Chemical Structures of 4-Oxo-Flavonoids in Relation to Inhibition of Oxidized Low-Density Lipoprotein (LDL)-Induced Vascular Endothelial Dysfunction
Abstract
:1. Introduction
2. Results
3. Experimental Section
3.1. Reagents
3.2. Oxidation of Low-Density Lipoprotein
3.3. Cell Culture
3.4. Cell Viability Measurement
3.5. Determination of MDA, NO and sICAM-1 Level
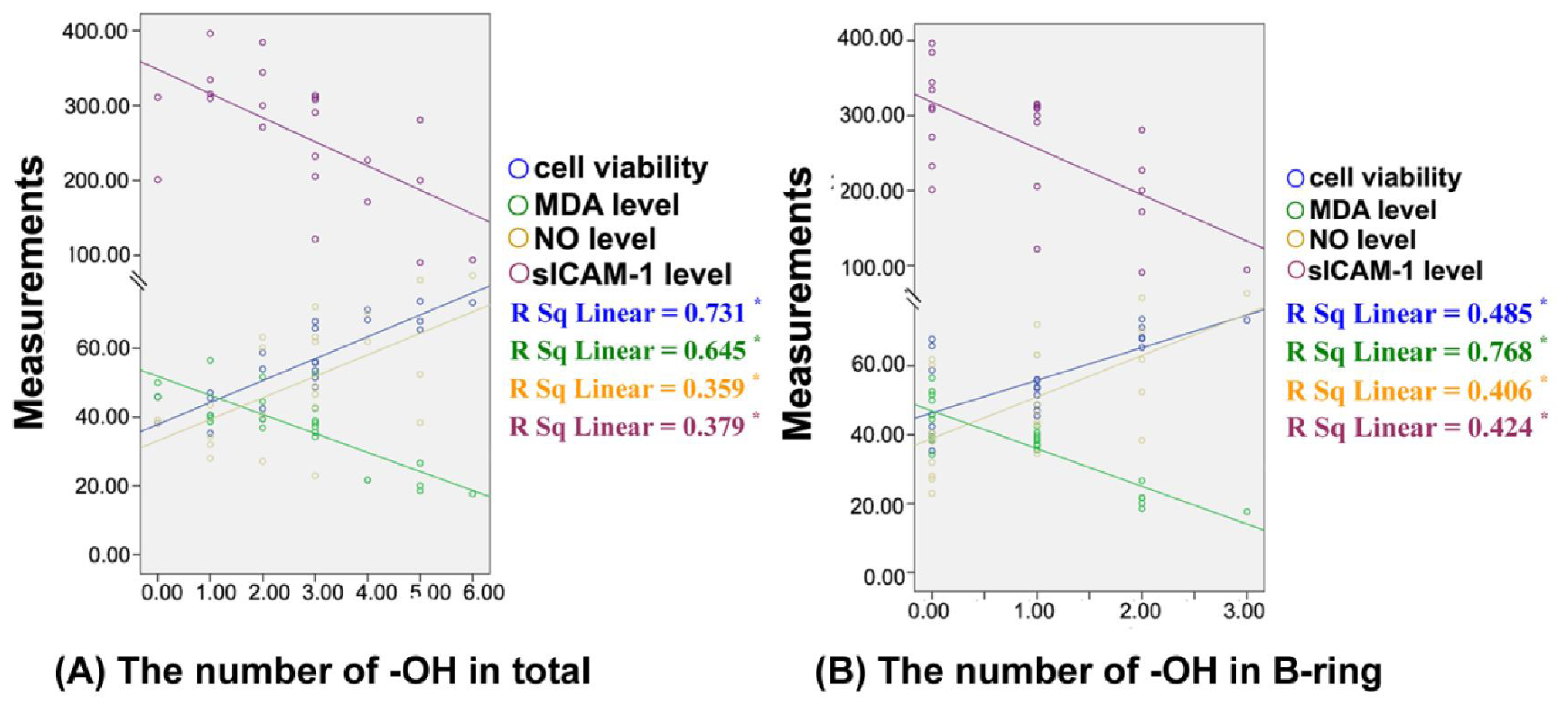
3.6. Structure-Activtity Relationship Analysis
3.7. Statistical Analysis
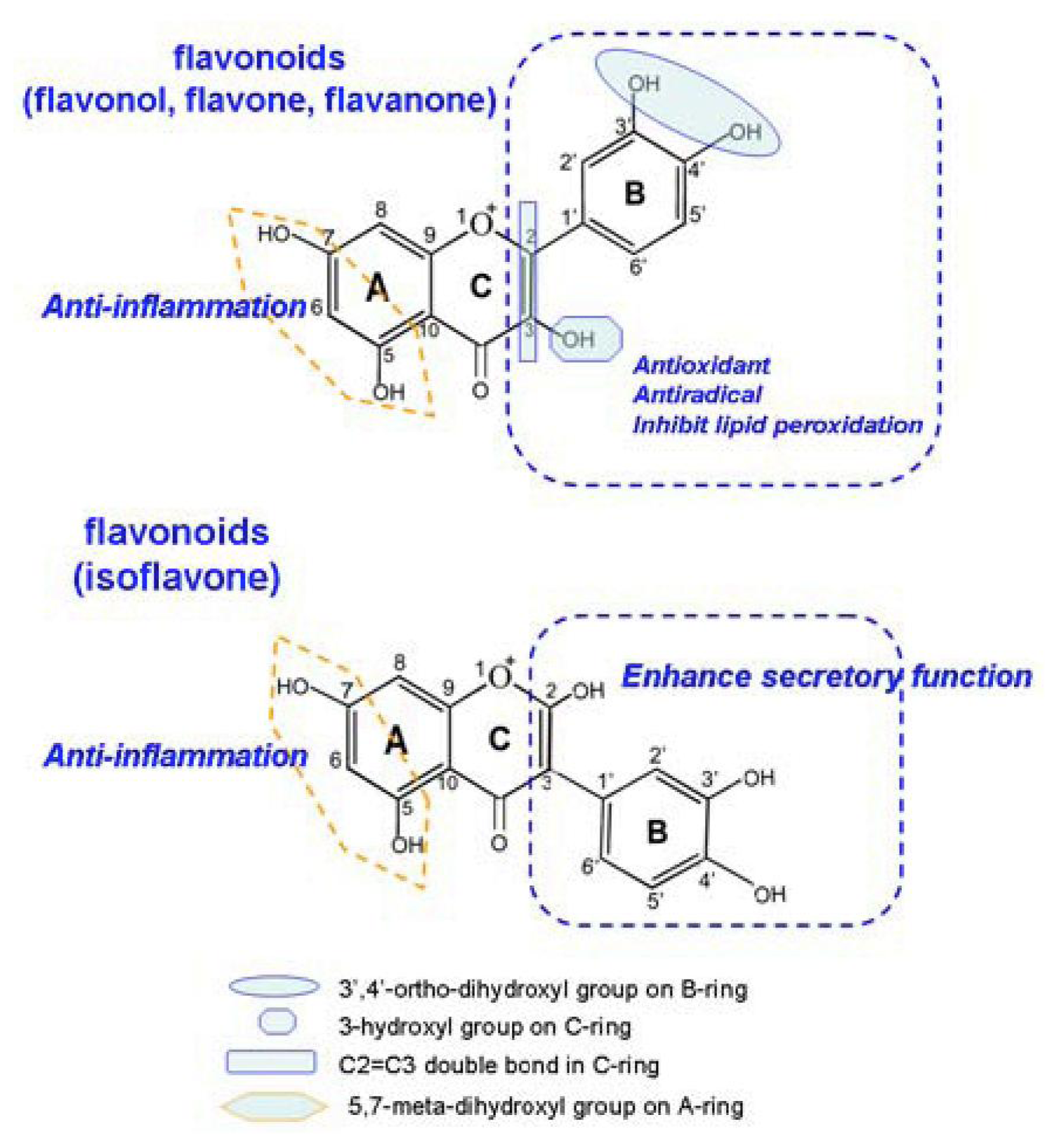
4. Discussion
5. Conclusions
Acknowledgments
References
- Badimon, JJ; Fuster, V; Chesebro, JH; Badimon, L. Coronary atherosclerosis. A multifactorial disease. Circulation 1993, 87, II3–II16. [Google Scholar]
- Chilton, RJ. Pathophysiology of coronary heart disease: A brief review. J Am Osteopath Assoc 2004, 104, S5–S8. [Google Scholar]
- Lusis, AJ. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar]
- Ahmadi, N; Tsimikas, S; Hajsadeghi, F; Saeed, A; Nabavi, V; Bevinal, MA; Kadakia, J; Flores, F; Ebrahimi, R; Budoff, MJ. Relation of oxidative biomarkers, vascular dysfunction, and progression of coronary artery calcium. Am J Cardiol 2010, 105, 459–466. [Google Scholar]
- Mehta, JL; Chen, J; Hermonat, PL; Romeo, F; Novelli, G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): A critical player in the development of atherosclerosis and related disorders. Cardiovasc Res 2006, 69, 36–45. [Google Scholar]
- Pennathurj, S; Heinecke, JW. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep 2007, 7, 257–264. [Google Scholar]
- Aherne, SA; O’Brien, NM. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar]
- Grassi, D; Desideri, G; Croce, G; Tiberti, S; Aggio, A; Ferri, C. Flavonoids, vascular function and cardiovascular protection. Curr Pharm Des 2009, 15, 1072–1084. [Google Scholar]
- Van Zanden, JJ; Geraets, L; Wortelboer, HM; van Bladeren, PJ; Rietjens, IM; Cnubben, NH. Structural requirements for the flavonoid-mediated modulation of glutathione S-transferase P1-1 and GS-X pump activity in MCF7 breast cancer cells. Biochem Pharmacol 2004, 67, 1607–1617. [Google Scholar]
- Xu, YC; Leung, SW; Yeung, DK; Hu, LH; Chen, GH; Che, CM; Man, RY. Structure-activity relationships of flavonoids for vascular relaxation in porcine coronary arter. Phytochemistry 2007, 68, 1179–1188. [Google Scholar]
