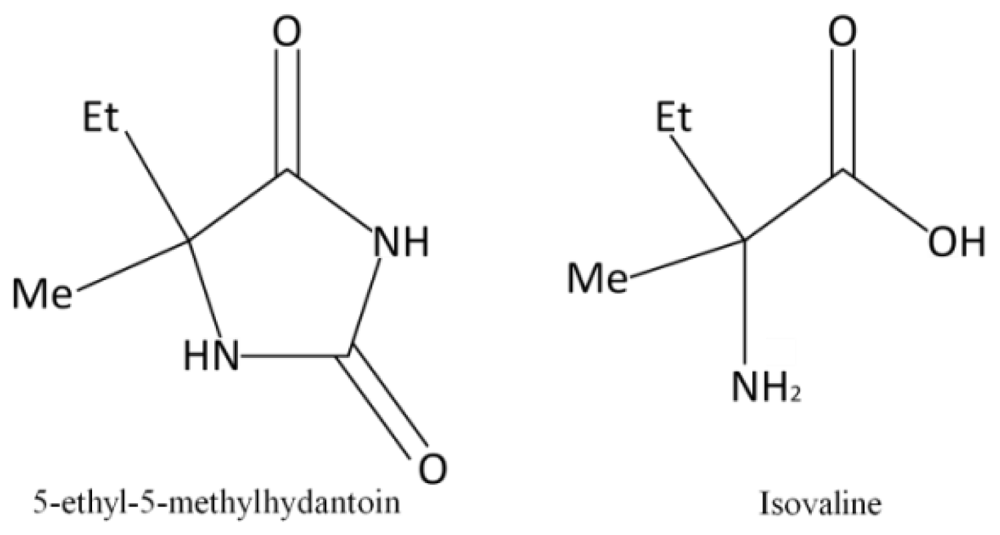
Photostability of Isovaline and its Precursor 5-Ethyl-5-methylhydantoin Exposed to Simulated Space Radiations
Abstract
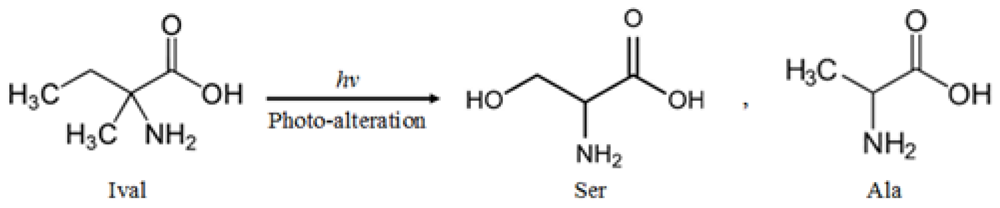
:1. Introduction
2. Results and Discussion
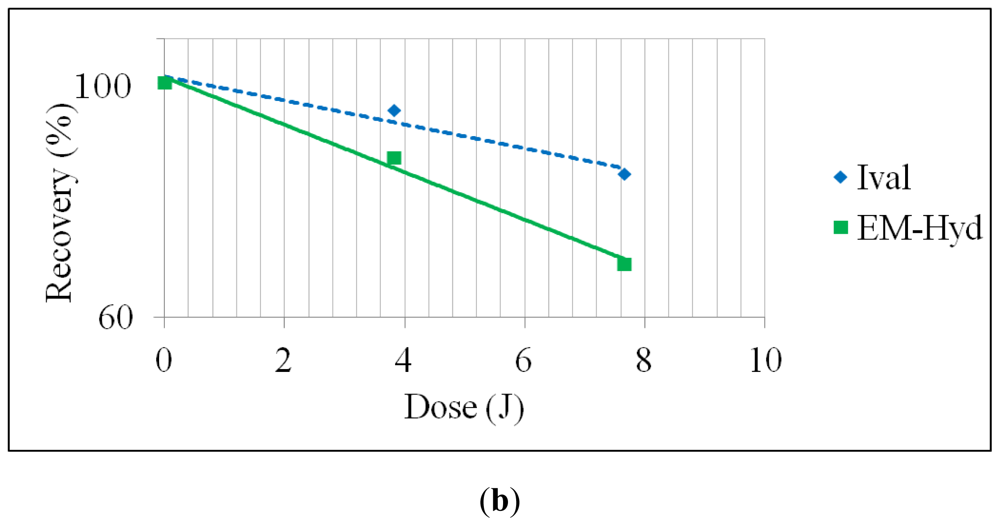
2.1. Stability of Ival and EM-Hyd Against UV Irradiation
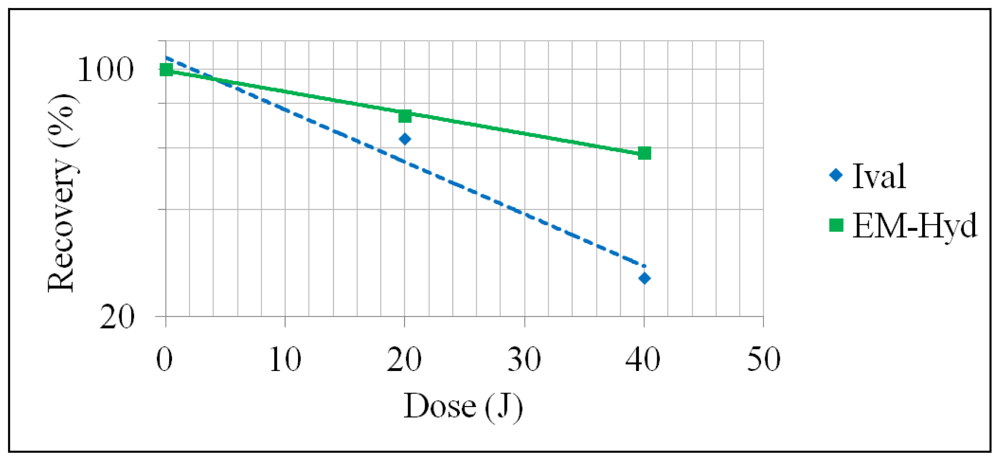
2.2. Stability of Ival and EM-Hyd Against γ-Ray Irradiation
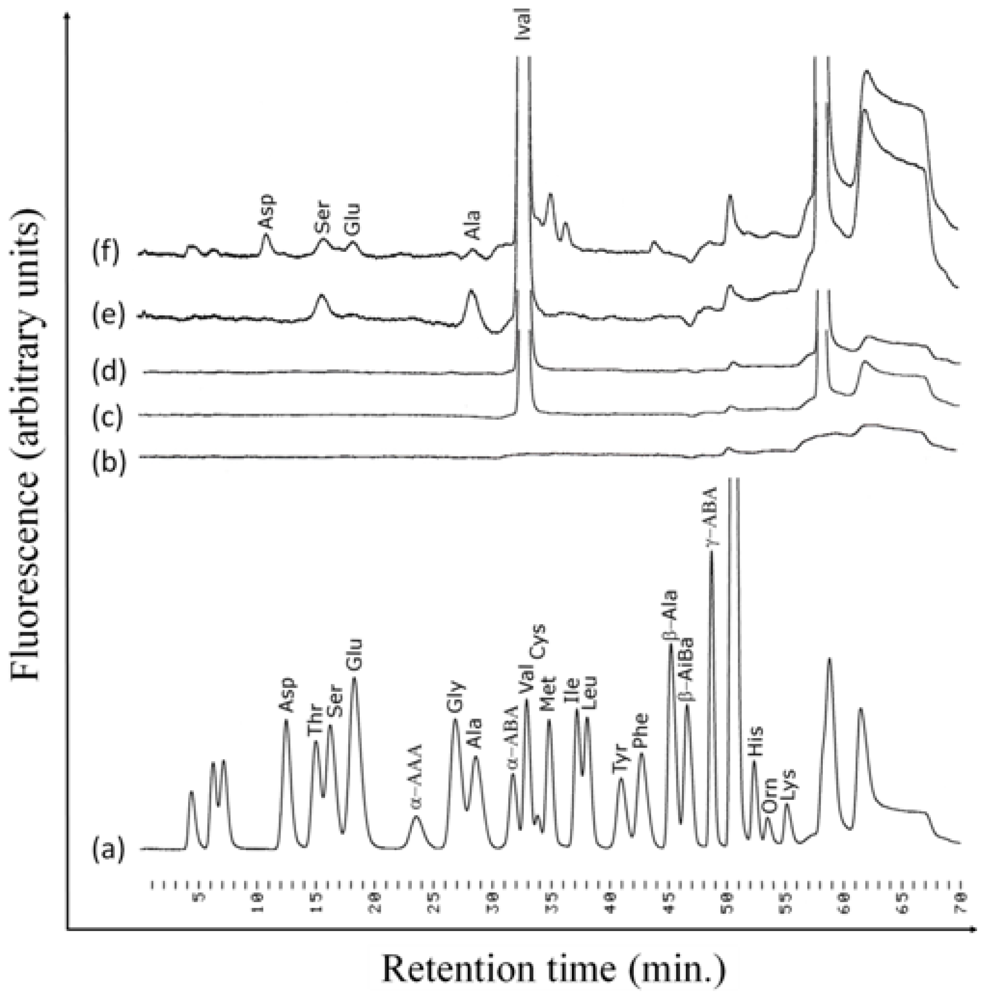
2.3. Photolysis Products of Irradiated Ival and EM-Hyd
3. Experimental Section
3.1. Chemicals
3.2. UV Absorption Measurement
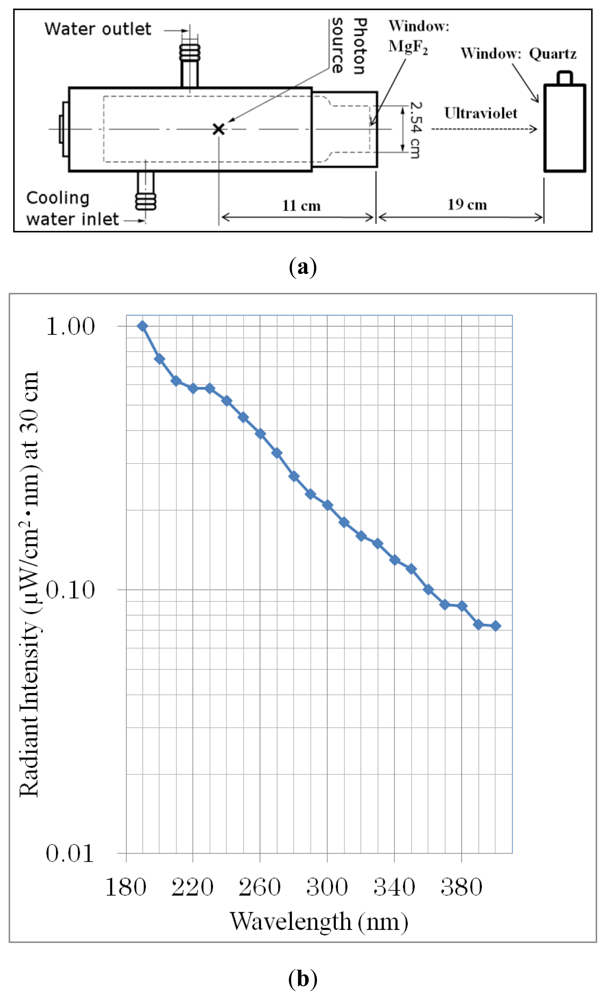
3.3. UV Irradiation
3.4. γ-Ray Irradiation
3.5. Analysis of Ival and EM-Hyd
4. Conclusions
Acknowledgments
References
- Schmitt-Kopplin, P.; Gabelica, Z.; Gougeon, R.D.; Fekete, A.; Kanawati, B.; Harir, M.; Gebefuegi, I.; Eckel, G.; Hertkorn, N. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar]
- Kvenvolden, K.; Lawless, J.; Pering, K.; Peterson, E.; Flores, J.; Ponnamperuma, C.; Kaplan, I.R.; Moore, C. Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature 1970, 228, 923–926. [Google Scholar]
- Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Cometary glycine detected in samples returned by Stardust. Meteorit. Planet. Sci 2009, 44, 1323–1330. [Google Scholar]
- Sandford, S.A.; Aléon, J.; Alexander, C.M.; Araki, T.; Bajt, S.; Baratta, G.A.; Borg, J.; Bradley, J.P.; Brownlee, D.E.; Brucato, J.R.; et al. Organics captured from comet 81P/Wild 2 by the Stardust spacecraft. Science 2006, 314, 1720–1724. [Google Scholar]
