Mechanisms of Action of (Meth)acrylates in Hemolytic Activity, in Vivo Toxicity and Dipalmitoylphosphatidylcholine (DPPC) Liposomes Determined Using NMR Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hemolytic Activity
2.2. In Vivo Toxicity
2.3. Interaction between DPPC Liposomes and (Meth)Acrylates
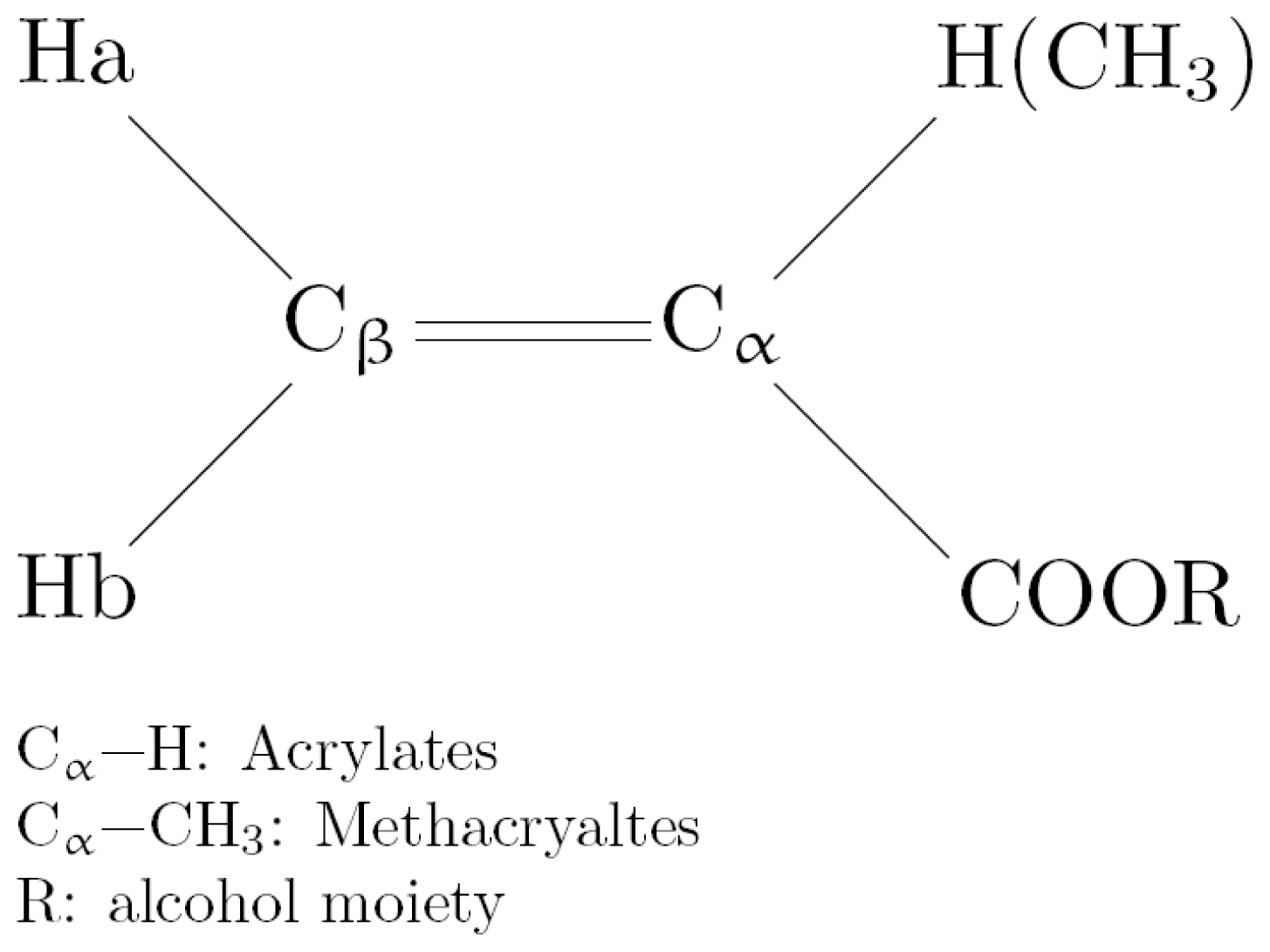
2.3.1. NMR Chemical Shifts of Ha
2.3.2. DSC Phase Transition Property
3. Experimental Section
3.1. Chemicals
3.2. NMR Spectra
3.3. NMR Study
3.4. DSC Study
3.5. Hemolytic Activity
3.6. In Vivo Toxicity
3.7. Theoretical Parameters
4. Conclusions
Acknowledgments
References
- Lawrence, W.H.; Bass, G.E.; Purcell, W.P.; Autian, J. Use of mathematical models in the study of structure-toxicity relationships of dental compounds: I. Esters of acrylic and methacrylic acids. J. Dent. Res 1972, 51, 526–535. [Google Scholar]
- Tanii, H.; Hashimoto, K. Structure-toxicity relationship of acrylates and methacrylates. Toxicol. Lett 1982, 11, 125–129. [Google Scholar]
- Dillingham, E.O.; Lawrence, W.H.; Autian, J. Acrylate and methacrylate esters: Relationship of hemolytic activity and in vivo toxicity. J. Biomed. Mater. Res 1983, 17, 945–957. [Google Scholar]
- Yoshii, E. Cytotoxic effects of acrylates and methacrylates: Relationships of monomer structures and cytotoxicity. J. Biomed. Mater. Res 1997, 37, 517–524. [Google Scholar]
- Chan, K.; O’Brien, P.J. Structure-activity relationships for hepatocyte toxicity and electrophilic reactivity of α,β-unsaturated esters, acrylates and methacrylates. J. Appl. Toxicol 2008, 28, 1004–1015. [Google Scholar]
- Cosmetic Ingredient Review. Final report of the safety assessment of methacrylic acid. Int. J. Toxicol. 2005, 24, 33–51.
- Bakopoulou, A.; Papadopoulos, T.; Garefis, P. Molecular toxicology of substances related from resin-based dental restorative materials. Int. J. Mol. Sci 2009, 10, 3861–3899. [Google Scholar]
- Tsukeoka, T.; Suzuki, M.; Ohtsuki, C.; Sugino, A.; Tsuneizumi, Y.; Miyagi, J.; Kuramoto, K.; Moriya, H. Mechanical and histological evaluation of a PMMA-based bone cement modified with γ-methacryloxypropyltrimethoxysilane and calcium acetate. Biomaterials 2006, 27, 3897–3903. [Google Scholar]