- Woodman, OL; Meeker, WF; Boujaoude, M. Vasorelaxant and antioxidant activity of flavonols and flavones: Structure-activity relationships. J Cardiovasc Pharmacol 2005, 46, 302–309. [Google Scholar]
- Chen, S; Zhang, F; Sherman, MA; Kijima, I; Cho, M; Yuan, YC; Toma, Y; Osawa, Y; Zhou, D; Eng, ET. Structure-function studies of aromatase and its inhibitors: A progress report. J Steroid Biochem Mol Biol 2003, 86, 231–237. [Google Scholar]
- Yi, L; Chen, CY; Jin, X; Mi, MT; Yu, B; Chang, H; Ling, WH; Zhang, T. Structural requirements of anthocyanins in relation to inhibition of endothelial injury induced by oxidized low-density lipoprotein and correlation with radical scavenging activity. FEBS Lett 2010, 584, 583–590. [Google Scholar]
- Sternberg, D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem 1997, 272, 20963–20966. [Google Scholar]
- Hou, G; Xue, L; Lu, Z; Fan, T; Tian, F; Xue, Y. An activated mTOR/p70S6K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett 2007, 253, 236–248. [Google Scholar]
- Wyllie, AH; Morris, RG; Smith, AL; Dunlop, D. Chromatin cleavage in apoptosis: Association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol 1984, 142, 67–77. [Google Scholar]
- Yang, Z; Gagarin, D; StLaurent, G, III; Hammell, N; Toma, I; Hu, CA; Iwasa, A; McCaffrey, TA. Cardiovascular inflammation and lesion cell apoptosis: A novel connection via the interferon-inducible immunoproteasome. Arterioscler Thromb Vasc Biol 2009, 29, 1213–1219. [Google Scholar]
- Wong, WT; Ng, CH; Tsang, SY; Huang, Y; Chen, ZY. Relative contribution of individual oxidized components in ox-LDL to inhibition on endothelium-dependent relaxation in rat aorta. Nutr Metab Cardiovasc Dis 2011, 21, 157–164. [Google Scholar]
- Sawamura, T; Kume, N; Aoyama, T; Moriwaki, H; Hoshikawa, H; Aiba, Y; Tanaka, T; Miwa, S; Katsura, Y; Kita, T; et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997, 386, 73–77. [Google Scholar]
- Morawietz, H. LOX-1 receptor as a novel target in endothelial dysfunction and atherosclerosis. Dtsch Med Wochenschr 2010, 135, 308–312. [Google Scholar]
- Ogura, S; Kakino, A; Sato, Y; Fujita, Y; Iwamoto, S; Otsui, K; Yoshimoto, R; Sawamura, T. Lox-1: The multifunctional receptor underlying cardiovascular dysfunction. Circ J 2009, 73, 1993–1999. [Google Scholar]
- Robbesyn, F; Salvayre, R; Negre-Salvayre, A. Dual role of oxidized LDL on the NF-kappaB signaling pathway. Free Radic Res 2004, 38, 541–551. [Google Scholar]
