Preparation of a Cu(II)-PVA/PA6 Composite Nanofibrous Membrane for Enzyme Immobilization
Abstract
:1. Introduction
2. Results and Discussion
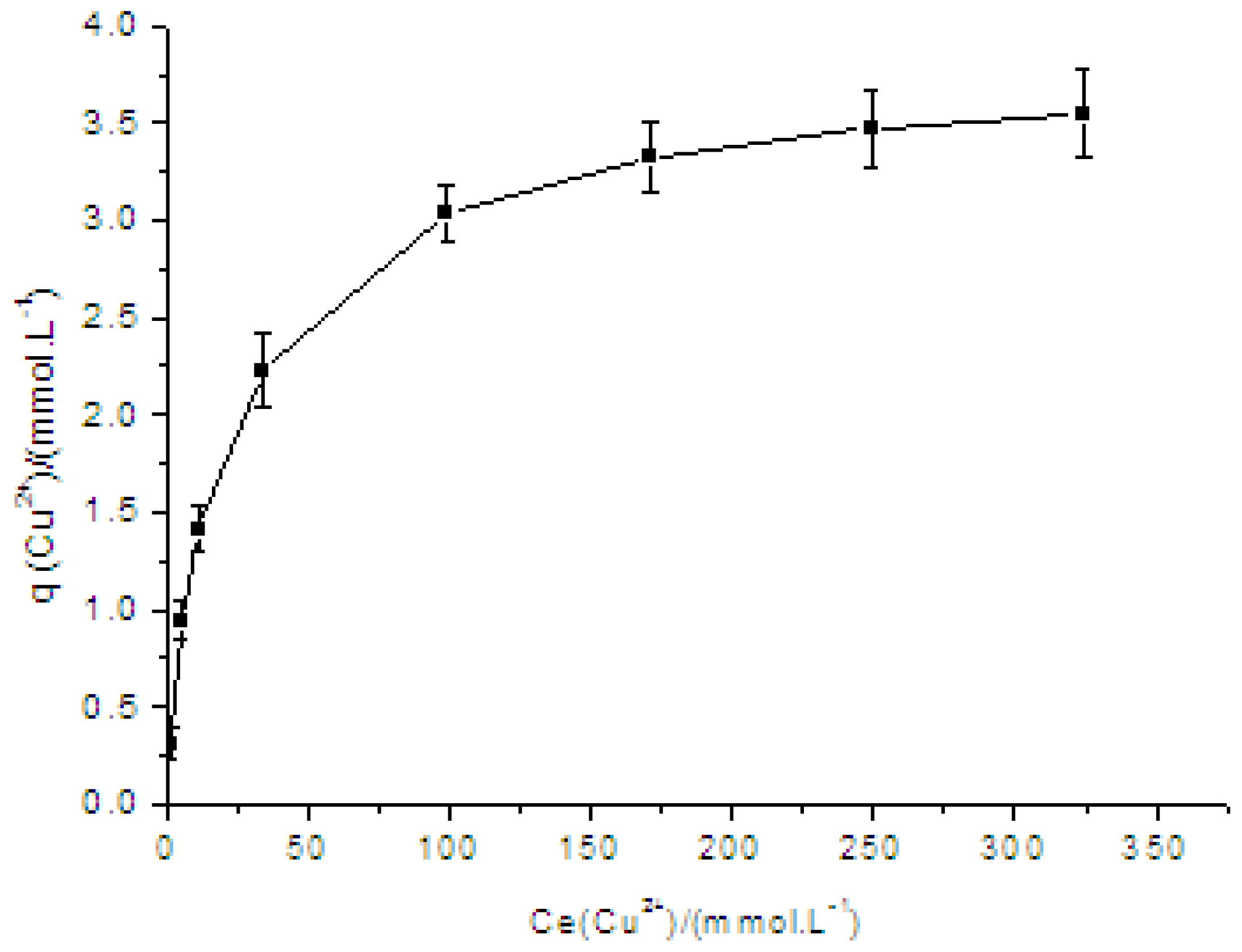
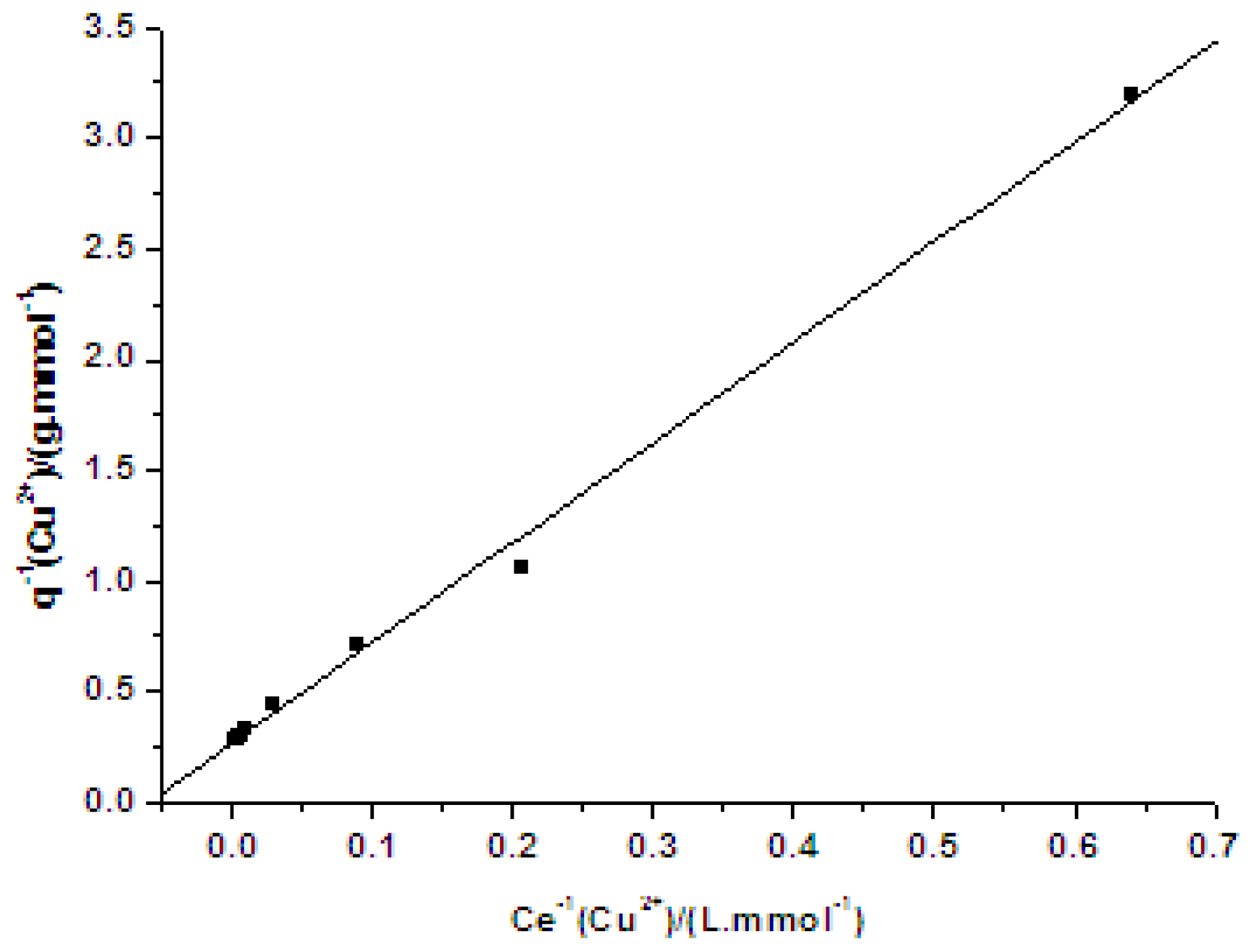
2.1. Adsorption Isotherm of Cu(II) on PVA/PA6 Composite Nanofibrous Membrane
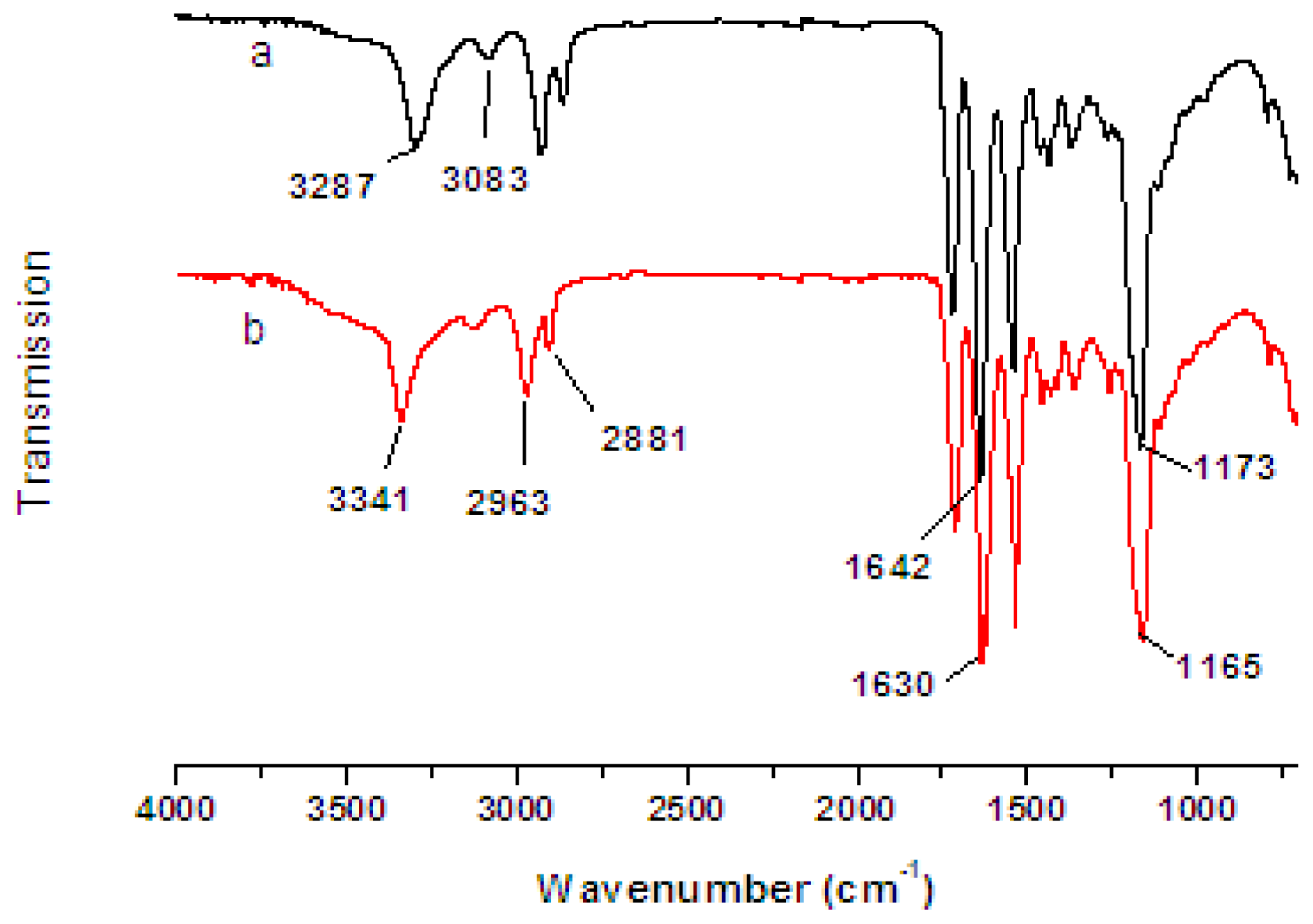
2.2. FTIR Spectra of Nanofibers
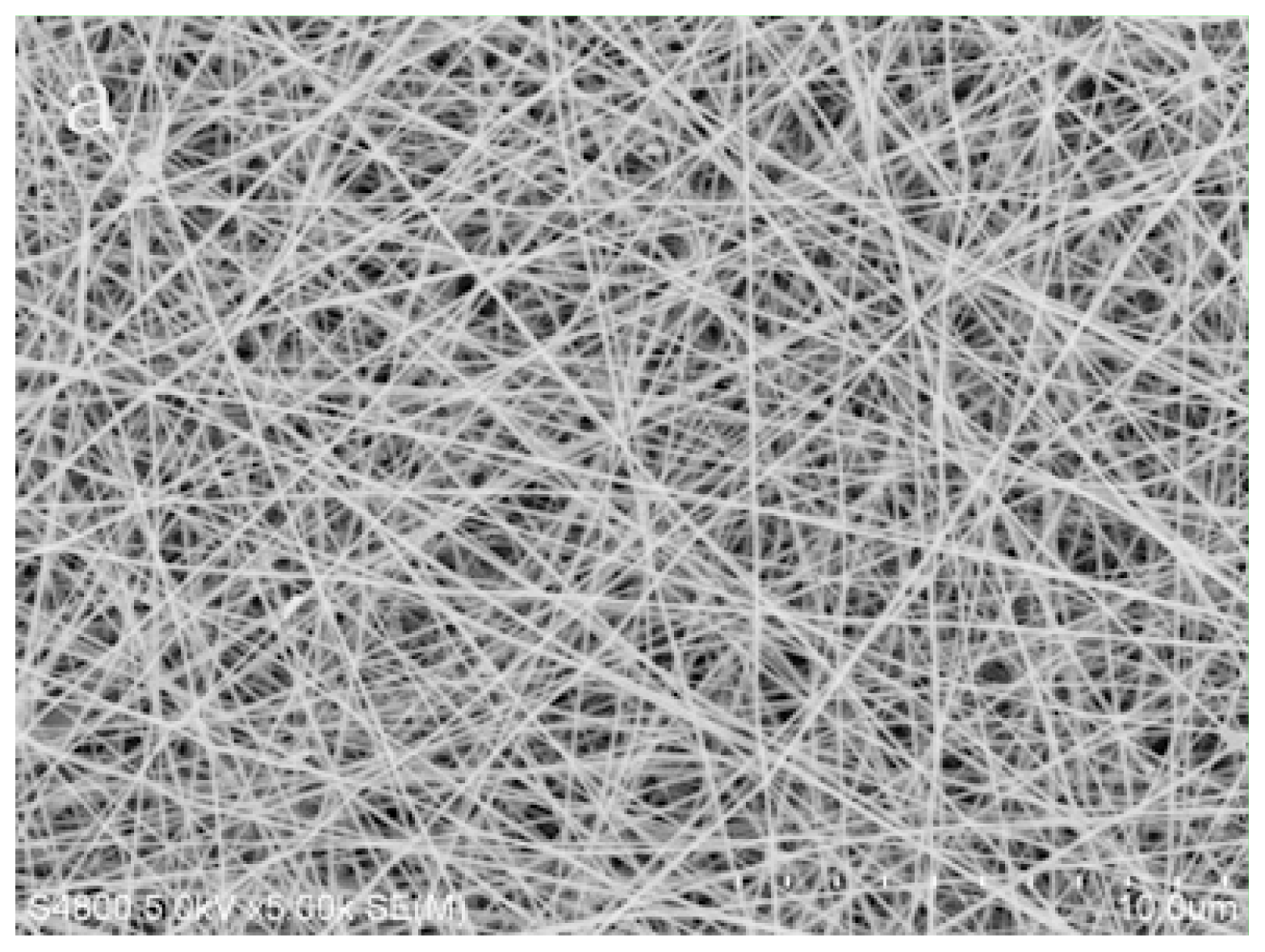
2.3. The Surface Morphologies of Nanofibrous Membrane
2.4. Kinetic Parameters of Immobilized and Free Catalases
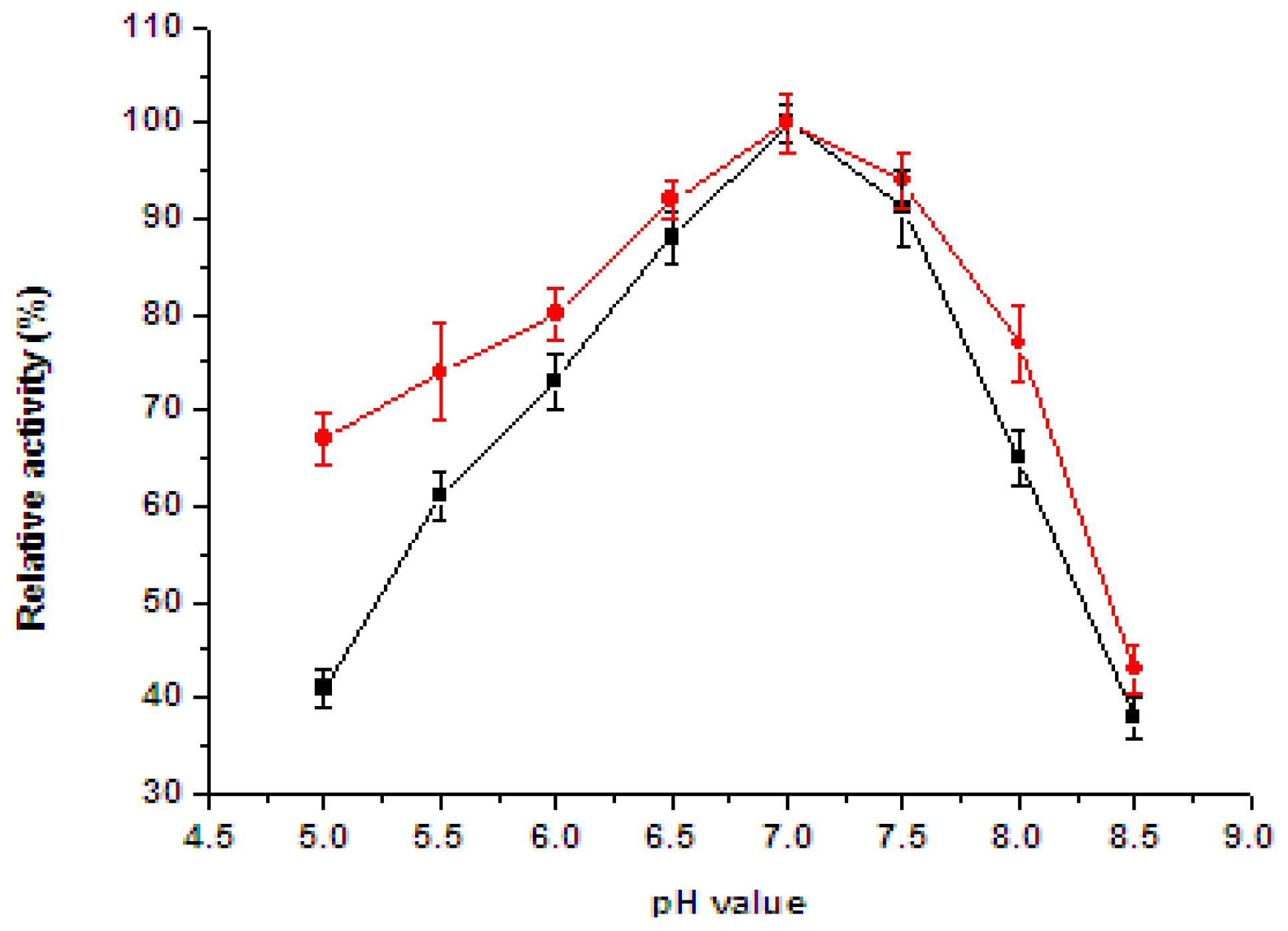
2.5. Effect of pH and Temperature on the Enzyme Activity
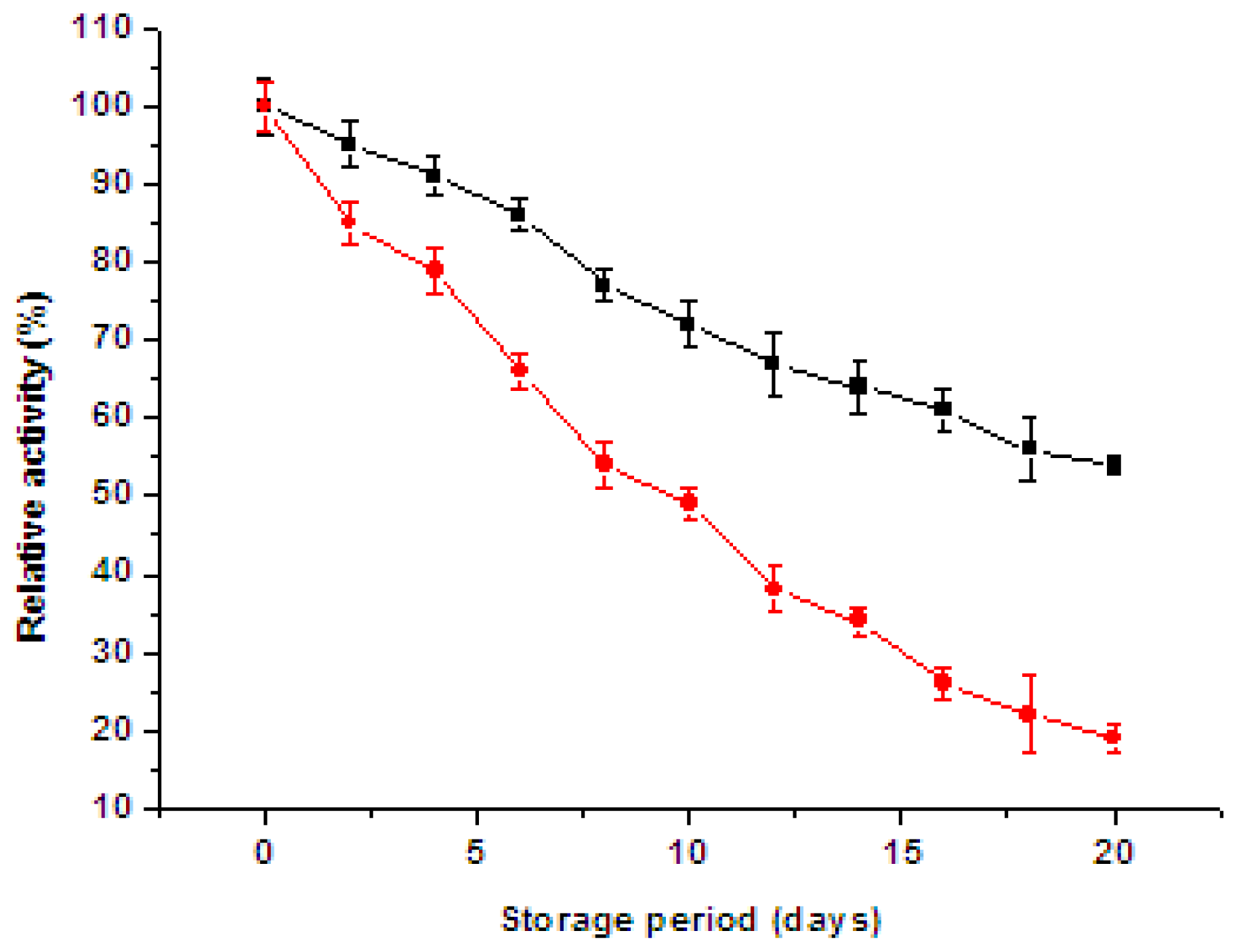
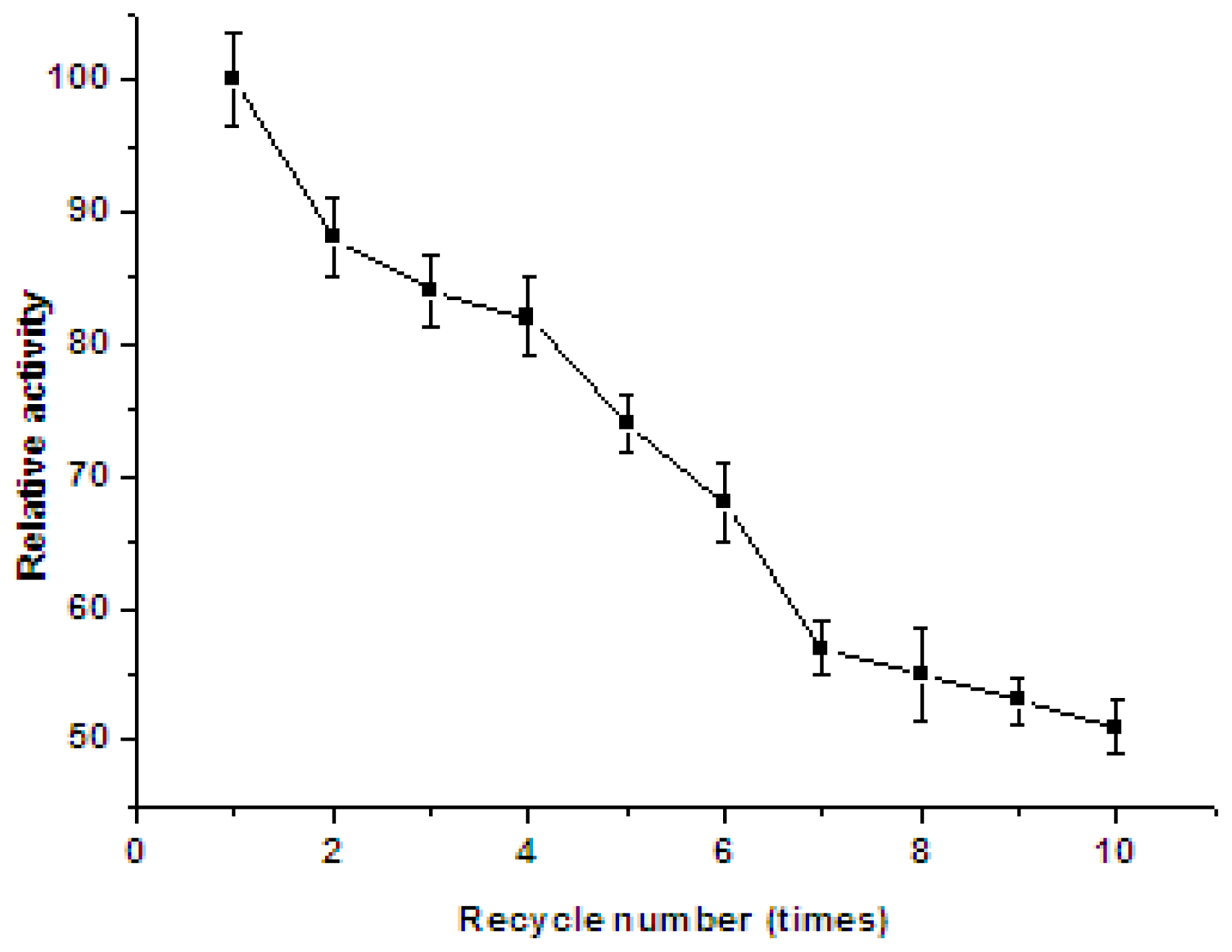
2.6. Storage Stability and Reusability
3. Materials and Methods
3.1. Materials
3.2. Electrospinning