- Hansch, C.; Fujita, T. p-σ-π Analysis. A method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc 1964, 86, 1616–1626. [Google Scholar]
- Linhart, I.; Vosmanská, M.; Smejkal, J. Biotransformation of acrylates. Excretion of mercapturic acids and changes in urinary carboxylic acid profile in rat dosed with ethyl and 1-butyl acrylate. Xenobiotica 1994, 24, 1043–1052. [Google Scholar]
- Freidig, A.P.; Verhaar, H.J.M.; Hermens, J.L.M. Quantitative structure-property relationships for the chemical reactivity of acrylates and methacrylates. Environ. Toxicol. Chem 1999, 18, 1133–1139. [Google Scholar]
- Cronin, M.T.; Walker, J.D.; Jaworska, J.S.; Comber, M.H.; Watts, C.D.; Worth, A.P. Use of QSARs in international decision-making frameworks to predict ecologic effects and environmental fate of chemical substances. Environ. Health Perspect 2003, 111, 1376–1390. [Google Scholar]
- Eroglu, E.; Palaz, S.; Oltulu, O.; Turkmen, H.; Ozaydın, C. Comparative QSTR study using semi-empirical and first principle methods based descriptors for acute toxicity of diverse organic compounds to the fathead minnow. Int. J. Mol. Sci 2007, 8, 1265–1283. [Google Scholar]
- Ishihara, M.; Fujisawa, S. Quantum-chemical descriptors for estimating hemolytic activity of aliphatic and aromatic methacrylates. Chemosphere 2008, 70, 1898–1902. [Google Scholar]
- Ishihara, M.; Fujisawa, S. A structure-activity relationship study on the mechanisms of methacrylate-induced toxicity using NMR chemical shift of β-carbon, RP-HPLC log P and semiempirical molecular descriptor. Dent. Mater. J 2009, 28, 113–120. [Google Scholar]
- Putz, M.V.; Ionaşcu, C.; Putz, A.M.; Ostafe, V. Alert-QSAR. Implications for electrophilic theory of chemical carcinogenesis. Int. J. Mol. Sci 2011, 12, 5098–5134. [Google Scholar]
- Fujisawa, S.; Kadoma, Y.; Ishihara, M.; Atsumi, T.; Yokoe, I. Dipalmitoylphosphatidylcholine (DPPC) and DPPC/cholesterol liposomes as predictors of the cytotoxicity of bis-GMA related compounds. J. Liposome Res 2004, 14, 39–49. [Google Scholar]