- Kobayashi, K.; Kasamatsu, T.; Kaneko, T.; Koike, J.; Oshima, T.; Saito, T.; Yamamoto, T.; Yanagawa, H. Formation of amino acid precursors in cometary ice environments by cosmic radiation. Adv. Space Res 1995, 16, 21–26. [Google Scholar]
- Takano, Y.; Takahashi, J.; Kaneko, T.; Marumo, K.; Kobayashi, K. Asymmetric synthesis of amino acid precursors in interstellar complex organics by circularly polarized light. Earth Planet. Sci. Lett 2007, 254, 106–114. [Google Scholar]
- Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Cooper, G.W.; Allamandola, L.J. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416, 401–403. [Google Scholar]
- Muñoz Caro, G.M.; Meierhenrich, U.J.; Schutte, W.A.; Barbier, B.; Arcones, S.A.; Rosenbauer, H.; Thiemann, W.H.-P.; Brack, A.; Greenberg, J.M. Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 2002, 416, 403–406. [Google Scholar]
- Nuevo, M.; Bredehöft, J.H.; Meierhenrich, U.J.; d’Hendecourt, L.; Thiemann, W.H. Urea, glycolic acid, and glycerol in an organic residue produced by ultraviolet irradiation of interstellar/pre-cometary ice analogs. Astrobiology 2010, 10, 245–256. [Google Scholar]
- de Marcellus, P.; Bertrand, M.; Nuevo, M.; Westall, F.; d’Hendecourt, L. Prebiotic significance of extraterrestrial ice photochemistry: Detection of hydantoin in organic residues. Astrobiology 2011, 11, 847–854. [Google Scholar]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 335, 125–132. [Google Scholar]
- Love, S.; Brownlee, D. A direct measurement of the terrestrial mass accretion rate of cosmic dust. Science 1993, 262, 550–553. [Google Scholar]
- Cronin, J.R.; Pizzarello, S. Amino acid enantiomer excesses in meteorites: Origin and significance. Adv. Space Res 1999, 23, 293–299. [Google Scholar]