3.3. Chelation of Cu(II)
3.4. Enzyme Immobilization
3.5. Activity Assays
3.6. Determination of Kinetic Parameters
3.7. Dependence of Temperature and Value of pH
3.8. Storage Stability and Reusability
4. Conclusions
Acknowledgments
References
- Hong, J.; Xu, D.M.; Gong, P.J.; Yu, J.H.; Ma, H.J.; Yao, S.D. Covalent-bonded immobilization of enzyme on hydrophilic polymercovering magneti cnanogels. Microporou. Mesoporous Mater 2008, 109, 470–477. [Google Scholar]
- Feng, Q.; Xia, X.; Wei, A.F.; Wang, X.Q.; Wei, Q.F. Preparation of Cu(II)-chelated poly(vinylalcohol) nanofibrous membranes for catalase immobilization. J. Appl. Polymer. Sci 2011, 120, 3291–3296. [Google Scholar]
- Ricca, E.; Calabrò, V.; Curcio, S.; Basso, A.; Gardossi, L.; Iorio, G. Fructose production by lnulinase covalently immobilized on sepabeads in batch and fluidized bed bioreactor. Int. J. Mol. Sci 2010, 11, 1180–1189. [Google Scholar]
- Park, H.; Ahn, J.; Lee, J.; Lee, H.; Kim, C.; Jung, J.-K.; Lee, H.; Lee, E.G. Expression, immobilization and enzymatic properties of glutamate decarboxylase fused to a cellulose-binding domain. Int. J. Mol. Sci 2012, 13, 358–368. [Google Scholar]
- Akgöl, S.; Öztürk, N.; Karagözler, A.A.; Aktas Uygun, D.; Uygun, M.; Denizli, A. A new metalchelated beads for reversible use in uricase adsorption. J. Mol. Catal. B Enzym 2008, 51, 36–41. [Google Scholar]
- Bayramoglu, G.; Arica, M.Y. Reversible immobilization of catalase on fibrous polymer grafted and metal chelated chitosan membrane. J. Mol. Catal. B Enzym 2010, 62, 297–304. [Google Scholar]
