Evaluation of a Centrifuged Double Y-Shape Microfluidic Platform for Simple Continuous Cell Environment Exchange
Abstract
:1. Introduction
2. Results and Discussion
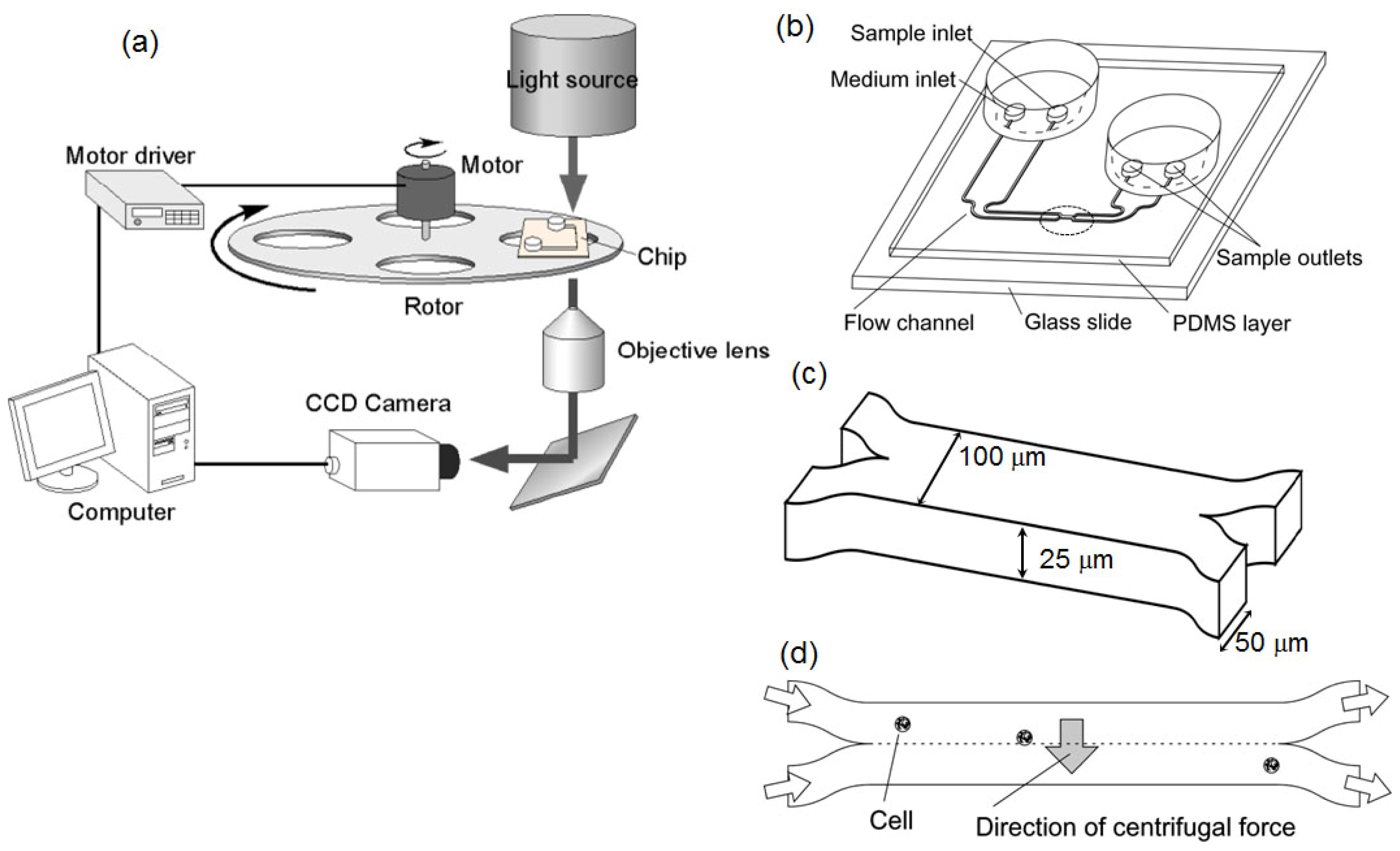
2.1. System Design
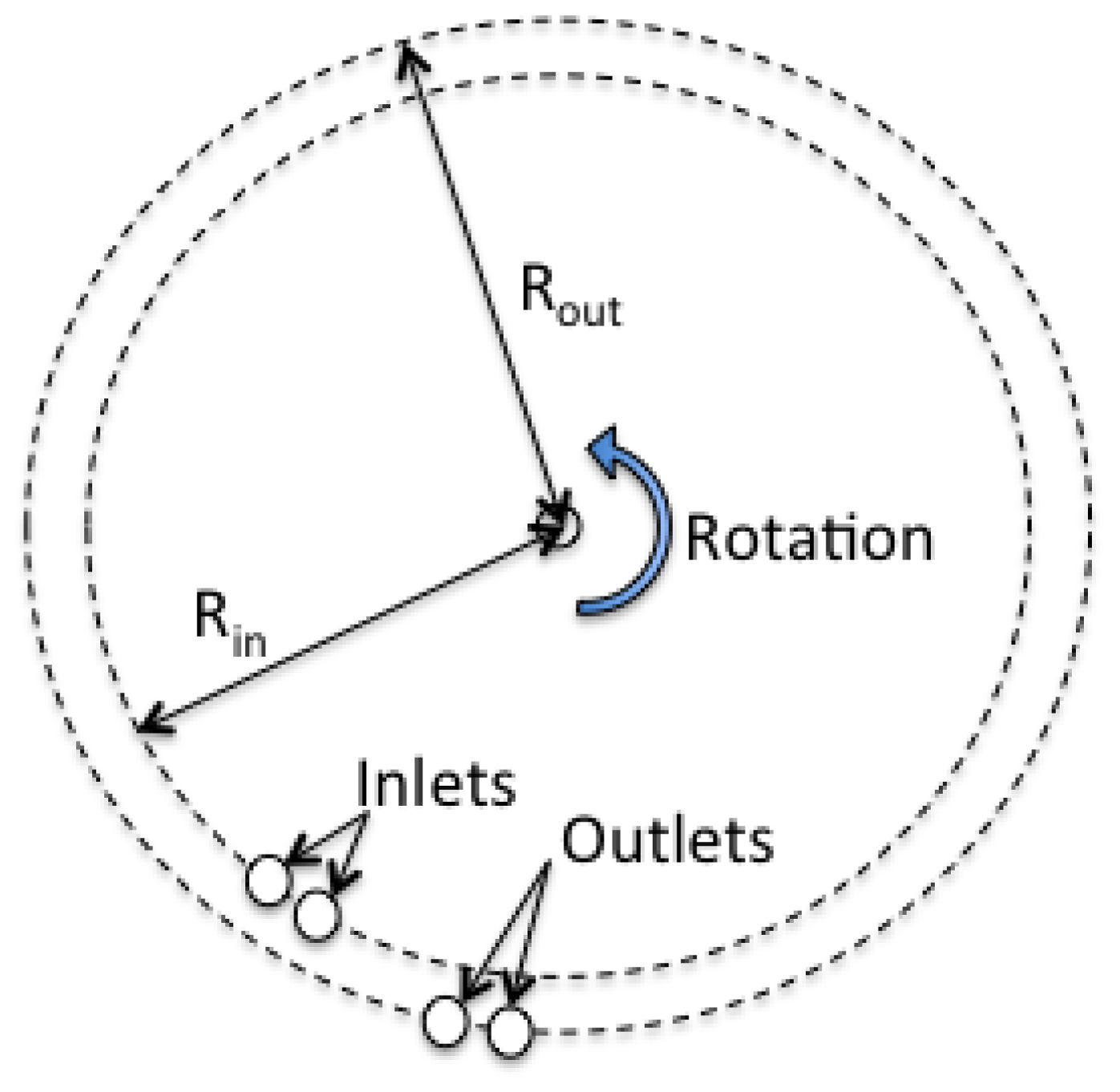
2.2. Rotation of Chip for Centrifugal Force Generation
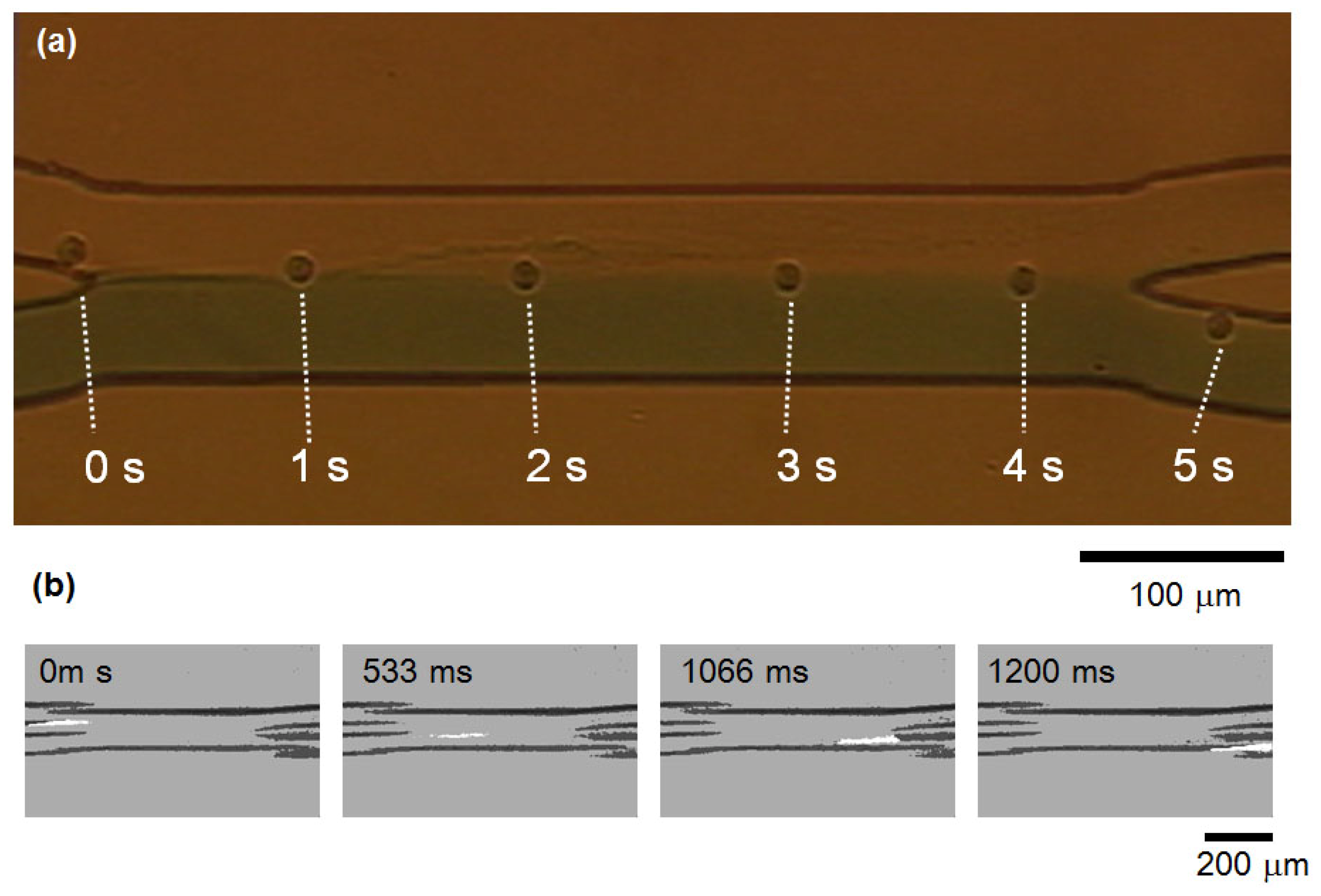
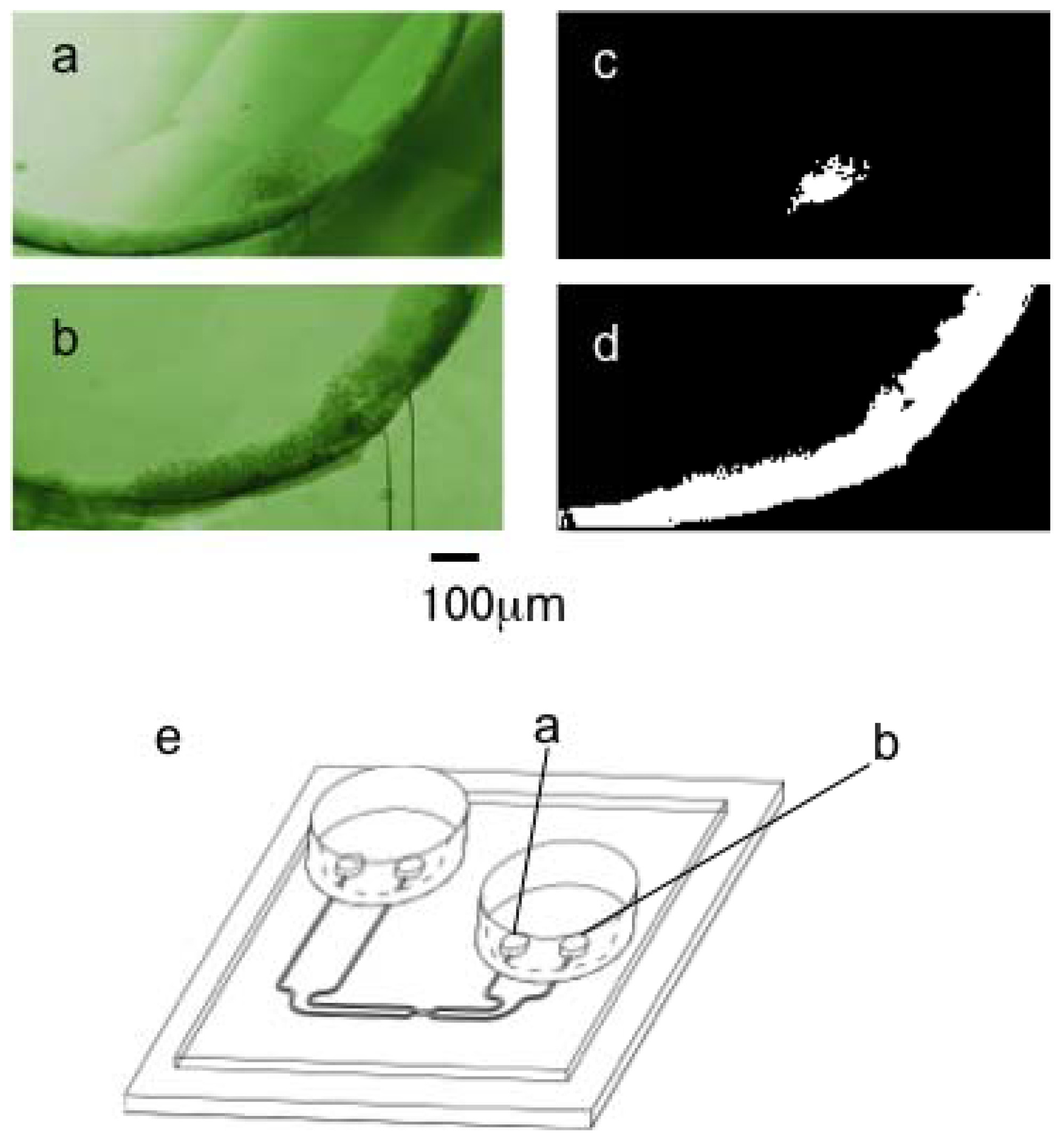
2.3. Cell Motion in Microfluidic Chip Under Centrifugation
2.4. Cell Transportation Between Two Laminar Flows
3. Experimental Section
3.1. Optical Setup
3.2. Samples
3.3. Experiment Procedure
3.4. Flow Velocity Estimation
3.5. Superimposed Image Acquision and Image Analysis
4. Conclusions
Acknowledgements
References
- Haeberle, S.; Brenner, T.; Zengerle, R.; Ducree, J. Centrifugal extraction of plasma from whole blood on a rotating disk. Lab Chip 2006, 6, 776–781. [Google Scholar]
- Haeberle, S.; Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar]
- Bhagat, A.A.; Kuntaegowdanahalli, S.S.; Papautsky, I. Continuous particle separation in spiral microchannels using Dean flows and differential migration. Lab Chip 2008, 8, 1906–1914. [Google Scholar]
- Hsu, C.H.; Di Carlo, D.; Chen, C.; Irimia, D.; Toner, M. Microvortex for focusing, guiding and sorting of particles. Lab Chip 2008, 8, 2128–2134. [Google Scholar]
