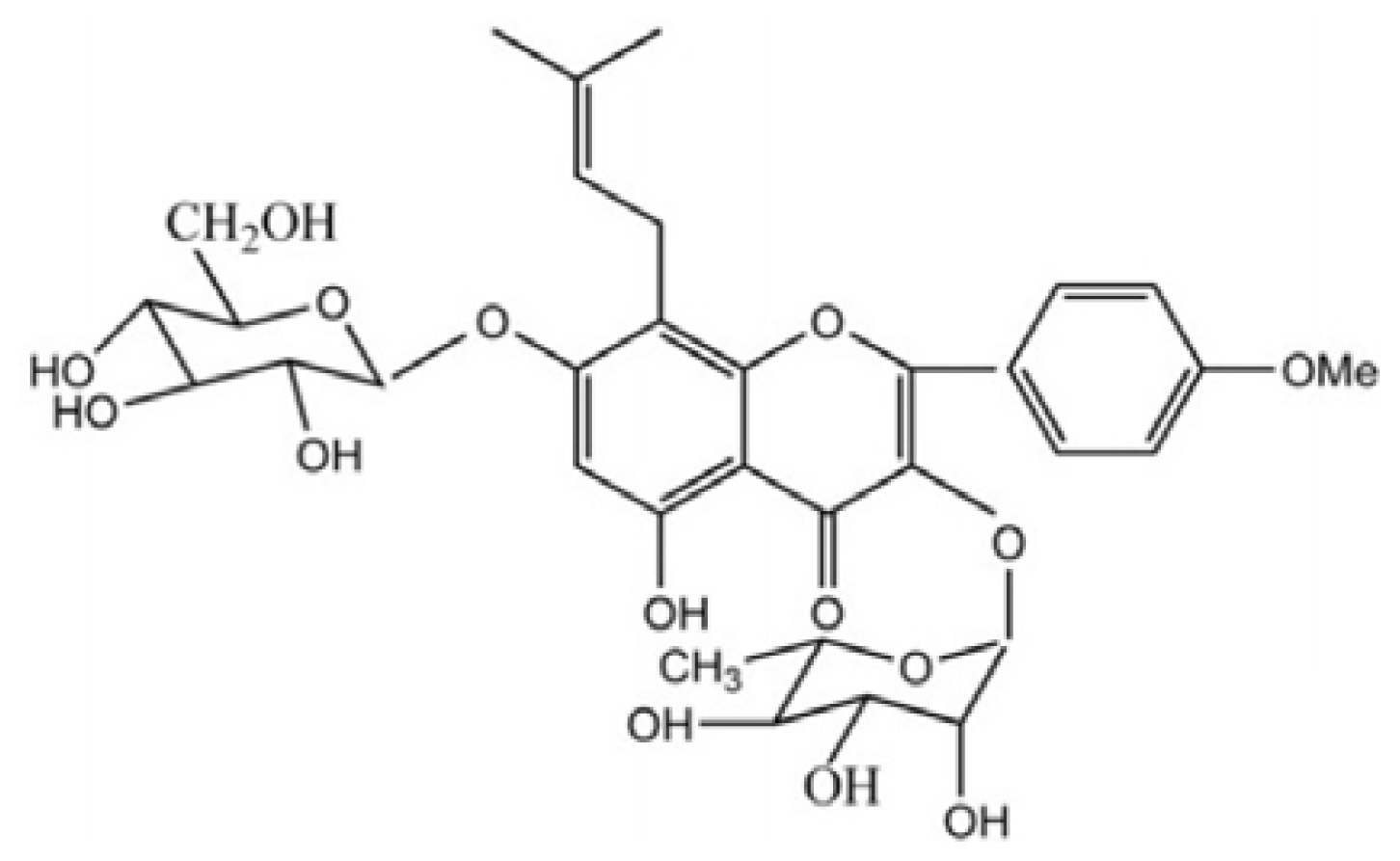
Icariin Ameliorates Streptozotocin-Induced Diabetic Retinopathy in Vitro and in Vivo
Abstract
:1. Introduction
2. Results
2.1. Metabolic and Physiological Variables
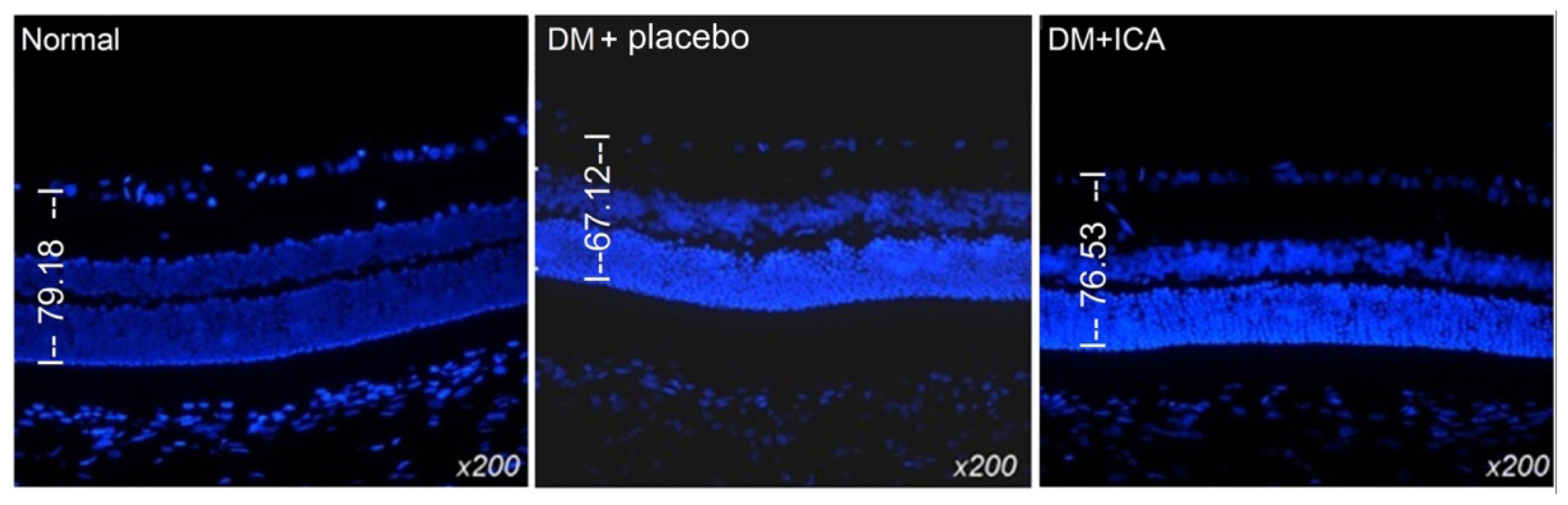
2.2. Morphological Changes of the Retina
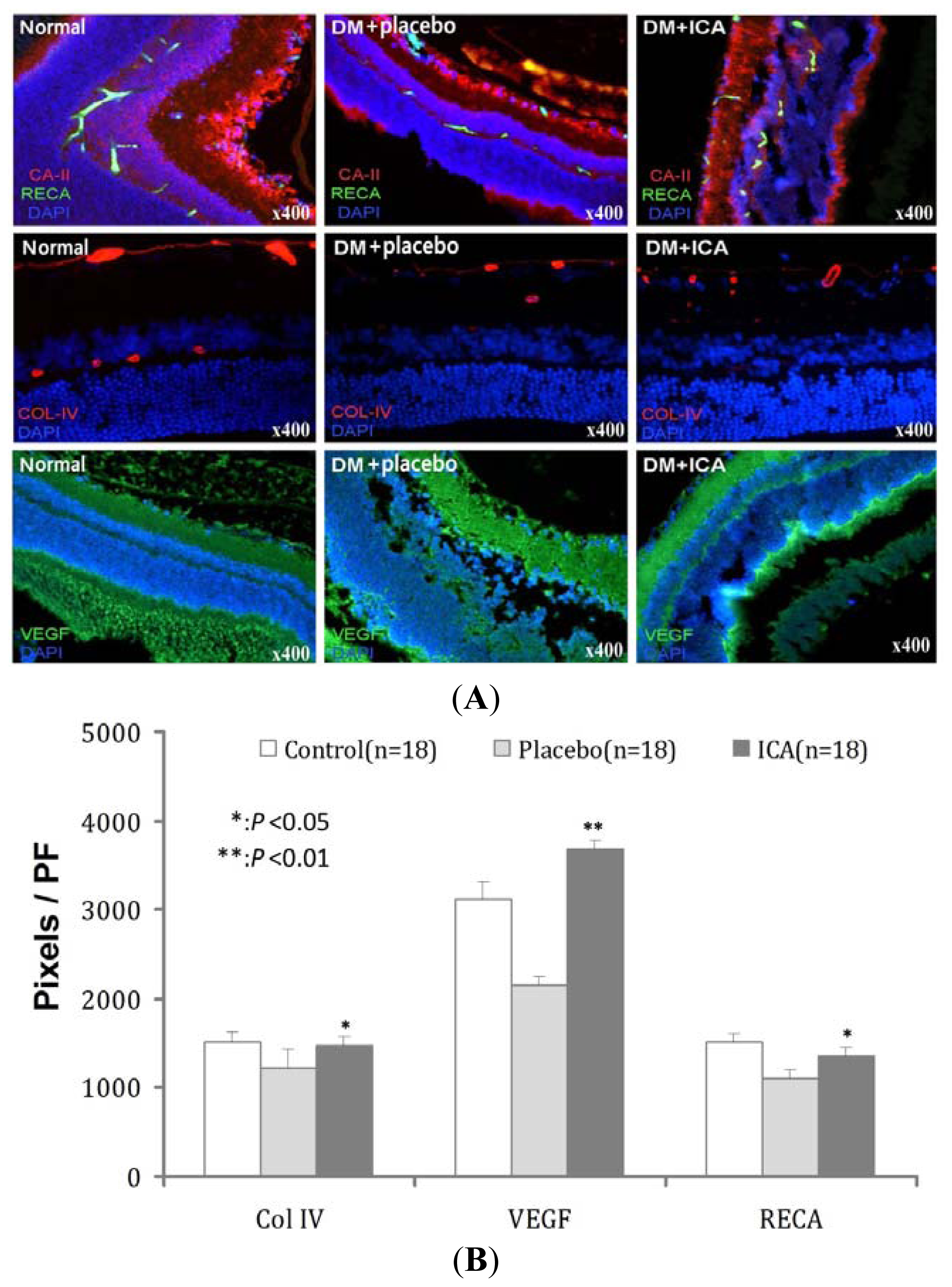
2.3. RECA, Col IV and VEGF Expression in Microvasculature of the Retina
2.4. Effects of ICA on Thy-1 and Brn3a Expression in RGCs of DR
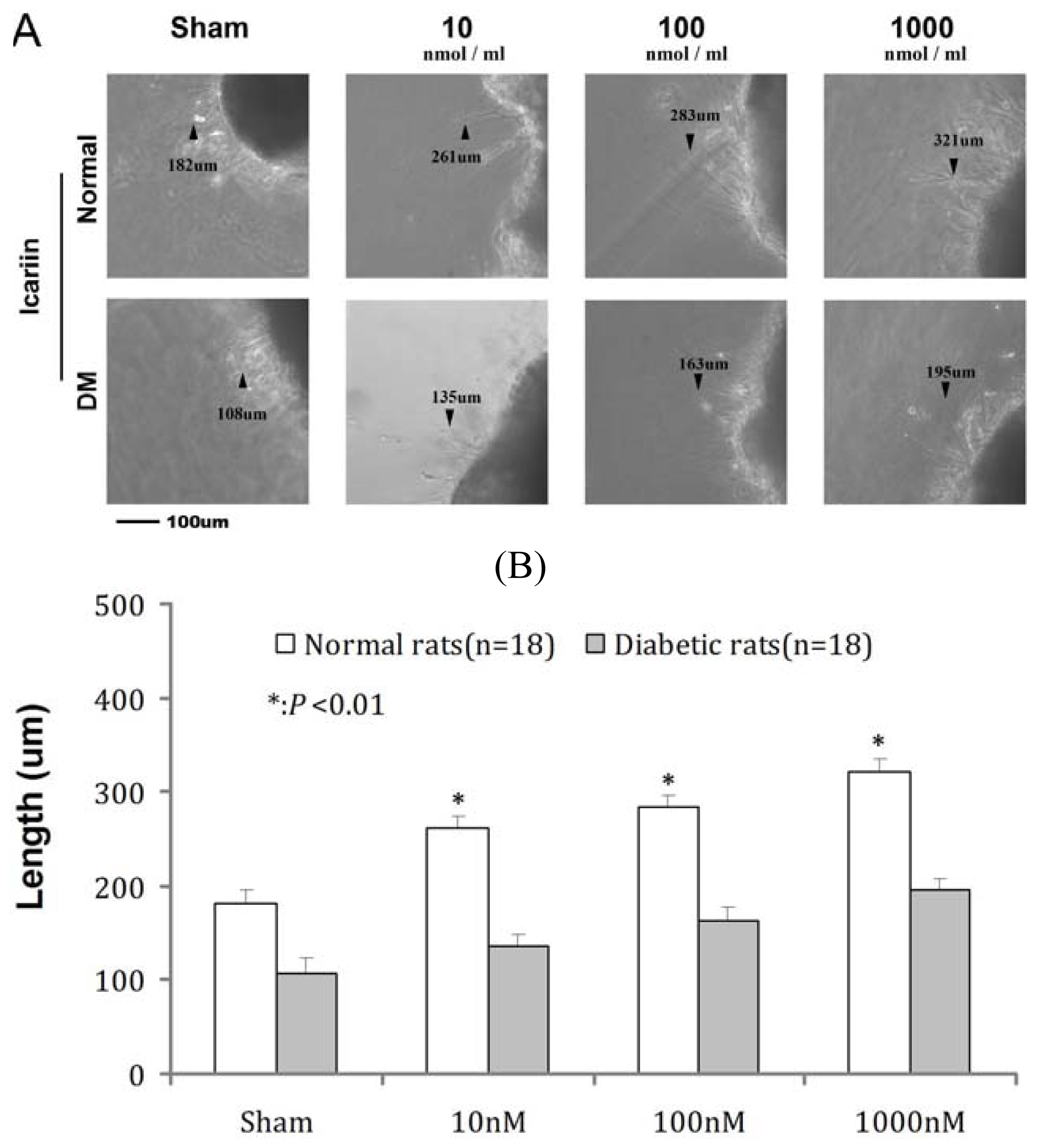
2.5. Effects of ICA on RGC Neurite Outgrowth from Retina in Vitro
3. Discussion
4. Materials and Methods
4.1. Animal and Treatment
4.2. Selection of Tissue Markers
4.3. Immunofluorescence Stain
4.4. Retina Ganglion Cell Tissue Culture in Vitro
4.5. Statistical Analysis
5. Conclusions
Acknowledgements
References
- Congdon, N.; O’Colmain, B.; Klaver, C.C.; Klein, R.; Muñoz, B.; Friedman, D.S.; Kempen, J.; Taylor, H.R.; Mitchell, P. Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol 2004, 122, 477–485. [Google Scholar]
- Marshall, S.M.; Flyvbjerg, A. Prevention and early detection of vascular complications of diabetes. Br. Med. J 2006, 333, 475–480. [Google Scholar]
- Klein, R.; Klein, B.E.; Moss, S.E. Epidemiology of proliferative diabetic retinopathy. Diabetes Care 1992, 15, 1875–1891. [Google Scholar]
