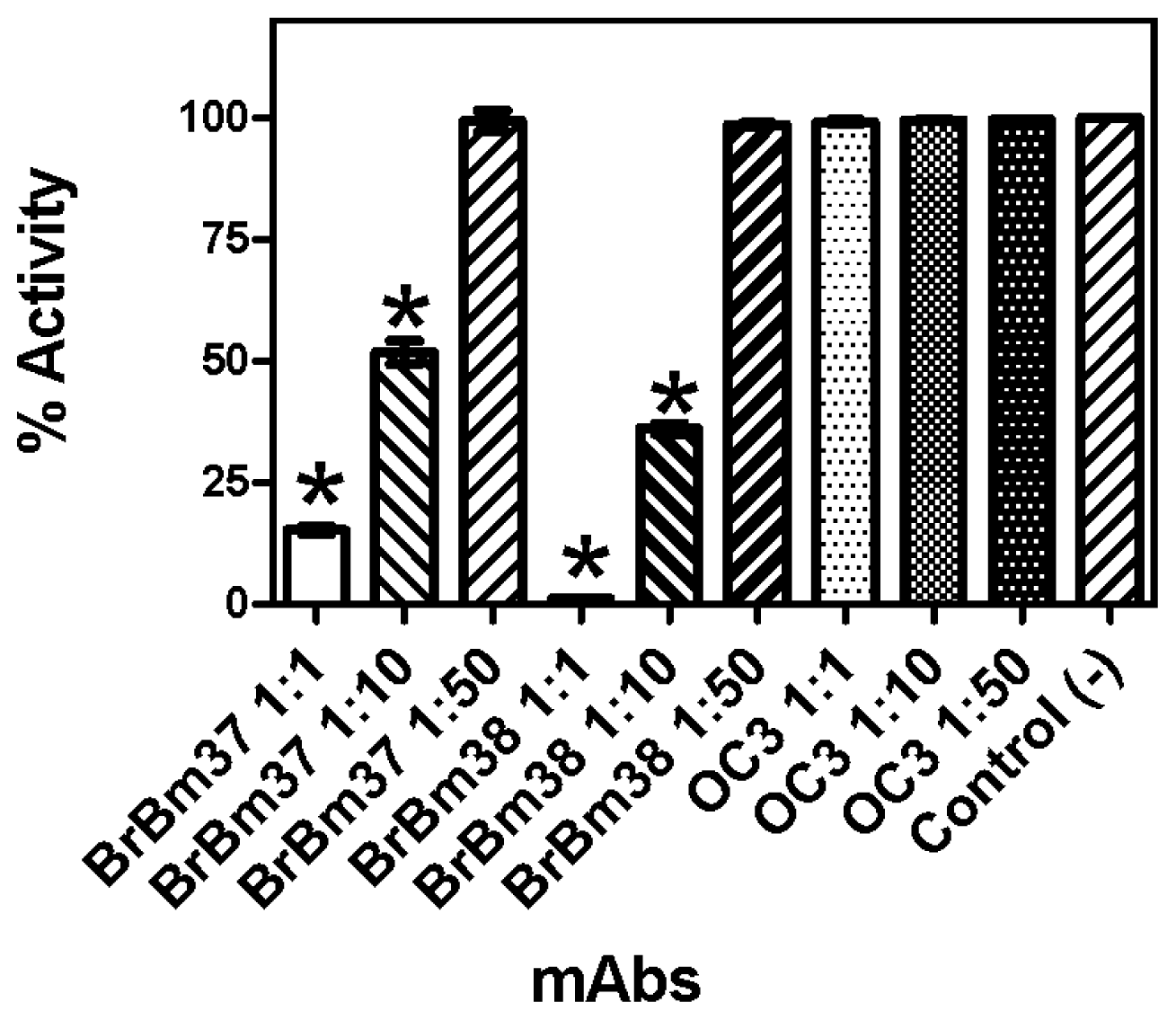
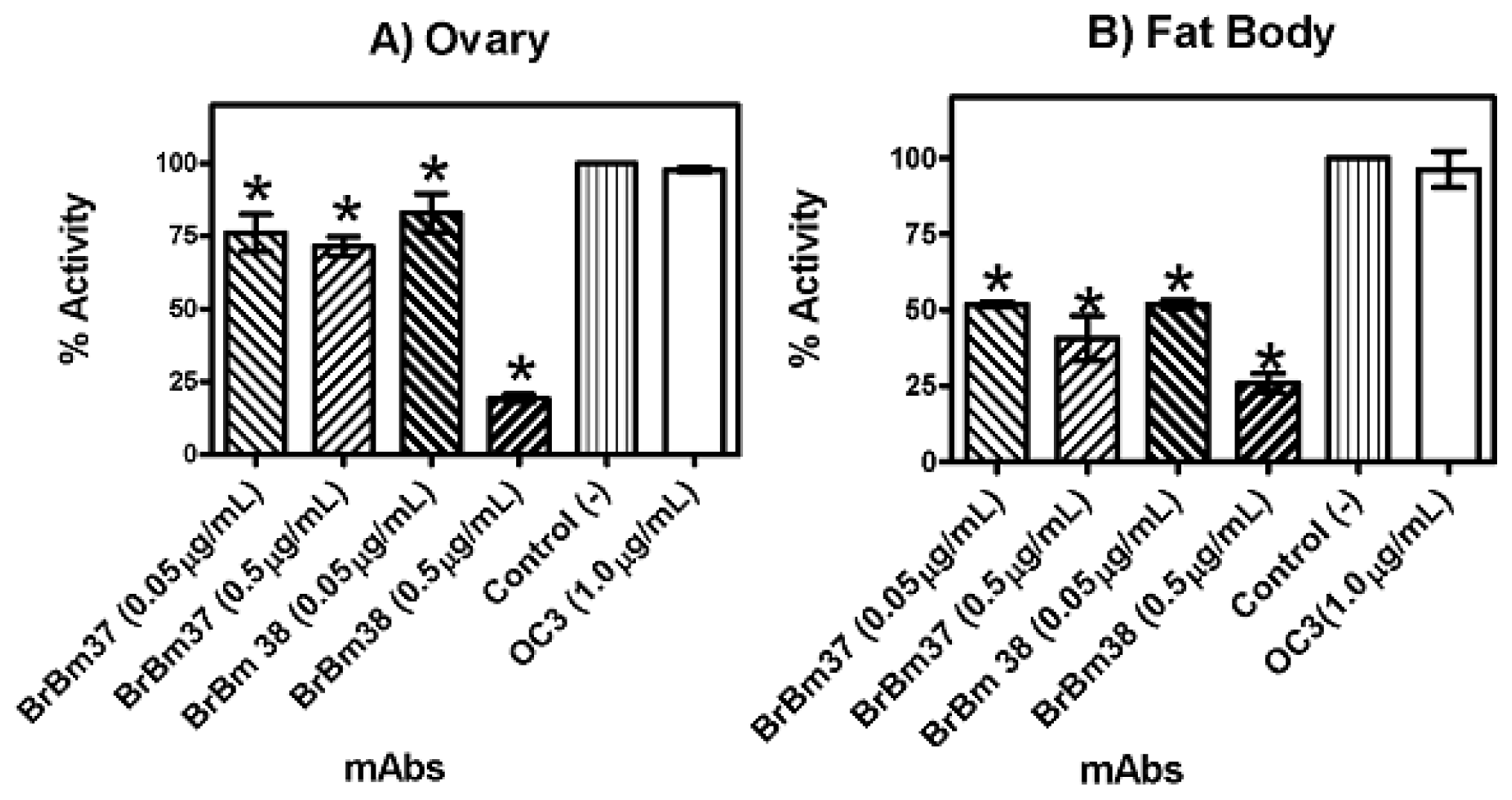
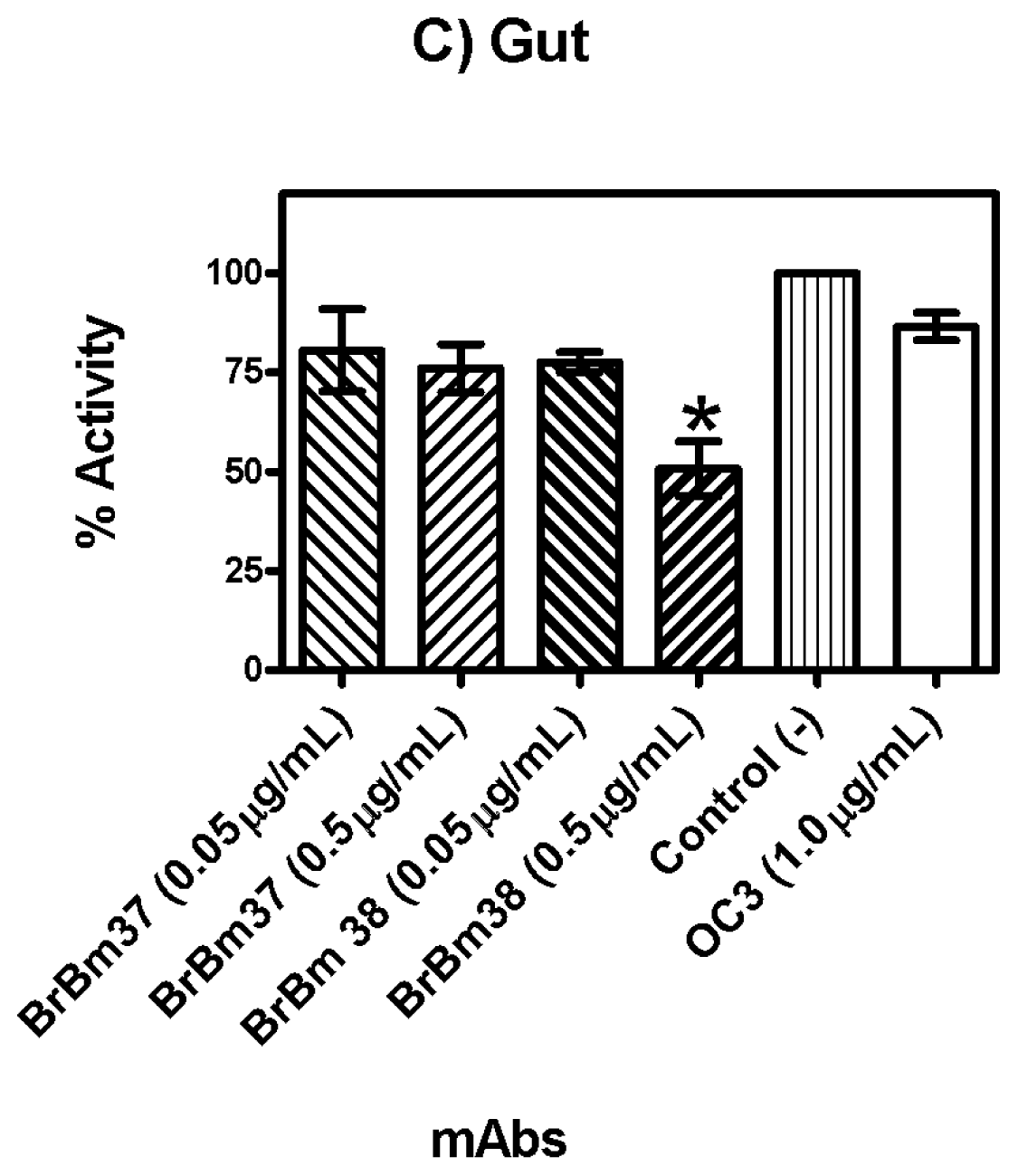
2.3. Monoclonal Antibody Inhibition of Triosephosphate Isomerase (TIM) Enzymatic Activity
2.4. Inhibitory Effect of mAb on Growth of BME26 Cells
3. Discussion
4. Experimental Section
4.1. Tissue Antigen Preparations
4.2. Production of Monoclonal Antibodies (mAbs)
4.3. ELISA
4.4. Immunoblot
4.5. Triosephosphate Isomerase Activity Assays
4.6. Evaluation of the Addition of mAb to BME26 Cell Cultures
5. Conclusions
Acknowledgements
References
- Jonsson, N.N. The productivity effects of cattle tick (Boophilus microplus) infestation on cattle, with particular reference to Bos indicus cattle and their crosses. Vet. Parasitol 2006, 137, 1–10. [Google Scholar]
- Dalgliesh, R.J.; Stewart, N.P. The Use of Tick transmission by Boophilus microplus to isolate pure strains of Babesia bovis, Babesia bigemina and Anaplasma marginale from cattle with mixed infections. Vet. Parasitol 1983, 13, 317–323. [Google Scholar]
- Jonsson, N.N.; Bock, R.E.; Jorgensen, W.K. Productivity and health effects of Anaplasmosis and Babesiosis on Bos indicus cattle and their crosses, and the effects of differing intensity of tick control in Australia. Vet. Parasitol 2008, 155, 1–9. [Google Scholar]
- Chevillon, C.; Ducornez, S.; de Meeûs, T.; Koffi, B.B.; Gaia, H.; Delathiere, J.M.; Barre, N. Accumulation of acaricide resistance mechanisms in Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) populations from New Caledonia island. Vet. Parasitol 2007, 147, 276–288. [Google Scholar]
- Rosario-Cruz, R.; Almazan, C.; Miller, R.J.; Dominguez-Garcia, D.I.; Hernandez-Ortiz, R.; de la Fuente, J. Genetic basis and impact of Tick Acaricide resistance. Front Biosci 2009, 14, 2657–2665. [Google Scholar]
- Morgan, J.A.; Corley, S.W.; Jackson, L.A.; Lew-Tabor, A.E.; Moolhuijzen, P.M.; Jonsson, N.N. Identification of a Mutation in the para sodium channel gene of the Cattle Tick Rhipicephalus (Boophilus) microplus associated with resistance to synthetic pyrethroid acaricides. Int. J. Parasitol 2009, 7, 775–779. [Google Scholar]
- Rosario-Cruz, R.; Guerrero, F.D.; Miller, R.J.; Rodriguez-Vivas, R.I.; Tijerina, M.; Dominguez-Garcia, D.I.; Hernandez-Ortiz, R.; Cornel, A.J.; McAbee, R.D.; Alonso-Diaz, M.A. Molecular survey of pyrethroid resistance mechanisms in mexican field Populations of Rhipicephalus (Boophilus) microplus. Parasitol. Res 2009, 105, 1145–1153. [Google Scholar]