- Meagher, R.J.; Light, Y.K.; Singh, A.K. Rapid, continuous purification of proteins in a microfluidic device using genetically-engineered partition tags. Lab Chip 2008, 8, 527–532. [Google Scholar]
- Tai, C.H.; Hsiung, S.K.; Chen, C.Y.; Tsai, M.L.; Lee, G.B. Automatic microfluidic platform for cell separation and nucleus collection. Biomed. Microdevices 2007, 9, 533–543. [Google Scholar]
- Chang, S.; Cho, Y.H. A continuous size-dependent particle separator using a negative dielectrophoretic virtual pillar array. Lab Chip 2008, 8, 1930–1936. [Google Scholar]
- Yasuda, K.; Umemura, S.-i.; Takeda, K. Concentration and fractionation of small particles in liquid by ultrasound. Jpn. J. Appl. Phys 1995, 34, 2715. [Google Scholar]
- Yasuda, K.; Kamakura, T. Acoustic radiation force on micrometer-size particles. Appl. Phys. Lett 1997, 71, 1771–1773. [Google Scholar]
- Yasuda, K.; Haupt, S.S.; Umemura, S.-i.; Yagi, T.; Nishida, M.; Shibata, Y. Using acoustic radiation force as a concentration method for erythrocytes. J. Acoust. Soc. Am 1997, 102, 642–645. [Google Scholar]
- Yasuda, K. Non-destructive, non-contact handling method for biomaterials in micro-chamber by ultrasound. Sens. Actuators B: Chem 2000, 64, 128–135. [Google Scholar]