- Morawietz, H. LOX-1 and atherosclerosis: Proof of concept in LOX-1-knockout mice. Circ Res 2007, 100, 1534–1536. [Google Scholar]
- Morazzoni, P; Livio, S; Scilingo, A; Malandrino, S. Vaccinium myrtillus anthocyanosides pharmacokinetics in rats. Arzneimittelforschung 1991, 41, 128–131. [Google Scholar]
- Tsuda, T; Horio, F; Osawa, T. Dietary cyanidin 3-O-beta-d-glucoside increases ex vivo oxidation resistance of serum in rats. Lipids 1998, 33, 583–588. [Google Scholar]
- Tsuda, T; Horio, F; Osawa, T. Absorption and metabolism of cyanidin 3-O-beta-d-glucoside in rats. FEBS Lett 1999, 449, 179–182. [Google Scholar]
- Tsuda, T; Kitoh, J; Osawa, T; Horio, F. Protective effects of dietary cyaniding 3-O-beta-d-glucoside on liver ischemia-reperfusion injury in rats. Arch Biochem Biophys 1999, 368, 361–326. [Google Scholar]
- Miyazawa, T; Nakagawa, K; Kudo, M; Muraishi, K; Someya, K. Direct intestinal absorption of red fruit anthocyanins; cyanidin-3-glucoside and cyanidin-3,5-diglucosideinto rats and humans. J Agric Food Chem 1999, 47, 1083–1091. [Google Scholar]
- Tian, Q; Giusti, MM; Stoner, GD; Schwartz, SJ. Urinary excretion of black raspberry (Rubus occidentalis) anthocyanins and their metabolites. J Agric Food Chem 2006, 54, 1467–1572. [Google Scholar]
- Youdim, KA; Martin, A; Joseph, JA. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidativestress. Free Radic Biol Med 2000, 29, 51–60. [Google Scholar]
- Furuno, K; Akasako, T; Sugihara, N. The contribution of the pyrogallol moiety to the superoxide radical scavenging activity of flavonoids. Biol Pharm Bull 2002, 25, 19–23. [Google Scholar]
- McPhail, DB; Hartley, RC; Gardner, PT; Duthie, GG. Kinetic and stoichiometric assessment of the antioxidant activity of flavonoids by electron spin resonance spectroscopy. J Agric Food Chem 2003, 51, 1684–1690. [Google Scholar]
- Fiorani, M; de Sanctis, R; de Bellis, R; Dachà, M. Intracellular flavonoids as electron donors for extracellular ferricyanide reduction in human erythrocytes. Free Radic Biol Med 2002, 32, 64–72. [Google Scholar]
- Leopoldini, M; Russo, N; Toscano, M. A comparative study of the antioxidant power of flavonoid catechin and its planar analogue. J Agric Food Chem 2007, 55, 7944–7949. [Google Scholar]
- Seeram, NP; Nair, MG. Inhibition of lipid peroxidation and structure-activity-related studies of the dietary constituents anthocyanins, anthocyanidins, and catechins. J Agric Food Chem 2002, 50, 5308–5312. [Google Scholar]