- Pohl, P.C.; Klafke, G.M.; Carvalho, D.D.; Martins, J.R.; Daffre, S.; da Silva, V.I., Jr; Masuda, A. ABC transporter efflux pumps: A defense mechanism against ivermectin in Rhipicephalus (Boophilus) microplus. Int. J. Parasitol 2011, 41, 1323–1333. [Google Scholar]
- Samish, M.; Ginsberg, H.; Glazer, I. Biological Control of Ticks. Parasitology 2004, 129, S389–S403. [Google Scholar]
- Rodriguez-Valle, M.; Lew-Tabor, A.; Gondro, C.; Moolhuijzen, P.; Vance, M.; Guerrero, F.D.; Bellgard, M.; Jorgensen, W. Comparative Microarray Analysis of Rhipicephalus (Boophilus) microplus Expression Profiles of Larvae Pre-Attachment and Feeding Adult Female Stages on Bos indicus and Bos taurus Cattle. BMC Genomics 2010, 11, 437. [Google Scholar]
- De la Fuente, J.; Almazan, C.; Canales, M.; Pérez de la Lastra, J.M.; Kocan, K.M.; Willadsen, P. A Ten-Year Review of Commercial Vaccine Performance for Control of Tick Infestations on Cattle. Anim Health Res. Rev 2007, 8, 23–28. [Google Scholar]
- Vercruysse, J.; Schetters, T.P.; Knox, D.P.; Willadsen, P.; Claerebout, E. Control of parasitic disease using vaccines: An answer to drug resistance? Rev. Sci. Tech 2007, 26, 105–115. [Google Scholar]
- Rodriguez, M.; Penichet, M.L.; Mouris, A.E.; Labarta, V.; Luaces, L.L.; Rubiera, R.; Cordoves, C.; Sanchez, P.A.; Ramos, E.; et al. Control of Boophilus microplus populations in grazing cattle vaccinated with a recombinant Bm86 antigen preparation. Vet. Parasitol 1995, 57, 339–349. [Google Scholar]
- Riding, G.A.; Jarmey, J.; McKenna, R.V.; Pearson, R.; Cobon, G.S.; Willadsen, P. A protective “Concealed” antigen from Boophilus microplus. Purification, localization, and possible function. J. Immunol 1994, 153, 5158–5166. [Google Scholar]
- Garcia-Garcia, J.C.; Montero, C.; Redondo, M.; Vargas, M.; Canales, M.; Boue, O.; Rodriguez, M.; Joglar, M.; Machado, H.; Gonzalez, I.L.; et al. Control of Ticks Resistant to Immunization With Bm86 in Cattle Vaccinated With the Recombinant Antigen Bm95 Isolated From the Cattle Tick, Boophilus microplus. Vaccine 2000, 18, 2275–2287. [Google Scholar]
- Da Silva Vaz, I., Jr; Logullo, C.; Sorgine, M.; Velloso, F.F.; Rosa de Lima, M.F.; Gonzales, J.C.; Masuda, H.; Oliveira, P.L.; Masuda, A. Immunization of Bovines With an Aspartic Proteinase Precursor Isolated From Boophilus microplus Eggs. Vet. Immunol. Immunopathol 1998, 66, 331–341. [Google Scholar]