- Wu, Z.C.; Zhang, Y.; Tao, T.X.; Zhang, L.F.; Fong, H. Silver nanoparticles on amidoximebers for photo-catalytic degradation of organic dyes in waste water. Appl. Surf. Sci 2010, 257, 1092–1097. [Google Scholar]
- Yew, P.L.; Lee, Y.H. A potentiometric formaldehyde biosensor based on immobilization of alcohol oxidase on acryloxysuccinimide-modified acrylic microspheres. Sensors 2010, 10, 9963–9981. [Google Scholar]
- Liu, X.Q.; Guan, Y.P.; Liu, H.Z.; Ma, Z.Y.; Yang, Y.; Wu, X.B. Preparation and characterization of magnetic polymer nanospheres with high protein binding capacity. J. Magn. Magn. Mater 2005, 293, 111–118. [Google Scholar]
- Wu, Z.C.; Zhang, B.; Yan, B. Regulation of enzyme activity through interactions with nanoparticles. Int. J. Mol. Sci 2009, 10, 4198–4209. [Google Scholar]
- Wan, L.S.; Ke, B.B.; Xu, Z.K. Electrospun nanofibrous membranes filled with carbon nanotubes for redox enzyme immobilization. Enzym. Microb. Tech 2008, 42, 332–339. [Google Scholar]
- Bayramoglu, G.; Arica, M.Y. Reversible immobilization of catalase on fibrous polymer grafted and metal chelated chitosan membrane. J. Mol. Catal. B Enzym. 2010, 62, 297–304. [Google Scholar]
- Ayse, D.; Azmi, T. Improving the stability of cellulase by immobilizationon modified polyvinyl alcohol coated chitosan beads. J. Mol. Catal. B Enzym 2007, 45, 10–14. [Google Scholar]
- Feng, Q.; Wang, X.Q.; Wei, Q.F.; Wang, X.; Hou, D. Preparation of Cd2+-PVA/PA6 metal chelated nanofibers and analysis of reaction kinetics. J. Textil. Res 2012, 33, 5–8. [Google Scholar]
- Saeed, K.; Haider, S.; Oh, T.J.; Park, S.Y. Preparation of amidoxime-modied polyacrylonitrile (PAN-oxime) nanofibers and their applications to metal ions adsorption. J. Membr. Sci 2008, 322, 400–405. [Google Scholar]