- Castell, O.K.; Allender, C.J.; Barrow, D.A. Novel biphasic separations utilising highly selective molecularly imprinted polymers as biorecognition solvent extraction agents. Biosens. Bioelectron 2006, 22, 526–533. [Google Scholar]
- Castell, O.K.; Allender, C.J.; Barrow, D.A. Continuous molecular enrichment in microfluidic systems. Lab Chip 2008, 8, 1031–1033. [Google Scholar]
- Castell, O.K.; Allender, C.J.; Barrow, D.A. Liquid-liquid phase separation: Characterisation of a novel device capable of separating particle carrying multiphase flows. Lab Chip 2009, 9, 388–396. [Google Scholar]
- Pamme, N.; Manz, A. On-chip free-flow magnetophoresis: Continuous flow separation of magnetic particles and agglomerates. Anal. Chem 2004, 76, 7250–7256. [Google Scholar]
- Yung, C.W.; Fiering, J.; Mueller, A.J.; Ingber, D.E. Micromagnetic-microfluidic blood cleansing device. Lab Chip 2009, 9, 1171–1177. [Google Scholar]
- Atencia, J.; Beebe, D.J. Steady flow generation in microcirculatory systems. Lab Chip 2006, 6, 567–574. [Google Scholar]
- Rhee, S.W.; Taylor, A.M.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. External force-assisted cell positioning inside microfluidic devices. Biomed. Microdevices 2007, 9, 15–23. [Google Scholar]