- Leal, A.T.; Pohl, P.C.; Ferreira, C.A.S.; Nascimento-Silva, M.C.L.; Sorgine, M.H.F.; Logullo, C.; Oliveira, P.L.; Farias, S.E.; Vaz, I.D.; Masuda, A. Purification and Antigenicity of Two Recombinant Forms of Boophilus microplus Yolk Pro-Cathepsin Expressed in Inclusion Bodies. Protein Expr. Purif 2006, 45, 107–114. [Google Scholar]
- Parizi, L.F.; Utiumi, K.U.; Imamura, S.; Onuma, M.; Ohashi, K.; Masuda, A.; da Silva, V.I., Jr. Cross Immunity with Haemaphysalis longicornis Glutathione S-Transferase Reduces an Experimental Rhipicephalus (Boophilus) microplus Infestation. Exp. Parasitol 2011, 127, 113–118. [Google Scholar]
- Seixas, A.; Leal, A.T.; Nascimento-Silva, M.C.; Masuda, A.; Termignoni, C.; da Silva, V.I., Jr. Vaccine Potential of a Tick Vitellin-Degrading Enzyme (VTDCE). Vet. Immunol. Immunopathol 2008, 124, 332–340. [Google Scholar]
- Seixas, A.; Oliveira, P.; Termignoni, C.; Logullo, C.; Masuda, A.; da Silva Vaz, I., Jr. Rhipicephalus (Boophilus) microplus Embryo Proteins as Target for Tick Vaccine. Vet. Immunol. Immunopathol 2012, 148, 149–156. [Google Scholar]
- Garza-Ramos, G.; Perez-Montfort, R.; Rojo-Dominguez, A.; de Gomez-Puyou, M.T.; Gomez-Puyou, A. Species-Specific Inhibition of Homologous Enzymes by Modification of Nonconserved Amino Acids Residues. The Cysteine Residues of Triosephosphate Isomerase. Eur. J. Biochem 1996, 241, 114–120. [Google Scholar]
- Maldonado, E.; Soriano-Garcia, M.; Moreno, A.; Cabrera, N.; Garza-Ramos, G.; de Gomez-Puyou, M.; Gomez-Puyou, A.; Perez-Montfort, R. Differences in the Intersubunit Contacts in Triosephosphate Isomerase From Two Closely Related Pathogenic Trypanosomes. J. Mol. Biol 1998, 283, 193–203. [Google Scholar]
- Gao, X.G.; Maldonado, E.; Perez-Montfort, R.; Garza-Ramos, G.; de Gomez-Puyou, M.T.; Gomez-Puyou, A.; Rodriguez-Romero, A. Crystal Structure of Triosephosphate Isomerase From Trypanosoma cruzi in Hexane. Proc. Natl. Acad. Sci. USA 1999, 96, 10062–10067. [Google Scholar]
- Hernandez-Alcantara, G.; Garza-Ramos, G.; Hernandez, G.M.; Gomez-Puyou, A.; Perez-Montfort, R. Catalysis and Stability of Triosephosphate Isomerase From Trypanosoma brucei With Different Residues at Position 14 of the Dimer Interface. Characterization of a Catalytically Competent Monomeric Enzyme. Biochemistry 2002, 41, 4230–4238. [Google Scholar]
- Zomosa-Signoret, V.; Hernandez-Alcantara, G.; Reyes-Vivas, H.; Martinez-Martinez, E.; Garza-Ramos, G.; Perez-Montfort, R.; Tuena, D.G.-P.; Gomez-Puyou, A. Control of the Reactivation Kinetics of Homodimeric Triosephosphate Isomerase From Unfolded Monomers. Biochemistry 2003, 42, 3311–3318. [Google Scholar]