- Frank, R.N. Diabetic retinopathy. N. Engl. J. Med 2004, 350, 48–58. [Google Scholar]
- Curtis, T.M.; Gardiner, T.A.; Stitt, A.W. Microvascular lesions of diabetic retinopathy: Clues towards understanding pathogenesis? Eye (Lond.) 2009, 23, 1496–1508. [Google Scholar]
- de la Cruz, J.P.; Gonzalez-Correa, J.A.; Guerrero, A.; de la Cuesta, F.S. Pharmacological approach to diabetic retinopathy. Diabetes Metab. Res. Rev 2004, 20, 91–113. [Google Scholar]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. JDRF Diabetic Retinopathy Center Group. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006, 55, 2401–2411. [Google Scholar]
- Xin, Z.C.; Kim, E.K.; Lin, C.S.; Liu, W.J.; Tian, L.; Yuan, Y.M.; Fu, J. Effects of icariin on cGMP-specific PDE5 and cAMP-specific PDE4 activities. Asian J. Androl 2003, 5, 15–18. [Google Scholar]
- Wang, H.; Liu, Y.; Huai, Q.; Cai, J.; Zoraghi, R.; Francis, S.H.; Corbin, J.D.; Robinson, H.; Xin, Z.; Lin, G.; Ke, H. Multiple conformations of phosphodiesterase-5: Implications for enzyme function and drug development. J. Biol. Chem 2006, 281, 21469–21479. [Google Scholar]
- Liu, W.J.; Xin, Z.C.; Xin, H.; Yuan, Y.M.; Tian, L.; Guo, Y.L. Effects of icariin on erectile function and expression of nitric oxide synthase isoforms in castrated rats. Asian J. Androl 2005, 7, 381–388. [Google Scholar]