- Collins, T; Read, MA; Neish, AS; Whitley, MZ; Thanos, D; Maniatis, T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. Faseb J 1995, 9, 899–909. [Google Scholar]
- Baeuerle, PA; Baltimore, D. NF-kappa B: Ten years after. Cell 1996, 87, 13–20. [Google Scholar]
- Accomando, S; Pellitteri, V; Corsello, G. Natural polyphenols as anti-inflammatory agents. Front Biosci (Schol Ed) 2010, 2, 318–331. [Google Scholar]
- Surh, YJ; Kundu, JK; Na, HK; Lee, JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr 2005, 135, 2993S–3001S. [Google Scholar]
- Crespo, I; García-Mediavilla, MV; Gutiérrez, B; Sánchez-Campos, S; Tuñón, MJ; González-Gallego, J. A comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Br J Nutr 2008, 100, 968–976. [Google Scholar]
- Aviram, M; Fuhrman, B. Wine flavonoids protect against LDL oxidation and atherosclerosis. Ann N Y Acad Sci 2002, 957, 146–161. [Google Scholar]
- Lotito, SB; Frei, B. Dietary flavonoids attenuate tumor necrosis factor alpha-induced adhesion molecule expression in human aortic endothelial cells. Structure-function relationships and activity after first pass metabolism. J Biol Chem 2006, 281, 37102–37110. [Google Scholar]
- McDonnell, DP; Norris, JD. Connections and regulation of the human estrogen receptor. Science 2002, 296, 1642–1644. [Google Scholar]
- Hisamoto, K; Bender, JR. Vascular cell signaling by membrane estrogen receptors. Steroids 2005, 70, 382–387. [Google Scholar]
- Sumi, D; Ignarro, LJ. Estrogen-related receptor alpha 1 up-regulates endothelial nitric oxide synthase expression. Proc Natl Acad Sci USA 2003, 100, 14451–14456. [Google Scholar]
- Borrás, C; Gambini, J; Gómez-Cabrera, MC; Sastre, J; Pallardó, FV; Mann, GE; Viña, J. 17beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging Cell 2005, 4, 113–118. [Google Scholar]
- Si, H; Liu, D. Phytochemical genistein in the regulation of vascular function: New insights. Curr Med Chem 2007, 14, 2581–2589. [Google Scholar]
- Cruz, MN; Agewall, S; Schenck-Gustafsson, K; Kublickiene, K. Acute dilatation to phytoestrogens and estrogen receptor subtypes expression in small arteries from women with coronary heart disease. Atherosclerosis 2008, 196, 49–58. [Google Scholar]
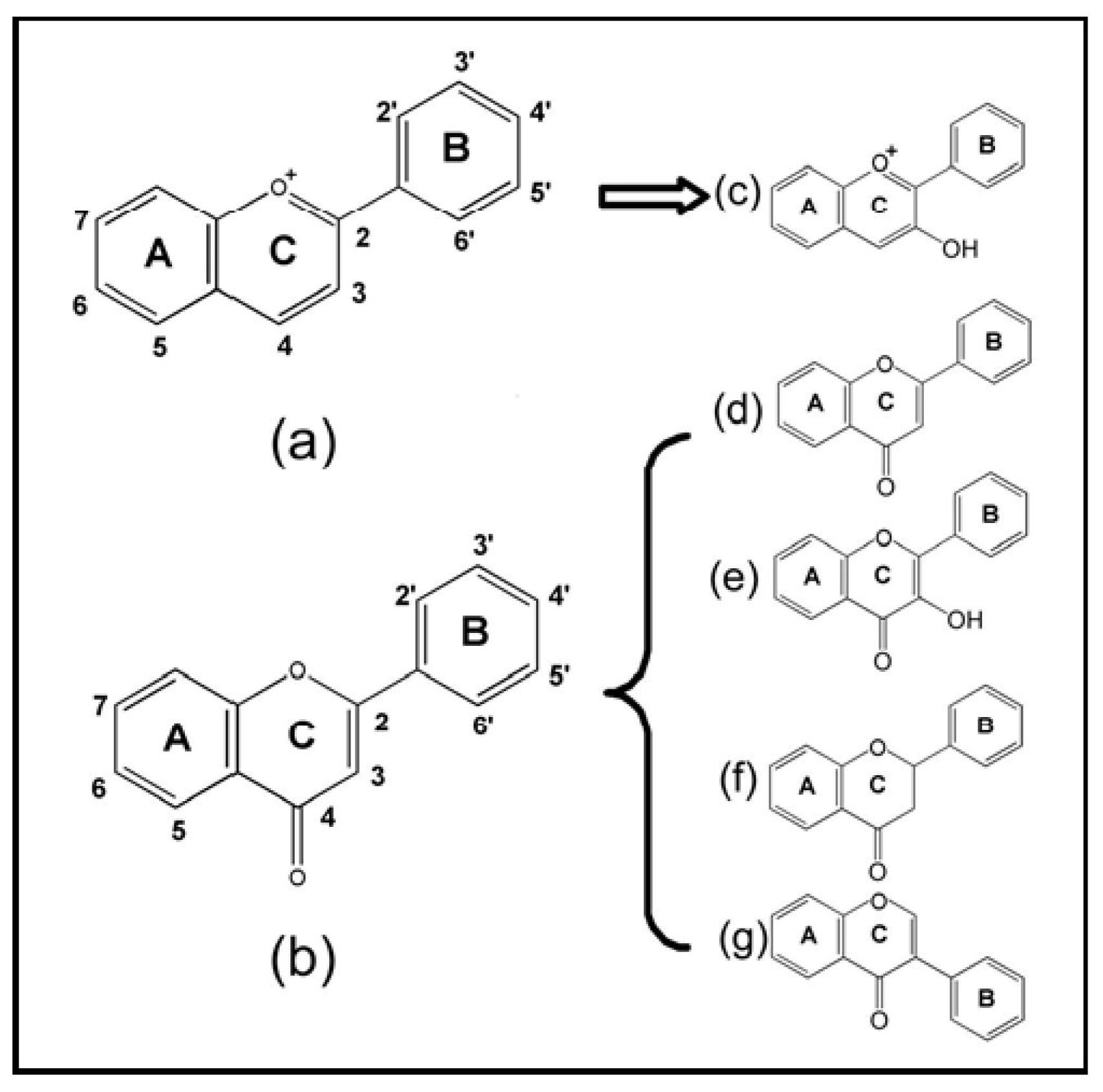

| No. | Chemicals | Source | B-ring
| C-ring
| A-ring
| 2,3-double bond | The number of −OH