- Gomez-Puyou, A.; Saavedra-Lira, E.; Becker, I.; Zubillaga, R.A.; Rojo-Dominguez, A.; Perez-Montfort, R. Using Evolutionary Changes to Achieve Species-Specific Inhibition of Enzyme Action—Studies With Triosephosphate Isomerase. Chem. Biol 1995, 2, 847–855. [Google Scholar]
- Zhu, Y.; Si, J.; Ham, D.A.; Yu, C.; He, W.; Hua, W.; Yin, X.; Liang, Y.; Xu, M.; Xu, R. The Protective Immunity Produced in Infected C57BL/6 Mice of a DNA Vaccine Encoding Schistosoma japonicum Chinese Strain Triose-Phosphate Isomerase. Southeast Asian J. Trop. Med. Public Health 2002, 33, 207–213. [Google Scholar]
- Jimenez, L.; Fernandez-Velasco, D.A.; Willms, K.; Landa, A. A Comparative Study of Biochemical and Immunological Properties of Triosephosphate Isomerase From Taenia solium and Sus scrofa. J. Parasitol 2003, 89, 209–214. [Google Scholar]
- Zhu, Y.; Si, J.; Harn, D.A.; Yu, C.; Liang, Y.; Ren, J.; Yin, X.; He, W.; Hua, W. The Protective Immunity of a DNA Vaccine Encoding Schistosoma japonicum Chinese Strain Triose-Phosphate Isomerase in Infected BALB/C Mice. Southeast Asian J. Trop. Med. Public Health 2004, 35, 518–522. [Google Scholar]
- Zhu, Y.; Si, J.; Harn, D.A.; Xu, M.; Ren, J.; Yu, C.; Liang, Y.; Yin, X.; He, W.; Cao, G. Schistosoma Japonicum Triose-Phosphate Isomerase Plasmid DNA Vaccine Protects Pigs Against Challenge Infection. Parasitology 2006, 132, 67–71. [Google Scholar]
- Reis, E.A.; Mauadi Carmo, T.A.; Athanazio, R.; Reis, M.G.; Harn, D.A., Jr. Schistosoma mansoni Triose Phosphate Isomerase Peptide MAP4 Is Able to Trigger Naive Donor Immune Response Towards a Type-1 Cytokine Profile. Scand. J. Immunol 2008, 68, 169–176. [Google Scholar]
- Jimenez, L.; Vibanco-Perez, N.; Navarro, L.; Landa, A. Cloning, Expression and Characterisation of a Recombinant Triosephosphate Isomerase From Taenia solium. Int. J. Parasitol 2000, 30, 1007–1012. [Google Scholar]
- Moraes, J.; Arreola, R.; Cabrera, N.; Saramago, L.; Freitas, D.; Masuda, A.; da Silva, V.I., Jr; Tuena, D.G.-P.; Perez-Montfort, R.; Gomez-Puyou, A.; et al. Biochemical Characterization of a Recombinant Triosephosphate Isomerase From Rhipicephalus (Boophilus) microplus. Insect Biochem. Mol. Biol 2011, 41, 400–409. [Google Scholar]
- Nejad-Moghaddam, A.; Abolhassani, M. Production and Characterization of Monoclonal Antibodies Recognizing a Common 57-KDa Antigen of Leishmania Species. Iran Biomed. J 2009, 13, 245–251. [Google Scholar]
- Nakajima, M.; Yanase, H.; Iwanaga, T.; Kodama, M.; Ohashi, K.; Onuma, M. Passive Immunization with Monoclonal Antibodies: Effects on Haemaphysalis longicornis Tick Infestation of BALB/c Mice. Jpn. J. Vet. Res 2003, 50, 157–163. [Google Scholar]