- Shindel, A.W.; Xin, Z.C.; Lin, G.; Fandel, T.M.; Huang, Y.C.; Banie, L.; Breyer, B.N.; Garcia, M.M.; Lin, C.S.; Lue, T.F. Erectogenic and neurotrophic effects of icariin, a purified extract of horny goat weed (Epimedium spp.) in vitro and in vivo. J. Sex. Med 2010, 7, 1518–1528. [Google Scholar]
- Liu, T.; Xin, H.; Li, W.R.; Zhou, F.; Li, G.Y.; Gong, Y.Q.; Gao, Z.Z.; Qin, X.C.; Cui, W.S.; Shindel, A.W.; Xin, Z.C. Effects of icariin on improving erectile function in streptozotocin-induced diabetic rats. J. Sex. Med 2011, 8, 2761–2772. [Google Scholar]
- Qi, M.Y.; Kai, C.; Liu, H.R.; Su, Y.H.; Yu, S.Q. Protective effect of Icariin on the early stage of experimental diabetic nephropathy induced by streptozotocin via modulating transforming growth factor beta(1) and type IV collagen expression in rats. J. Ethnopharmacol 2011, 138, 731–736. [Google Scholar]
- Reichenbach, A.; Wurm, A.; Pannicke, T.; Iandiev, I.; Wiedemann, P.; Bringmann, A. Muller cells as players in retinal degeneration and edema. Graefes Arch. Clin. Exp. Ophthalmol 2007, 245, 627–636. [Google Scholar]
- Pambianco, G.; Costacou, T.; Ellis, D.; Becker, D.J.; Klein, R.; Orchard, T.J. The 30-year natural history of type 1 diabetes complications—The Pittsburgh epidemiology of diabetes complications study experience. Diabetes 2006, 55, 1463–1469. [Google Scholar]