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C2′ | C3′ | C4′ | C5′ | C6′ | C2 | C3 | C5 | C6 | C7 | C8 | In total | A-ring | B-ring | C-ring | ||||
| flavones: | ||||||||||||||||||
| 1 | flavone | Sigma | −H | −H | −H | −H | −H | B-ring | −H | −H | −H | −H | −H | √ | 0 | 0 | 0 | 0 |
| 2 | chrysin | Sigma | −H | −H | −H | −H | −H | B-ring | −H | −OH | −H | −OH | −H | √ | 2 | 2 | 0 | 0 |
| 3 | apigenin | Sigma | −H | −H | −OH | −H | −H | B-ring | −H | −OH | −H | −OH | −H | √ | 3 | 2 | 1 | 0 |
| 4 | luteolin | Sigma | −H | −OH | −OH | −H | −H | B-ring | −H | −OH | −H | −OH | −H | √ | 4 | 2 | 2 | 0 |
| 5 | 6-hydroxyflavone | Sigma | −H | −H | −H | −H | −H | B-ring | −H | −H | −OH | −H | −H | √ | 1 | 1 | 0 | 0 |
| 6 | baicalein | Sigma | −H | −H | −H | −H | −H | B-ring | −H | −OH | −OH | −OH | −H | √ | 3 | 3 | 0 | 0 |
| 7 | 7-hydroxyflavone | Sigma | −H | −H | −H | −H | −H | B-ring | −H | −H | −H | −OH | −H | √ | 1 | 1 | 0 | 0 |
| isoflavones: | ||||||||||||||||||
| 8 | genistein | Sigma | −H | −H | −OH | −H | −H | −H | B−ring | −OH | −H | −OH | −H | √ | 3 | 2 | 1 | 0 |
| 9 | daidzein | Sigma | −H | −H | −OH | −H | −H | −H | B−ring | −H | −H | −OH | −H | √ | 2 | 1 | 1 | 0 |
| flavanones: | ||||||||||||||||||
| 10 | hesperetin | Sigma | −H | −OH | −OCH3 | −H | −H | B-ring | −H | −OH | −H | −OH | −H | − | 3 | 2 | 1 | 0 |
| 11 | flavanone | Sigma | −H | −H | −H | −H | −H | B-ring | −H | −H | −H | −H | −H | − | 0 | 0 | 0 | 0 |
| 12 | naringenin | Sigma | −H | −H | −OH | −H | −H | B-ring | −H | −OH | −H | −OH | −H | − | 3 | 2 | 1 | 0 |
| 13 | 2′-hydroxyflavanone | Sigma | −OH | −H | −H | −H | −H | B-ring | −H | −H | −H | −H | −H | − | 1 | 0 | 1 | 0 |
| 14 | 4′-hydroxyflavanone | Sigma | −H | −H | −OH | −H | −H | B-ring | −H | −H | −H | −H | −H | − | 1 | 0 | 1 | 0 |
| flavonols: | ||||||||||||||||||
| 15 | galangin | Sigma | −H | −H | −H | −H | −H | B-ring | −OH | −OH | −H | −OH | −H | √ | 3 | 2 | 0 | 1 |
| 16 | morin | Sigma | −OH | −H | −OH | −H | −H | B-ring | −OH | −OH | −H | −OH | −H | √ | 5 | 2 | 2 | 1 |
| 17 | myricetin | Sigma | −H | −OH | −OH | −OH | −H | B-ring | −OH | −OH | −H | −OH | −H | √ | 6 | 2 | 3 | 1 |
| 18 | fisetin | Sigma | −H | −OH | −OH | −H | −H | B-ring | −OH | −H | −H | −OH | −H | √ | 4 | 1 | 2 | 1 |
| 19 | geraldol | Sigma | −H | −OCH3 | −OH | −H | −H | B-ring | −OH | −H | −H | −OH | −H | √ | 3 | 1 | 1 | 1 |
| 20 | quercetin | Sigma | −H | −OH | −OH | −H | −H | B-ring | −OH | −OH | −H | −OH | −H | √ | 5 | 2 | 2 | 1 |