- Gonsioroski, A.V.; Bezerra, I.A.; Utiumi, K.U.; Driemeier, D.; Farias, S.E.; da Silva, V.I., Jr; Masuda, A. Anti-Tick Monoclonal Antibody Applied by Artificial Capillary Feeding in Rhipicephalus (Boophilus) microplus Females. Exp. Parasitol 2012, 130, 359–363. [Google Scholar]
- Richard, J.P. A Paradigm for Enzyme-Catalyzed Proton Transfer at Carbon: Triosephosphate Isomerase. Biochemistry 2012, 51, 2652–2661. [Google Scholar]
- Zomosa-Signoret, V.; Guirre-Lopez, B.; Hernandez-Alcantara, G.; Perez-Montfort, R.; de Gomez-Puyou, M.T.; Gomez-Puyou, A. Crosstalk Between the Subunits of the Homodimeric Enzyme Triosephosphate Isomerase. Proteins 2007, 67, 75–83. [Google Scholar]
- Sorgine, M.H.; Logullo, C.; Zingali, R.B.; Paiva-Silva, G.O.; Juliano, L.; Oliveira, P.L. A Heme-Binding Aspartic Proteinase from the Eggs of the Hard Tick Boophilus microplus. J. Biol. Chem 2000, 275, 28659–28665. [Google Scholar]
- Parizi, L.F.; Pohl, P.C.; Masuda, A.; Vaz Ida, S., Jr. New Approaches Toward Anti-Rhipicephalus (Boophilus) microplus Tick Vaccine. Rev. Bras. Parasitol. Vet 2009, 18, 1–7. [Google Scholar]
- Leder, L.; Berger, C.; Bornhauser, S.; Wendt, H.; Ackermann, F.; Jelesarov, I.; Bosshard, H.R. Spectroscopic, Calorimetric, and Kinetic Demonstration of Conformational Adaptation in Peptide-Antibody Recognition. Biochemistry 1995, 34, 16509–16518. [Google Scholar]
- Weber-Bornhauser, S.; Eggenberger, J.; Jelesarov, I.; Bernard, A.; Berger, C.; Bosshard, H.R. Thermodynamics and Kinetics of the Reaction of a Single-Chain Antibody Fragment (ScFv) With the Leucine Zipper Domain of Transcription Factor GCN4. Biochemistry 1998, 37, 13011–13020. [Google Scholar]
- Katschke, K.J., Jr; Stawicki, S.; Yin, J.; Steffek, M.; Xi, H.; Sturgeon, L.; Hass, P.E.; Loyet, K.M.; Deforge, L.; Wu, Y.; et al. Functional Analysis of a C3b-Specific Antibody That Selectively Inhibits the Alternative Pathway of Complement. J. Biol. Chem 2009, 284, 10473–10479. [Google Scholar]
- Park, S.S.; Fujino, T.; West, D.; Guengerich, F.P.; Gelboin, H.V. Monoclonal Antibodies That Inhibit Enzyme Activity of 3-Methylcholanthrene-Induced Cytochrome P-450. Cancer Res 1982, 42, 1798–1808. [Google Scholar]
- Park, S.S.; Cha, S.J.; Miller, H.; Persson, A.V.; Coon, M.J.; Gelboin, H.V. Monoclonal Antibodies to Rabbit Liver Cytochrome P-450LM2 and Cytochrome P-450LM4. Mol. Pharmacol 1982, 21, 248–258. [Google Scholar]