- Zhang, W.; Liu, H.; Al-Shabrawey, M.; Caldwell, R.W.; Caldwell, R.B. Inflammation and diabetic retinal microvascular complications. J. Cardiovasc. Dis. Res 2011, 2, 96–103. [Google Scholar]
- Zong, H.; Ward, M.; Stitt, A.W. AGEs, RAGE, and diabetic retinopathy. Curr. Diabetes Rep 2011, 11, 244–252. [Google Scholar]
- Edwards, P.J.; Barnard, L.; Leonard, V.K.; Yi, J.S.; Moloney, K.P.; Kongnakorn, T.; Jacko, J.A.; Sainfort, F. Understanding users with diabetic retinopathy: Factors that affect performance in a menu selection task. Behav. Inf. Technol 2005, 24, 175–186. [Google Scholar]
- Shahidi, A.M.; Sampson, G.P.; Pritchard, N.; Edwards, K.; Russell, A.; Malik, R.A.; Efron, N. Exploring retinal and functional markers of diabetic neuropathy. Clin. Exp. Optom 2010, 93, 309–323. [Google Scholar] [Green Version]
- Chrissobolis, S.; Miller, A.A.; Drummond, G.R.; Kemp-Harper, B.K.; Sobey, C.G. Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front. Biosci 2011, 16, 1733–1745. [Google Scholar]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar]
- Ceriello, A. Hypothesis: The “metabolic memory”, the new challenge of diabetes. Diabetes Res. Clin. Pract 2009, 86, S2–S6. [Google Scholar]
- Li, Q.; Ao, X.; Du, Y.; Li, Y.; Ou, Y.; Gong, R.; Sun, X.; Yang, Y.X.; Wen, G. Effects of aminoguanidine and vitamin C on collagen type IV in diabetic nephropathy rats. Endocrine 2011, 39, 251–258. [Google Scholar]