- Hatada, K.; Kitayama, T.; Nishiura, T.; Shibuya, W. Relation between reactivities of vinyl monomers and their NMR spectra. Curr. Org. Chem 2002, 6, 121–153. [Google Scholar]
- Fujisawa, S.; Kadoma, Y. Prediction of the reduced glutathione (GSH) reactivity of dental methacrylate monomers using NMR spectra—Relationship between toxicity and GSH reactivity. Dent. Mater. J 2009, 28, 722–729. [Google Scholar]
- McCarthy, T.J.; Hayes, E.P.; Schwartz, C.S.; Witz, G. The reactivity of selected acrylate esters toward glutathione and deoxyribonucleosides in vitro: Structure-activity relationships. Fundam. Appl. Toxicol 1994, 22, 543–548. [Google Scholar]
- Verhaar, H.J.M.; van Leeuwen, C.J.; Hermens, J.L.M. Classifying environmental pollutants. 1: Structure-activity relationships for prediction of aquatic toxicity. Chemosphere 1992, 25, 471–491. [Google Scholar]
- Russom, C.L.; Bradbury, S.P.; Broderius, S.J.; Hammermeister, D.E.; Drummond, R.A. Predicting modes of toxic action from chemical structure: Acute toxicity in the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem 1997, 16, 948–967. [Google Scholar]
- Koleva, Y.K.; Madden, J.C.; Cronin, M.T. Formation of categories from structure-activity relationships to allow read-across for risk assessment: Toxicity of α,β-unsaturated carbonyl compounds. Chem. Res. Toxicol 2008, 21, 2300–2312. [Google Scholar]
- del Re, G. A simple MO-LCAO method for the calculation of charge distributions in saturated organic molecules. J. Chem. Soc 1958, 4031–4040. [Google Scholar] [CrossRef]
- Talalay, P.; de Long, M.J.; Prochaska, H.J. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc. Natl. Acad. Sci. USA 1988, 85, 8261–8265. [Google Scholar]
- The University of Oxford. Department of Chemistry MSDS web resource. http://msds.chem.ox.uk/AC/acrolein.html accessed on 19 October 2011.
- The University of Oxford. Department of Chemistry MSDS web resource. http://msds.chem.ox.uk/AC/acrylonitrile.html accessed on 15 November 2011.
- The University of Oxford. Department of Chemistry MSDS web resource. http://msds.chem.ox.uk/AC/acrylamide.html accessed on 7 February 2005.