- Sephton, M.A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep 2002, 19, 292–311. [Google Scholar]
- Bailey, J.; Chrysostomou, A.; Hough, J.H.; Gledhill, T.M.; McCall, A.; Clark, S.; Menard, F.; Tamura, M. Circular polarization in star-formation regions: Implications for biomolecular homochirality. Science 1998, 281, 672–674. [Google Scholar]
- Bailey, J. Astronomical sources of circularly polarized light and the origin of homochirality. Orig. Life Evol. Biosph 2001, 31, 167–183. [Google Scholar]
- Buschermohle, M.; Whittet, D.C.B.; Chrysostomou, A.; Hough, J.H.; Lucas, P.W.; Adamson, A.J.; Whitney, B.A.; Wolff, M.J. An extended search for circularly polarized infrared radiation from the OMC-1 region of Orion. Astrophys. J 2005, 624, 821–826. [Google Scholar]
- Kobayashi, K.; Tonishi, H.; Tsuboi, T.; Suzuki, N.; Kaneko, T.; Takano, Y.; Muramatsu, Y.Y.; Hashimoto, H.; Yamashita, M. Formation and stability of complex organic compounds in space environments. Biol. Sci. Space 2004, 18, 179–180. [Google Scholar]
- Takano, Y.; Kaneko, T.; Kobayashi, K.; Hiroishi, D.; Ikeda, H.; Marumo, K. Experimental verification of photostability for free- and bound-amino acids exposed to γ-rays and UV irradiation. Earth Planets Space 2004, 56, 669–674. [Google Scholar]
- Ehrenfreund, P.; Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Allamandola, L.J. The photostability of amino acids in space. Astrophys. J 2001, 550, L95–L99. [Google Scholar]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space microbiology. Microbiol. Mol. Biol. Rev 2010, 74, 121–156. [Google Scholar]