- Jonas, J.B. Clinical implications of peripapillary atrophy in glaucoma. Curr. Opin. Ophthalmol 2005, 16, 84–88. [Google Scholar]
- Usta, M.F.; Kendirci, M.; Gur, S.; Foxwell, N.A.; Bivalacqua, T.J.; Cellek, S.; Hellstrom, W.J. The breakdown of preformed advanced glycation end products reverses erectile dysfunction in streptozotocin-induced diabetic rats: Preventive versus curative treatment. J. Sex. Med 2006, 3, 242–252. [Google Scholar]
- Chen, Y.; Yang, R.; Yao, L.; Sun, Z.; Wang, R.; Dai, Y. Differential expression of neurotrophins in penises of streptozotocin-induced diabetic rats. J. Androl 2007, 28, 306–312. [Google Scholar]
- Li, J.; Wang, J.J.; Yu, Q.; Chen, K.; Mahadev, K.; Zhang, S.X. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: Role of NADPH oxidase 4. Diabetes 2010, 59, 1528–1538. [Google Scholar]
- Sivakumar, V.; Zhang, Y.; Ling, E.A.; Foulds, W.S.; Kaur, C. Insulin-like growth factors, angiopoietin-2, and pigment epithelium-derived growth factor in the hypoxic retina. J. Neurosci. Res 2008, 86, 702–711. [Google Scholar]
- Sanchez-Migallon, M.C.; Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Brain derived neurotrophic factor maintains Brn3a expression in axotomized rat retinal ganglion cells. Exp. Eye Res 2011, 92, 260–267. [Google Scholar]