- Djavadi-Ohaniance, L.; Friguet, B.; Goldberg, M.E. Structural and Functional Influence of Enzyme-Antibody Interactions: Effects of Eight Different Monoclonal Antibodies on the Enzymatic Activity of Escherichia coli Tryptophan Synthase. Biochemistry 1984, 23, 97–104. [Google Scholar]
- Larvor, M.P.; Djavadi-Ohaniance, L.; Friguet, B.; Baleux, F.; Goldberg, M.E. Peptide/Antibody Recognition: Synthetic Peptides Derived From the E. Coli Tryptophan Synthase Beta 2 Subunit Interact With High Affinity With an Anti-Beta 2 Monoclonal Antibody. Mol. Immunol 1991, 28, 523–531. [Google Scholar]
- Rowley, G.L.; Rubenstein, K.E.; Huisjen, J.; Ullman, E.F. Mechanism by Which Antibodies Inhibit Hapten-Malate Dehydrogenase Conjugates. An Enzyme Immunoassay for Morphine. J. Biol. Chem 1975, 250, 3759–3766. [Google Scholar]
- Gregory, K.F.; Ng, C.W.; Pantekoek, J.F. Antibody to Lactate Dehydrogenase. I. Inhibition of Glycolysis in Tumor and Liver Homogenates. Biochim. Biophys. Acta 1966, 130, 469–476. [Google Scholar]
- Rardin, M.J.; Wiley, S.E.; Naviaux, R.K.; Murphy, A.N.; Dixon, J.E. Monitoring Phosphorylation of the Pyruvate Dehydrogenase Complex. Anal. Biochem 2009, 389, 157–164. [Google Scholar]
- Labarta, V.; Rodriguez, M.; Penichet, M.; Lleonart, R.; Luaces, L.L.; de la, F.J. Simulation of Control Strategies for the Cattle Tick Boophilus microplus Employing Vaccination With a Recombinant Bm86 Antigen Preparation. Vet. Parasitol 1996, 63, 131–160. [Google Scholar]
- De la Fuente, J.; Rodriguez, M.; Montero, C.; Redondo, M.; Garcia-Garcia, J.C.; Mendez, L.; Serrano, E.; Valdes, M.; Enriquez, A.; Canales, M.; et al. Vaccination Against Ticks (Boophilus Spp.): the Experience With the Bm86-Based Vaccine Gavac. Genet. Anal 1999, 15, 143–148. [Google Scholar]
- Bergquist, N.R. Schistosomiasis Vaccine Development: Approaches and Prospects. Mem. Inst. Oswaldo Cruz 1995, 90, 221–227. [Google Scholar]
- Cortes-Figueroa, A.A.; Perez-Torres, A.; Salaiza, N.; Cabrera, N.; Escalona-Montano, A.; Rondan, A.; guirre-Garcia, M.; Gomez-Puyou, A.; Perez-Montfort, R.; Becker, I. A monoclonal antibody that inhibits Trypanosoma cruzi growth in vitro and its reaction with intracellular triosephosphate isomerase. Parasitol. Res 2008, 102, 635–643. [Google Scholar]
- Lee, W.H.; Choi, J.S.; Byun, M.R.; Koo, K.T.; Shin, S.; Lee, S.K.; Surh, Y.J. Functional inactivation of triosephosphate isomerase through phosphorylation during etoposide-induced apoptosis in HeLa cells: Potential role of Cdk2. Toxicology 2010, 278, 224–228. [Google Scholar]