- Horneck, G.; Mileikowsky, C.; Melosh, H.J.; Wilson, J.W.; Cucinotta, F.A.; Gladman, B. Viable Transfer of Microorganisms in the Solar System and Beyond. In Astrobiology: The Quest for the Conditions of Life; Horneck, G., Baumstark-Khan, C., Eds.; Springer-Verlag Berlin: Heidelberg, Germany, 2002. [Google Scholar]
- Cottin, H.; Coll, P.; Coscia, D.; Fray, N.; Guan, Y.Y.; Macari, F.; Raulin, F.; Rivron, C.; Stalport, F.; Szopa, C.; et al. Heterogeneous solid/gas chemistry of organic compounds related to comets, meteorites, Titan, and Mars: Laboratory and in lower Earth orbit experiments. Adv. Space Res 2008, 42, 2019–2035. [Google Scholar]
- Rubenstein, E.; Bonner, W.A.; Noyes, H.P.; Brown, G.S. Supernovae and life. Nature 1983, 306, 118. [Google Scholar]
- Bonner, W.A. The origin and amplification of biomolecular chirality. Orig. Life Evol. Biosph 1991, 21, 59–111. [Google Scholar]
- de Marcellus, P.; Meinert, C.; Nuevo, M.; Filippi, J.-J.; Danger, G.; Deboffle, D.; Nahon, L.; d’Hendecourt, L.; Meierhenrich, U.J. Non-racemic amino acid production by ultraviolet irradiation of achiral interstellar ice analogs with circularly polarized light. Astrophys. J. Lett 2011, 727. [Google Scholar] [CrossRef]
- Fukue, T.; Tamura, M.; Kandori, R.; Kusakabe, N.; Hough, J.H.; Bailey, J.; Whittet, D.C.; Lucas, P.W.; Nakajima, Y.; Hashimoto, J. Extended high circular polarization in the orion massive star forming region: Implications for the origin of homochirality in the solar system. Orig. Life Evol. Biosph 2010, 40, 335–346. [Google Scholar]
- Flores, J.J.; Bonner, W.A.; Massey, G.A. Asymmetric photolysis of (R,S)-leucine with circularly polarized light. J. Am. Chem. Soc 1977, 99, 3622–3625. [Google Scholar]