- Tumturk, H.; Karaca, N.; Demirel, G. Preparation and application of poly(N, Ndimethylacrylamide- co-acrylamide) and poly (N-isopropylacrylamide-co-acrylamide)/- carrageenan hydrogels for immobilization of lipase. Int. J. Biol. Macromol 2007, 40, 281–285. [Google Scholar]
- Senay, A.C.; Ebru, S.; Dursun, S. Preparation of Cu(II) adsorbed chitosan beads for catalase immobilization. Food Chem 2009, 114, 962–969. [Google Scholar]
- Sinan, A.; Nevra, O.; Adil, D. New generation polymeric nanospheres for catalase immobilization. J. Appl. Polymer. Sci 2009, 114, 962–970. [Google Scholar]
- Nursel, P.; Bekir, S.; Olgun, G. Activity studies of glucose oxidase immobilized onto poly(Nvinylimidazole) and metal ion-chelated poly(N-vinylimidazole) hydrogels. J. Mol. Catal. B: Enzym 2003, 21, 273–282. [Google Scholar]
- Wang, Z.G.; Xu, Z.K.; Wan, L.S.; Wu, J. Nanofibrous membranes containing carbon nanotubes: Electrospun for redox enzyme immobilization. Macromol. Rapid Comm 2006, 27, 516–521. [Google Scholar]


| Amount of bound enzyme (mg/g fibers) | Specific activity (Units/mg) | Km (mM) | Vmax (μmol/mg·min) | |
|---|---|---|---|---|
| Free catalase | 3400 | 26.815 | 4878 | |
| Immobilized catalases | −64 | 2150 | 41.132 | 3774 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Feng, Q.; Tang, B.; Wei, Q.; Hou, D.; Bi, S.; Wei, A. Preparation of a Cu(II)-PVA/PA6 Composite Nanofibrous Membrane for Enzyme Immobilization. Int. J. Mol. Sci. 2012, 13, 12734-12746. https://doi.org/10.3390/ijms131012734
Feng Q, Tang B, Wei Q, Hou D, Bi S, Wei A. Preparation of a Cu(II)-PVA/PA6 Composite Nanofibrous Membrane for Enzyme Immobilization. International Journal of Molecular Sciences. 2012; 13(10):12734-12746. https://doi.org/10.3390/ijms131012734
Chicago/Turabian StyleFeng, Quan, Bin Tang, Qufu Wei, Dayin Hou, Songmei Bi, and Anfang Wei. 2012. "Preparation of a Cu(II)-PVA/PA6 Composite Nanofibrous Membrane for Enzyme Immobilization" International Journal of Molecular Sciences 13, no. 10: 12734-12746. https://doi.org/10.3390/ijms131012734
APA StyleFeng, Q., Tang, B., Wei, Q., Hou, D., Bi, S., & Wei, A. (2012). Preparation of a Cu(II)-PVA/PA6 Composite Nanofibrous Membrane for Enzyme Immobilization. International Journal of Molecular Sciences, 13(10), 12734-12746. https://doi.org/10.3390/ijms131012734