- Jin, Y.H.; Yoo, K.J.; Lee, Y.H.; Lee, S.K. Caspase 3-mediated cleavage of P21WAF1/CIP1 associated with the cyclin A-cyclin-dependent kinase 2 complex is a prerequisite for apoptosis in SK-HEP-1 cells. J. Biol. Chem 2000, 275, 30256–30263. [Google Scholar]
- Jin, Y.H.; Yim, H.; Park, J.H.; Lee, S.K. Cdk2 Activity is associated with depolarization of mitochondrial membrane potential during apoptosis. Biochem. Biophys. Res. Commun 2003, 305, 974–980. [Google Scholar]
- Ahmed, N.; Battah, S.; Karachalias, N.; Babaei-Jadidi, R.; Horanyi, M.; Baroti, K.; Hollan, S.; Thornalley, P.J. Increased formation of methylglyoxal and protein glycation, oxidation and nitrosation in triosephosphate isomerase deficiency. Biochim. Biophys. Acta 2003, 1639, 121–132. [Google Scholar]
- Fraval, H.N.; McBrien, D.C. The effect of methyl glyoxal on cell Division and the synthesis of protein and DNA in synchronous and asynchronous Cultures of Escherichia coli B/r. J. Gen. Microbiol 1980, 117, 127–134. [Google Scholar]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Methylglyoxal comes of AGE. Cell 2006, 124, 258–260. [Google Scholar]
- Dhar-Chowdhury, P.; Harrell, M.D.; Han, S.Y.; Jankowska, D.; Parachuru, L.; Morrissey, A.; Srivastava, S.; Liu, W.; Malester, B.; Yoshida, H.; et al. The glycolytic enzymes, glyceraldehyde- 3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the K(ATP) channel macromolecular complex and regulate its function. J. Biol. Chem 2005, 280, 38464–38470. [Google Scholar]
- Da Silva, V.I., Jr; Ozaki, L.S.; Masuda, A. Serum of Boophilus microplus infested cattle reacts with different tick tissues. Vet. Parasitol 1994, 52, 71–78. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- Kohler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. [Google Scholar]
- Harlow, E.; Lane, D. Antibodies: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1988; p. 123. [Google Scholar]
- Crowther, J.R.; Farias, S.; Carpenter, W.C.; Samuel, A.R. Identification of a Fifth Neutralizable Site on Type O Foot-and-Mouth Disease Virus Following Characterization of Single and Quintuple Monoclonal Antibody Escape Mutants. J. Gen. Virol 1993, 74, 1547–1553. [Google Scholar]
- Dunn, S.D. Effects of the Modification of Transfer Buffer Composition and the Renaturation of Proteins in Gels on the Recognition of Proteins on Western Blots by Monoclonal Antibodies. Anal. Biochem 1986, 157, 144–153. [Google Scholar]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic Transfer of Proteins from Polyacrylamide Gels to Nitrocellulose Sheets: Procedure and Some Applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar]