| 21 | gossypin | Sigma | −H | −OH | −OH | −H | −H | B-ring | −OH | −OH | −H | −OH | O-glucoside | √ | 5 | 2 | 2 | 1 |
| 22 | 3,6-dihydroxyflavone | Sigma | −H | −H | −H | −H | −H | B-ring | −OH | −H | −OH | −H | −H | √ | 2 | 1 | 0 | 1 |
| 23 | 3,7-dihydroxyflavone | Sigma | −H | −H | −H | −H | −H | B-ring | −OH | −H | −H | −OH | −H | √ | 2 | 1 | 0 | 1 |
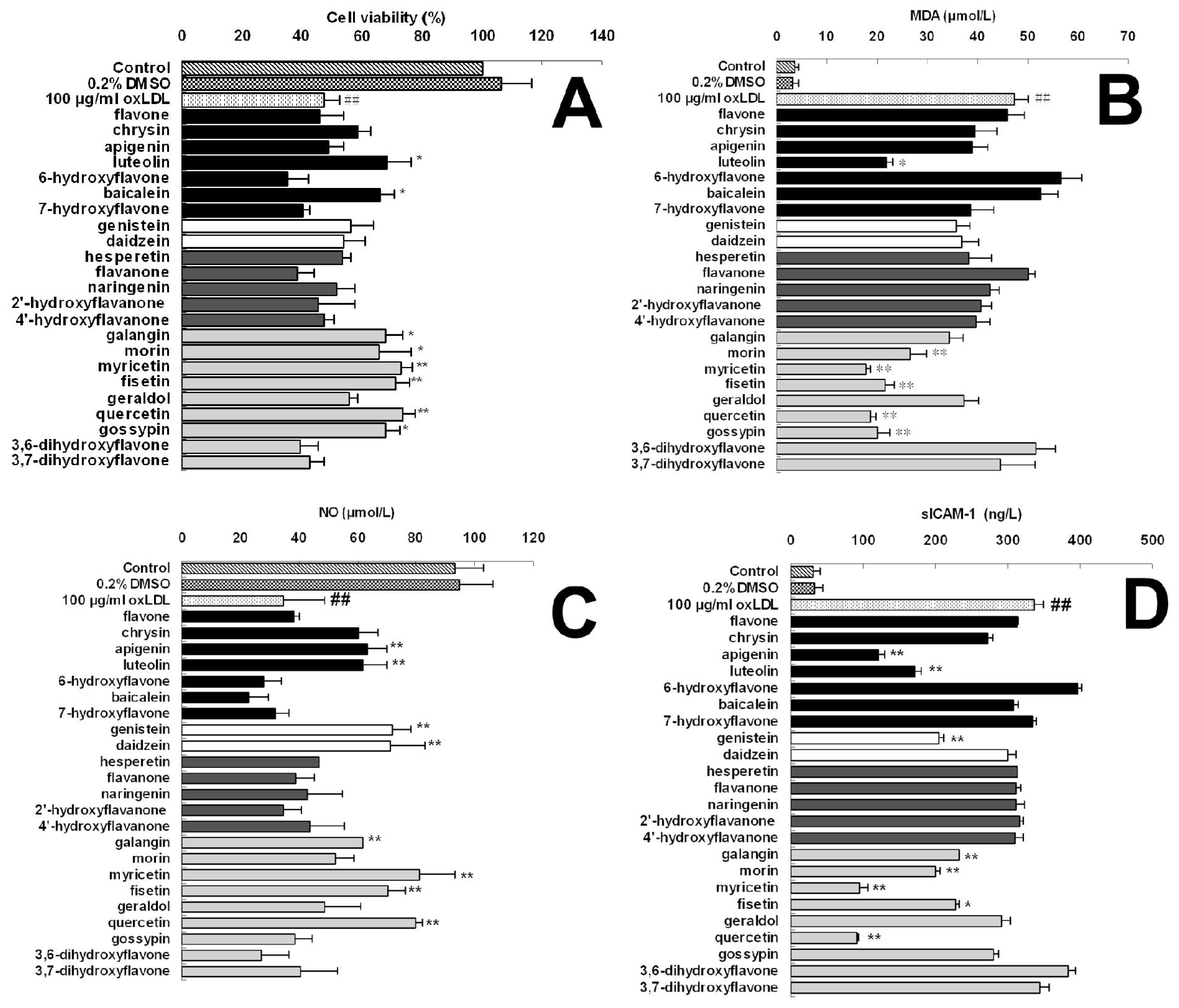
| Groups | Cell viability (% of control)
| |||||
|---|---|---|---|---|---|---|
| 5 μM | 10 μM | 20 μM | 40 μM | 80 μM | ||
| Control | 100.00 | |||||
| 0.2% DMSO | 106.36 ± 10.32 | |||||
| 100 μg/mL oxLDL | 47.11± 5.19 ## | |||||
| flavones: | ||||||
| flavone | 47.76 ± 4.77 | 48.27 ± 5.91 | 45.22 ± 5.50 | 45.88 ± 7.92 | 40.65 ± 6.53 | |
| chrysin | 48.08 ± 3.83 | 47.29 ± 8.56 | 53.98± 5.64 | 58.73 ± 4.16 | 45.12 ± 2.94 | |
| apigenin | 53.86 ± 2.89 | 50.67 ± 6.25 | 54.64± 8.36 | 48.73 ± 5.08 | 42.79 ± 5.35 | |
| luteolin | 55.96 ± 1.99 | 59.48 ± 6.32 | 61.04 ± 6.74 * | 68.27 ± 8.22 * | 76.44 ± 4.46 ** | |
| 6-hydroxyflavone | 46.99 ± 7.92 | 43.90 ± 4.19 | 40.60 ± 6.04 | 35.34 ± 6.59 | 29.51 ± 4.84 | |
| baicalein | 48.62 ± 2.28 | 50.25 ± 6.87 | 57.17± 6.38 | 65.81 ± 4.90 * | 57.76 ± 5.51 | |
| 7-hydroxyflavone | 50.34 ± 3.31 | 49.12 ± 8.92 | 44.46 ± 9.43 | 40.47 ± 2.32 | 33.23 ± 3.36 | |
| isoflavones: | ||||||
| genistein | 51.15 ± 2.72 | 46.53 ± 6.70 | 52.81± 4.68 | 56.03 ± 7.71 | 43.46 ± 4.65 | |
| daidzein | 47.88 ± 4.22 | 47.94 ± 8.29 | 46.85± 11.10 | 53.94 ± 6.72 | 35.38 ± 9.70 | |
| flavanones: | ||||||
| hesperetin | 49.72 ± 3.25 | 47.45 ± 3.03 | 57.32± 4.29 | 53.49 ± 2.89 | 45.89 ± 6.56 | |
| flavanone | 44.69 ± 5.27 | 48.48 ± 6.40 | 42.94± 4.56 | 38.20 ± 5.77 | 34.37 ± 3.31 | |
| naringenin | 49.47 ± 3.18 | 49.41 ± 7.63 | 54.10± 4.90 | 51.52 ± 6.26 | 40.41 ± 6.79 | |
| 2′-hydroxyflavanone | 49.21 ± 3.02 | 51.41 ± 6.40 | 44.29± 3.01 | 45.51 ± 11.98 | 41.70 ± 6.59 | |
| 4′-hydroxyflavanone | 51.64 ± 6.31 | 54.01 ± 5.54 | 49.50± 10.23 | 47.08 ± 3.38 | 43.23 ± 3.81 | |
| flavonols: | ||||||
| galangin | 52.15 ± 3.57 | 56.59 ± 3.56 | 62.29± 4.35 * | 67.78 ± 5.69 * | 68.56 ± 2.24 * | |
| morin | 53.24 ± 3.51 | 54.00 ± 7.73 | 59.72± 3.85 | 65.37 ± 11.06 * | 71.60 ± 4.45 ** | |
| myricetin | 53.07 ± 2.00 | 60.19 ± 5.12 | 68.41± 4.60 * | 73.26 ± 3.61 ** | 82.58 ± 3.67 ** | |
| fisetin | 51.66 ± 3.97 | 58.92 ± 3.44 | 64.49± 8.1 * | 71.22 ± 4.73 ** | 79.40 ± 5.55 ** | |