- Bonner, W.A.; Bean, B.D. Asymmetric photolysis with elliptically polarized light. Orig. Life Evol. Biosph 2000, 30, 513–517. [Google Scholar]
- Nishino, H.; Kosaka, A.; Hembury, G.A.; Shitomi, H.; Onuki, H.; Inoue, Y. Mechanism of pH-dependent photolysis of aliphatic amino acids and enantiomeric enrichment of racemic leucine by circularly polarized light. Org. Lett 2001, 3, 921–924. [Google Scholar]
- Meierhenrich, U.J.; Nahon, L.; Alcarez, C.; Bredeht, J.H.; Hoffmann, S.K.; Barbier, B.; Brack, A. Asymmetric vacuum UV photolysis of the amino acids leucine in solid state. Angew. Chem. Int. Ed 2005, 44, 5630–5636. [Google Scholar]
- Takahashi, J.; Shinojima, H.; Seyama, M.; Ueno, Y.; Kaneko, T.; Kobayashi, K.; Mita, H.; Adachi, M.; Hosaka, M.; Katoh, M. Chirality emergence in thin solid films of amino acids by polarized light from synchrotron radiation and free electron laser. Int. J. Mol. Sci 2009, 10, 3044–3064. [Google Scholar]
- Pizzarello, S.; Zolensky, M.; Turk, K.A. Nonracemic isovaline in the Murchison meteorite: Chiral distribution and mineral association. Geochim. Cosmochim. Acta 2003, 67, 1589–1595. [Google Scholar]
- Glavin, D.P.; Dworkin, J.P. Enrichment of the amino acid l-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl. Acad. Sci. USA 2009, 106, 5487–5492. [Google Scholar]
- Shimoyama, A.; Ogasawara, R. Dipeptides and diketopiperazines in the Yamato-791198 and Murchison carbonaceous chondrites. Orig. Life Evol. Biosph 2002, 32, 165–179. [Google Scholar]
- Olson, E.S. Amino acids from coal gasification. Nature 1992, 357. [Google Scholar] [CrossRef]