- Dickinson, D.A.; Forman, H.J. Cellular glutathione and thiols metabolism. Biochem. Pharmacol 2002, 64, 1019–1026. [Google Scholar]
- Geurtsen, W.; Leyhausen, G. Chemical-biological interactions of the resin monomer triethyleneglycol-dimethacrylate (TEGDMA). J. Dent. Res 2001, 80, 2046–2050. [Google Scholar]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res 2006, 85, 870–877. [Google Scholar]
- Ishikawa, T.; Li, Z.S.; Lu, Y.P.; Rea, P.A. The GS-X pump in plant, yeast, and animal cells: Structure, function, and gene expression. Biosci. Rep 1997, 17, 189–207. [Google Scholar]
- Tanel, A.; Averill-Bates, D.A. The aldehyde acrolein induces apoptosis via activation of the mitochondrial pathway. Biochim. Biophys. Acta 2005, 1743, 255–267. [Google Scholar]
- Zhang, H.; Kamendulis, L.M.; Jiang, J.; Xu, Y.; Klaunig, J.E. Acrylonitrile-induced morphological transformation in Syrian hamster embryo cells. Carcinogenesis 2000, 21, 727–733. [Google Scholar]
- Zitting, A.; Heinonen, T. Decrease of reduced glutathione in isolated rat hepatocytes caused by acrolein, acrylonitrile, and the thermal degradation products of styrene copolymers. Toxicology 1980, 17, 333–341. [Google Scholar]
- Besaratinia, A.; Pfeifer, G.P. A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis 2007, 28, 519–528. [Google Scholar]
- Papahadjopoulos, D. Liposomes and their uses in biology and medicine. Ann. N. Y. Acad. Sci 1978, 308, 1–462. [Google Scholar]
- Fujisawa, S.; Kadoma, Y.; Masuhara, E. A calorimetric study of the interaction of synthetic phospholipid liposomes with vinyl monomers, acrylates and methacrylates. J. Biomed. Mater. Res 1984, 18, 1105–1114. [Google Scholar]
- Fujisawa, S.; Atsumi, T.; Kadoma, Y. Cytotoxicity of methyl methacrylate (MMA) and related compounds and their interaction with dipalmitoylphosphatidylcholine (DPPC) liposomes as a model for biomembranes. Oral Dis 2000, 6, 215–221. [Google Scholar]
- Fujisawa, S.; Atsumi, T.; Kadoma, Y. Cytotoxicity and phospholipid-liposome phase-transition properties of 2-hydroxyethyl methacrylate (HEMA). Artif. Cells Blood Substit. Immobil. Biotechnol 2001, 29, 245–261. [Google Scholar]
- Lasic, D.D. Magnetic resonance methods in the studies of liposomes. Bull. Magn. Reson 1991, 13, 3–13. [Google Scholar]
- Cruciani, O.; Mannina, L.; Sobolev, A.P.; Cametti, C.; Segre, A. An improved NMR study of liposomes using 1-palmitoyl-2-oleoyl-sn-glycero-3-phospatidylcholine as model. Molecules 2006, 11, 334–344. [Google Scholar]
- Jain, M.K.; Wu, N.Y.; Wray, L.V. Drug-induced phase change in bilayer as possible mode of action of membrane expanding drugs. Nature 1975, 255, 494–496. [Google Scholar]