| geraldol | 49.35 ± 3.52 | 50.63 ± 7.49 | 45.57± 2.57 | 55.81 ± 2.60 | 40.24 ± 3.75 | |
| quercetin | 50.75 ± 2.13 | 59.49 ± 7.71 | 66.32± 6.4 * | 73.63 ± 3.87 ** | 81.53 ± 6.18 ** | |
| gossypin | 48.99 ± 3.27 | 58.15 ± 3.34 | 65.31± 6.5 * | 67.87 ± 4.50 * | 78.78 ± 4.42 ** | |
| 3,6- dihydroxyflavone | 50.66 ± 6.11 | 50.11 ± 5.70 | 46.15± 7.99 | 39.38 ± 6.14 | 34.07 ± 4.31 | |
| 3,7- dihydroxyflavone | 45.74 ± 4.81 | 50.57 ± 7.01 | 42.87± 4.28 | 42.42 ± 4.67 | 39.12 ± 3.03 | |
| Paired comparison
| Cell viability | MDA level | NO level | sICAM-1level | |||
|---|---|---|---|---|---|---|---|
| A | B | ||||||
| (a) | B-ring | ||||||
| flavanone |  | 2′-hydroxyflavanone (2′-hydroxyl) | ↑ | ↓ | ↓ | ↑ | |
| morin(2′,4′-dihydroxyl) |  | quercetin(3′,4′-dihydroxy) | ↑ * | ↓ * | ↑ * | ↓ * | |
| geraldol (3′-methoxy, 4′-hydroxyl) |  | fistin (3′,4′-dihydroxyl) | ↑ ** | ↓ * | ↑ ** | ↓ | |
| apigenin (4′-hydroxyl) |  | luteolin (3′,4′-dihydroxyl) | ↑ ** | ↓ * | ↓ | ↑ | |
| 3,7-dihydroxyflavone |  | fistin (3′,4′-dihydroxyl) | ↑ ** | ↓ ** | ↑ ** | ↓ ** | |
| flavanone |  | 4′-hydroxyflavanone (4′-hydroxyl) | ↑ | ↓ * | ↑ | ↓ | |
| chrysin |  | apigenin (4′-hydroxyl) | ↓ | ↓ * | ↑ | ↓ * | |
| chrysin |  | luteolin (3′,4′-dihydroxyl) | ↑ * | ↓ * | ↑ | ↓ * | |
| galangin |  | morin (2′,4′-dihydroxyl) | ↓ | ↓ | ↓ | ↓ | |
| galangin |  | myricetin (3′,4′,5′-trihydroxyl) | ↑ * | ↓ ** | ↑ * | ↓ ** | |
| galangin |  | quercetin (3′,4′-dihydroxyl) | ↑ * | ↓ ** | ↑ * | ↓ * | |
| morin (2′,4′-dihydroxyl) |  | myricetin (3′,4′,5′-trihydroxyl) | ↑ * | ↓ * | ↑ * | ↓ ** | |
| morin (2′,4′-dihydroxyl) |  | quercetin (3′,4′-dihydroxyl) | ↑ * | ↓ * | ↑ ** | ↓ ** | |
| 3,7-dihydroxyflavone |  | fistin (3′,4′-dihydroxyl) | ↑ ** | ↓ ** | ↑ ** | ↓ * | |
| 3,7-dihydroxyflavone |  | geraldol (3′-methoxyl, 4′-hydroxyl) | ↑ | ↓* | ↑ | ↓ | |
| chrysin (2′,4′ - dihydroxyl) |  | luteolin (3′,4′-dihydroxyl) | ↑ * | ↓ ** | ↑ | ↓ * | |
| quercetin (3′,4′-dihydroxyl) |  | myricetin (3′,4′,5′-trihydroxyl) | ↓ | ↓ | ↑ | ↑ | |
| (b) | A-ring | ||||||
| flavone |  | chrysin (5,7-dihydroxyl) | ↑ | ↓ | ↑ ** | ↓ | |
| flavone |  | 6-hydroxyflavone (6-hydroxyl) | ↓ * | ↑ * | ↓ * | ↑ * | |
| flavone |  | baicalein (5,6,7-trihydroxyl) | ↑ * | ↑ | ↓ * | ↓ | |
| flavone |  | 7-hydroxyflavone (7-hydroxyl) | ↓ | ↓ | ↓ | ↑ | |
| chrysin (5,7-dihydroxyl) |  | baicalein (5,6,7-trihydroxyl) | ↑ * | ↑ * | ↓ ** | ↑ | |