- Min, J.; Kim, J.H.; Lee, Y.; Namkoong, K.; Im, H.C.; Kim, H.N.; Kim, H.Y.; Huh, N.; Kim, Y.R. Functional integration of DNA purification and concentration into a real time micro-PCR chip. Lab Chip 2011, 11, 259–265. [Google Scholar]
- Gong, M.; Bohn, P.W.; Sweedler, J.V. Centrifugal sedimentation for selectively packing channels with silica microbeads in three-dimensional micro/nanofluidic devices. Anal. Chem 2009, 81, 2022–2026. [Google Scholar]
- Lai, S.; Wang, S.; Luo, J.; Lee, L.J.; Yang, S.T.; Madou, M.J. Design of a compact disk-like microfluidic platform for enzyme-linked immunosorbent assay. Anal. Chem 2004, 76, 1832–1837. [Google Scholar]
- Grumann, M.; Steigert, J.; Riegger, L.; Moser, I.; Enderle, B.; Riebeseel, K.; Urban, G.; Zengerle, R.; Ducree, J. Sensitivity enhancement for colorimetric glucose assays on whole blood by on-chip beam-guidance. Biomed. Microdevices 2006, 8, 209–214. [Google Scholar]
- Chen, B.; Zhou, X.; Li, C.; Wang, Q.; Liu, D.; Lin, B. Rapid screening of phenylketonuria using a CD microfluidic device. J. Chromatogr. A 2011, 1218, 1907–1912. [Google Scholar]
- Haeberle, S.; Brenner, T.; Zengerle, R.; Ducree, J. Centrifugal extraction of plasma from whole blood on a rotating disk. Lab Chip 2006, 6, 776–781. [Google Scholar]