- Burnette, W.N. “Western Blotting”: Electrophoretic Transfer of Proteins From Sodium Dodecyl Sulfate--Polyacrylamide Gels to Unmodified Nitrocellulose and Radiographic Detection With Antibody and Radioiodinated Protein A. Anal. Biochem 1981, 112, 195–203. [Google Scholar]
- Esteves, E.; Lara, F.A.; Lorenzini, D.M.; Costa, G.H.; Fukuzawa, A.H.; Pressinotti, L.N.; Silva, J.R.; Ferro, J.A.; Kurtti, T.J.; Munderloh, U.G.; et al. Cellular and Molecular Characterization of an Embryonic Cell Line (BME26) From the Tick Rhipicephalus (Boophilus) microplus. Insect Biochem. Mol. Biol 2008, 38, 568–580. [Google Scholar]
- De Abreu, L.A.; Fabres, A.; Esteves, E.; Masuda, A.; da Silva, V.I., Jr; Daffre, S.; Logullo, C. Exogenous Insulin Stimulates Glycogen Accumulation in Rhipicephalus (Boophilus) microplus Embryo Cell Line BME26 Via PI3K/AKT Pathway. Comp. Biochem. Physiol B Biochem. Mol. Biol 2009, 153, 185–190. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saramago, L.; Franceschi, M.; Logullo, C.; Masuda, A.; Vaz, I.D.S., Jr.; Farias, S.E.; Moraes, J. Inhibition of Enzyme Activity of Rhipicephalus (Boophilus) microplus Triosephosphate Isomerase and BME26 Cell Growth by Monoclonal Antibodies. Int. J. Mol. Sci. 2012, 13, 13118-13133. https://doi.org/10.3390/ijms131013118
Saramago L, Franceschi M, Logullo C, Masuda A, Vaz IDS Jr., Farias SE, Moraes J. Inhibition of Enzyme Activity of Rhipicephalus (Boophilus) microplus Triosephosphate Isomerase and BME26 Cell Growth by Monoclonal Antibodies. International Journal of Molecular Sciences. 2012; 13(10):13118-13133. https://doi.org/10.3390/ijms131013118
Chicago/Turabian StyleSaramago, Luiz, Mariana Franceschi, Carlos Logullo, Aoi Masuda, Itabajara Da Silva Vaz, Jr., Sandra Estrazulas Farias, and Jorge Moraes. 2012. "Inhibition of Enzyme Activity of Rhipicephalus (Boophilus) microplus Triosephosphate Isomerase and BME26 Cell Growth by Monoclonal Antibodies" International Journal of Molecular Sciences 13, no. 10: 13118-13133. https://doi.org/10.3390/ijms131013118
APA StyleSaramago, L., Franceschi, M., Logullo, C., Masuda, A., Vaz, I. D. S., Jr., Farias, S. E., & Moraes, J. (2012). Inhibition of Enzyme Activity of Rhipicephalus (Boophilus) microplus Triosephosphate Isomerase and BME26 Cell Growth by Monoclonal Antibodies. International Journal of Molecular Sciences, 13(10), 13118-13133. https://doi.org/10.3390/ijms131013118