| chrysin (5,7-dihydroxyl) |  | 7-hydroxyflavone (7-hydroxyl) | ↓ * | ↓ | ↓ ** | ↑ * | |
| chrysin (5,7-dihydroxyl) |  | 6-hydroxyflavone (6-hydroxyl) | ↓ ** | ↑ ** | ↓ ** | ↑ ** | |
| genistein |  | daidzein | ↓ | ↑ | ↓ | ↑ * | |
| fistin (7-hydroxyl) |  | quercetin(5,7-dihydroxyl) | ↑ | ↓ | ↑ | ↓ ** | |
| fistin (7-hydroxyl) |  | gossypin (5,7-dihydroxyl, 8-O-glucoside) | ↓ | ↓ | ↓ ** | ↑ | |
| 6-hydroxyflavone (6- hydroxyl) |  | baicalein (5,6,7-trihydroxyl) | ↑ ** | ↓ | ↓ | ↓* | |
| 6-hydroxyflavone (6- hydroxyl) |  | 7-hydroxyflavone (7-hydroxyl) | ↑ | ↓ * | ↓ | ↓ * | |
| quercetin (5,7- dihydroxyl) |  | gossypin (5,7-dihydroxyl, 8-O-glucoside) | ↓ | ↑ | ↓ ** | ↑ ** | |
| 3,6-dihydroxyflavone (6-hydroxyl) |  | 3,7-dihydroxyflavone (7-hydroxyl) | ↑ | ↓ | ↑ * | ↓ * | |
| (c) | C-ring | ||||||
| chrysin |  | galangin (3-hydroxyl) | ↑ * | ↓ | ↑ | ↓ * | |
| luteolin |  | quercetin(3-hydroxyl) | ↑ | ↓ * | ↑ * | ↓ ** | |
| 7-hydroxyflavone (7- hydroxyl) |  | 3,7-dihydroxyflavone (3,7-dihydroxyl) | ↑ | ↑ | ↑ | ↑ | |
| (d) | 2,3-double bond | ||||||
| flavanone |  | flavone (2,3-double bond) | ↑ * | ↓ | ↓ | ↓ * | |
| naringenin |  | apigenin (2,3-double bond) | ↓ | ↓ * | ↑ * | ↓ ** | |
| (e) | Substituted position of B-ring | ||||||
| apigenin (C2) |  | genistein (C3) | ↑ | ↓ | ↑ * | ↑ * | |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yi, L.; Jin, X.; Chen, C.-Y.; Fu, Y.-J.; Zhang, T.; Chang, H.; Zhou, Y.; Zhu, J.-D.; Zhang, Q.-Y.; Mi, M.-T. Chemical Structures of 4-Oxo-Flavonoids in Relation to Inhibition of Oxidized Low-Density Lipoprotein (LDL)-Induced Vascular Endothelial Dysfunction. Int. J. Mol. Sci. 2011, 12, 5471-5489. https://doi.org/10.3390/ijms12095471
Yi L, Jin X, Chen C-Y, Fu Y-J, Zhang T, Chang H, Zhou Y, Zhu J-D, Zhang Q-Y, Mi M-T. Chemical Structures of 4-Oxo-Flavonoids in Relation to Inhibition of Oxidized Low-Density Lipoprotein (LDL)-Induced Vascular Endothelial Dysfunction. International Journal of Molecular Sciences. 2011; 12(9):5471-5489. https://doi.org/10.3390/ijms12095471
Chicago/Turabian StyleYi, Long, Xin Jin, Chun-Ye Chen, Yu-Jie Fu, Ting Zhang, Hui Chang, Yong Zhou, Jun-Dong Zhu, Qian-Yong Zhang, and Man-Tian Mi. 2011. "Chemical Structures of 4-Oxo-Flavonoids in Relation to Inhibition of Oxidized Low-Density Lipoprotein (LDL)-Induced Vascular Endothelial Dysfunction" International Journal of Molecular Sciences 12, no. 9: 5471-5489. https://doi.org/10.3390/ijms12095471