- Chen, H.; Li, X.; Wang, L.; Li, P.C. A rotating microfluidic array chip for staining assays. Talanta 2010, 81, 1203–1208. [Google Scholar]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc 2010, 5, 491–502. [Google Scholar]
- Hattori, A.; Yasuda, K. Comprehensive study of microgel electrode for on-chip electrophoretic cell sorting. Jpn. J. Appl. Phys 2010, 49, 06GM04:1–06GM04:4. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hattori, A.; Yasuda, K. Evaluation of a Centrifuged Double Y-Shape Microfluidic Platform for Simple Continuous Cell Environment Exchange. Int. J. Mol. Sci. 2012, 13, 819-827. https://doi.org/10.3390/ijms13010819
Hattori A, Yasuda K. Evaluation of a Centrifuged Double Y-Shape Microfluidic Platform for Simple Continuous Cell Environment Exchange. International Journal of Molecular Sciences. 2012; 13(1):819-827. https://doi.org/10.3390/ijms13010819
Chicago/Turabian StyleHattori, Akihiro, and Kenji Yasuda. 2012. "Evaluation of a Centrifuged Double Y-Shape Microfluidic Platform for Simple Continuous Cell Environment Exchange" International Journal of Molecular Sciences 13, no. 1: 819-827. https://doi.org/10.3390/ijms13010819