- Marique-Moreno, M.; Suwalsky, M.; Villena, F.; Garidel, P. Effects of the nonsteroidal anti-inflammatory drug naproxen on human erythrocytes and on cell membrane molecular models. Biophys. Chem 2010, 147, 53–58. [Google Scholar]


| Compound | log 1/H50 (mole/L) a | 7-days ipLD50 (mole/106 g) a | δHa (ppm) b | δCβ (ppm) b |
|---|---|---|---|---|
| Methyl acrylate (MA) | 0.63 | 2.95 | 5.82 | 130.56 |
| Ethyl acrylate (EA) | 0.95 | 5.98 | 5.807 | 130.24 |
| n-Propyl acrylate (nPA) | 1.59 | 5.80 | 5.809 | 130.22 |
| n-Butyl acrylate (nBA) | 2.61 | 6.64 | 5.805 | 130.21 |
| Isobutyl acrylate (IBA) | 2.84 | 5.92 | 5.813 | 130.23 |
| Methyl methacrylate (MMA) | 1.05 | 10.88 | 5.555 | 125.23 |
| Ethyl methacrylate (EMA) | 1.44 | 7.89 | 5.541 | 124.97 |
| n-Propyl methacrylate (nPMA) | 2.17 | 11.63 | 5.54 | 124.95 |
| n-Butyl methacrylate (nBMA) | 3.42 | 10.47 | 5.532 | 124.70 |
| Com. a | Log P b | MR b | Vm (cm2/mole) b | Qσ (C) c | Rate constant (kapp) (liter mol−1min−1) d |
|---|---|---|---|---|---|
| MA | 0.625 | 21.85 | 49.02 | 0.1666 | 52.0 |
| EA | 1.165 | 26.03 | 59.25 | 0.1662 | 26.6 |
| nPA | 1.705 | 26.5 | 69.47 | -- | -- |
| nBA | 2.245 | 31.15 | 79.70 | 0.1662 | 38.7 |
| IBA | 2.245 | 31.15 | 79.70 | 0.1662 | -- |
| MMA | 0.945 | 27.5 | 59.25 | 0.1638 | 0.325 |
| EMA | 1.485 | 31.68 | 69.48 | 0.1634 | 0.139 |
| nPMA | 2.025 | 32.15 | 79.70 | -- | -- |
| nBMA | 2.565 | 36.8 | 89.94 | 0.1634 | No appreciable rate |
| Comp. | Heat of formation (HF) kcal/mol | EHOMO eV | η eV | χ eV | ω eV |
|---|---|---|---|---|---|
| MA | −67.387 | −11.066 | 5.492 | 5.574 | 2.829 |
| EA | −72.173 | −11.040 | 5.495 | 5.546 | 2.799 |
| nPA | −77.404 | −11.044 | 5.495 | 5.550 | 2.803 |
| nBA | −82.791 | −11.045 | 5.495 | 5.550 | 2.803 |
| IBA | −82.435 | −11.042 | 5.495 | 5.548 | 2.801 |
| MMA | −74.768 | −10.548 | 5.245 | 5.303 | 2.681 |
| EMA | −79.542 | −10.524 | 5.249 | 5.278 | 2.654 |
| nPMA | −84.767 | −10.529 | 5.248 | 5.281 | 2.657 |
| nBMA | −90.156 | −10.530 | 5.248 | 5.282 | 2.658 |
| (A) | |
| Equation (1) | |
| Equation (2) | |
| Equation (3) | |
| Equation (4) | |
| For (meth)acrylates: | |
| kapp = −941.33 (±9.43) + 7.53 (±1.64) δCβ (n = 5, r2 = 0.88, p < 0.05) | QSPR 1 |
| For MA, EA, MMA and EMA at 40 mM DPPC: | |
| ΔδHa = −0.320 (±0.012) − 0.005 (±0.001) HF (n = 4, r2 = 0.92, p < 0.05) | QSPR 2 |
| (B) | |
| For (meth)acrylates: | |
| Log 1/H50 = −0.44 (±0.24) − 0.36 (±0.12) log P (n = 9, r2 = 0.95, p < 0.001) | QSAR 1 |
| Log 1/H50 = −5.55 (±0.27) − 0.09 (±0.14) HF (n = 9, r2 = 0.86, p < 0.001) | QSAR 2 |
| ipLD50 = 123.0 (±1.5) − 0.9 (±0.2) δCβ (n = 9, r2 = 0.78, p < 0.01) | QSAR 3 |
| ipLD50 = 109.0 (±1.5) − 17.8 (±3.6) δHa (n = 9, r2 = 0.78, p < 0.01) | QSAR 4 |