- Yamagishi, A.; Yano, H.; Okudaira, K.; Kobayashi, K.; Yokobori, S.; Tabata, M.; Kawai, H.; Yamashita, M.; Hashimoto, H.; Naraoka, H.; et al. Tanpopo: Astrobiology exposure and micrometeoroid capture experiments. Trans. JSASS Space Tech. Jpn 2009, 7, Tk:49–Tk:55. [Google Scholar]

| Types of irradiation | Total energy dose (J) | Recovery (%) | |
|---|---|---|---|
| Ival | EM-Hyd | ||
| UV (continuous, 190–400 nm) | 3.8 | 94 ± 0.24 | 85 ± 0.95 |
| 7.6 | 82 ± 0.07 | 67 ± 0.96 | |
| γ-rays | 20 | 64 ± 0.02 | 74 ± 1.5 |
| 40 | 26 ± 0.44 | 58 ± 2.3 | |
| Types of irradiation | Quantum yield of photolysis, Φ (molecule/photon) | |
|---|---|---|
| Ival | EM-Hyd | |
| UV (continuous, 190–400 nm) | 0.89 | 0.80 |
| γ-rays | 4.4 × 104 | 2.8 × 104 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sarker, P.K.; Takahashi, J.-i.; Kawamoto, Y.; Obayashi, Y.; Kaneko, T.; Kobayashi, K. Photostability of Isovaline and its Precursor 5-Ethyl-5-methylhydantoin Exposed to Simulated Space Radiations. Int. J. Mol. Sci. 2012, 13, 1006-1017. https://doi.org/10.3390/ijms13011006
Sarker PK, Takahashi J-i, Kawamoto Y, Obayashi Y, Kaneko T, Kobayashi K. Photostability of Isovaline and its Precursor 5-Ethyl-5-methylhydantoin Exposed to Simulated Space Radiations. International Journal of Molecular Sciences. 2012; 13(1):1006-1017. https://doi.org/10.3390/ijms13011006
Chicago/Turabian StyleSarker, Palash K., Jun-ichi Takahashi, Yukinori Kawamoto, Yumiko Obayashi, Takeo Kaneko, and Kensei Kobayashi. 2012. "Photostability of Isovaline and its Precursor 5-Ethyl-5-methylhydantoin Exposed to Simulated Space Radiations" International Journal of Molecular Sciences 13, no. 1: 1006-1017. https://doi.org/10.3390/ijms13011006