- Zhang, X.M.; Li Liu, D.T.; Chiang, S.W.; Choy, K.W.; Pang, C.P.; Lam, D.S.; Yam, G.H. Immunopanning purification and long-term culture of human retinal ganglion cells. Mol. Vis 2010, 16, 2867–2872. [Google Scholar]
- Bian, Z.M.; Elner, S.G.; Elner, V.M. Regulation of VEGF mRNA expression and protein secretion by TGF-beta2 in human retinal pigment epithelial cells. Exp. Eye Res 2007, 84, 812–822. [Google Scholar]

| Variable | Diabetic ED Model | Sham (n = 12) | |
|---|---|---|---|
| placebo (n = 18) | ICA (n = 18) | ||
| Initial weight(g) | 253.5 ± 7.2 | 251.3 ± 11.9 | 264.1 ± 12.4 |
| Final weight(g) | 247.6 ± 12.2 ** | 254.2 ± 15.2 ** | 568.2 ± 16.4 |
| Initial fasting glucose(mg/dL) | 107.1 ± 4.8 | 102.4 ± 5.3 | 115.2 ± 6.1 |
| Initial postprandial glucose(mg/dL) | 128.2 ± 8.2 | 119.5 ± 11.4 | 131.9 ± 14.6 |
| Final fasting glucose(mg/dL) | 382.4 ± 31.4 ** | 392.6 ± 32.4 ** | 102 ± 13.6 |
| Final postprandial glucose(mg/dL) | 485.2 ± 31.7 ** | 516.7 ± 23.6 ** | 136.1 ± 11.8 |
| Groups | Col IV | VEGF | RECA |
|---|---|---|---|
| Normal | 1510 ± 121 | 3128 ± 181 | 1521 ± 95 |
| DM + placebo | 1227 ± 198 | 2145 ± 97 | 1103 ± 101 |
| DM + ICA | 1466 ± 101 * | 3678 ± 110 ** | 1359 ± 87 * |
| P value | <0.05 | <0.01 | <0.05 |
| Groups | CA-II | Thy-1 | Brna3 |
|---|---|---|---|