| ipLD50 = 1.02 (±0.26) − 0.01 (±0.03) δCβ + 1.40(±0.14) log P (n = 9, r2 = 0.94, p < 0.001) | QSAR 5 |
| ipLD50 = −1.1 + 8.8 (±2.0) log P − 2.1 (±0.6) log P2 (n = 9, r2 = 0.78, p < 0.01) | QSAR 6 |
| ipLD50 = 270.2 (±16) − 1592.8 (±429.9) Qσ(C) (n = 7, r2 = 0.73, p < 0.05) | QSAR 7 |
| ipLD50 = 111.2 (±1.5) − 19.3 (±4.2) η (n = 9, r2 = 0.75, p < 0.01) | QSAR 8 |
| ipLD50 = 105.1 (±1.5) − 17.5 (±3.7) χ (n = 9, r2 = 0.75, p < 0.01) | QSAR 9 |
| ipLD50 = 98.5 (±1.4) − 33.1 (±6.6) ω (n = 9, r2 = 0.78, p < 0.01) | QSAR 10 |
| For MA, EA, MMA and EMA: | |
| Log 1/H50 = 0.57 (±0.13) + 117.55 (±27.48) ΔδHa (n = 4, r2 = 0.90, p < 0.05) | QSAR 11 |
| Name | Acrylate | Concentration of QR | kapp | Reported oral-LD50, (mg kg−1) | NMR chemical shift h |
|---|---|---|---|---|---|
| Structure | (mM) a | (M−1min−1) b | (ipLD50, mol kg−1) c | δHa(δCβ), ppm | |
| MA | CH2=CHCOOCH3 | 20 | 41.8 | 857 (5.5) d | 5.825(130.56) |
| MMA | CH2=C(CH3)COOCH3 | I | 16.8 | 5,197 (10.3) d | 5.555(125.23) |
| Acrolein | CH2=CHCHO | 130 | 94.6 | 40 (0.5) e | 6.495(137.57) |
| Acrylonitrile | CH2=CHC≡N | 50 | 91.4 | 27 (0.8) f | 6.083(137.14) |
| Acrylamide | CH2=CHCONH2 | I | 17.9 | 107 (8.4) g | 5.700(127.38) |
| MMA, structure and numbering | H attached to the carbon | ΔδHa, ppm | |
|---|---|---|---|
| 25 °C | 50 °C | ||
 | Ha | −0.01 | −0.05 |
| Hb | −0.005 | −0.01 | |
| 5H | −0.004 | 0.00 | |
| 2H | −0.001 | 0.03 | |
| Compound | Phase transition temperature (T) °C | Enthalpy (ΔH) kcal/mol | Entropy (ΔE) cal mo1−1K−1 |
|---|---|---|---|
| Control | 41.0 | 8.8 | 28.03 |
| MA a | 33.5 | 7.9 | 25.77 |
| MMA a | 39.5 | 6.7 | 21.44 |
| Control | 41.5 | 8.9 | 28.30 |
| EA b | 32.5 | 7.7 | 25.20 |
| nBA b | 31.5 | 4.0 | 13.13 |
| EMA b | 40.5 | 5.7 | 18.18 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fujisawa, S.; Kadoma, Y. Mechanisms of Action of (Meth)acrylates in Hemolytic Activity, in Vivo Toxicity and Dipalmitoylphosphatidylcholine (DPPC) Liposomes Determined Using NMR Spectroscopy. Int. J. Mol. Sci. 2012, 13, 758-773. https://doi.org/10.3390/ijms13010758
Fujisawa S, Kadoma Y. Mechanisms of Action of (Meth)acrylates in Hemolytic Activity, in Vivo Toxicity and Dipalmitoylphosphatidylcholine (DPPC) Liposomes Determined Using NMR Spectroscopy. International Journal of Molecular Sciences. 2012; 13(1):758-773. https://doi.org/10.3390/ijms13010758
Chicago/Turabian StyleFujisawa, Seiichiro, and Yoshinori Kadoma. 2012. "Mechanisms of Action of (Meth)acrylates in Hemolytic Activity, in Vivo Toxicity and Dipalmitoylphosphatidylcholine (DPPC) Liposomes Determined Using NMR Spectroscopy" International Journal of Molecular Sciences 13, no. 1: 758-773. https://doi.org/10.3390/ijms13010758