| Normal | 1728 ± 101 | 2138 ± 143 | 1923 ± 98 |
| DM + placebo | 1219 ± 192 | 1106 ± 87 | 870 ± 65 |
| DM + ICA | 1601 ± 121 * | 1820 ± 110 ** | 1213 ± 126 ** |
| P value | <0.05 | <0.01 | <0.01 |
| Groups | Sham | 10 nM | 100 nM | 1000 nM |
|---|---|---|---|---|
| Normal | 182 ± 4.1 ** | 261 ± 2.1 ** | 283 ± 3.3 ** | 321 ± 4.6 ** |
| DM | 108 ± 5.3 | 135 ± 2.3 | 163 ± 4.1 | 195 ± 3.8 |
| P value | <0.01 | <0.01 | <0.01 | <0.01 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xin, H.; Zhou, F.; Liu, T.; Li, G.-Y.; Liu, J.; Gao, Z.-Z.; Bai, G.-Y.; Lu, H.; Xin, Z.-C. Icariin Ameliorates Streptozotocin-Induced Diabetic Retinopathy in Vitro and in Vivo. Int. J. Mol. Sci. 2012, 13, 866-878. https://doi.org/10.3390/ijms13010866
Xin H, Zhou F, Liu T, Li G-Y, Liu J, Gao Z-Z, Bai G-Y, Lu H, Xin Z-C. Icariin Ameliorates Streptozotocin-Induced Diabetic Retinopathy in Vitro and in Vivo. International Journal of Molecular Sciences. 2012; 13(1):866-878. https://doi.org/10.3390/ijms13010866
Chicago/Turabian StyleXin, Hua, Feng Zhou, Tao Liu, Guang-Yong Li, Jing Liu, Zhe-Zhu Gao, Guang-Yi Bai, Hong Lu, and Zhong-Cheng Xin. 2012. "Icariin Ameliorates Streptozotocin-Induced Diabetic Retinopathy in Vitro and in Vivo" International Journal of Molecular Sciences 13, no. 1: 866-878. https://doi.org/10.3390/ijms13010866




