Spin Transition Sensors Based on β-Amino-Acid 1,2,4-Triazole Derivative
Abstract
:1. Introduction
2. Results and Discussion
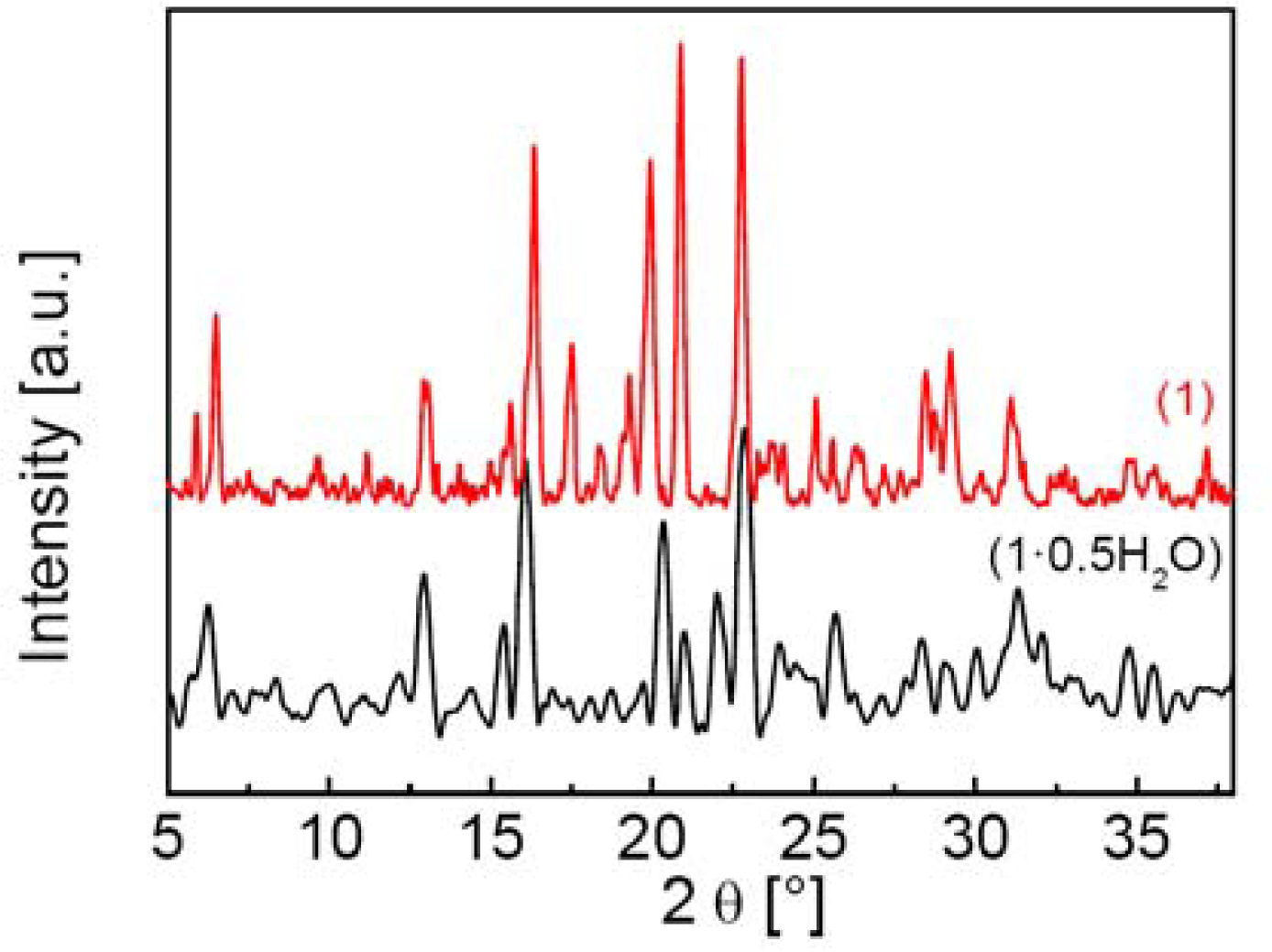
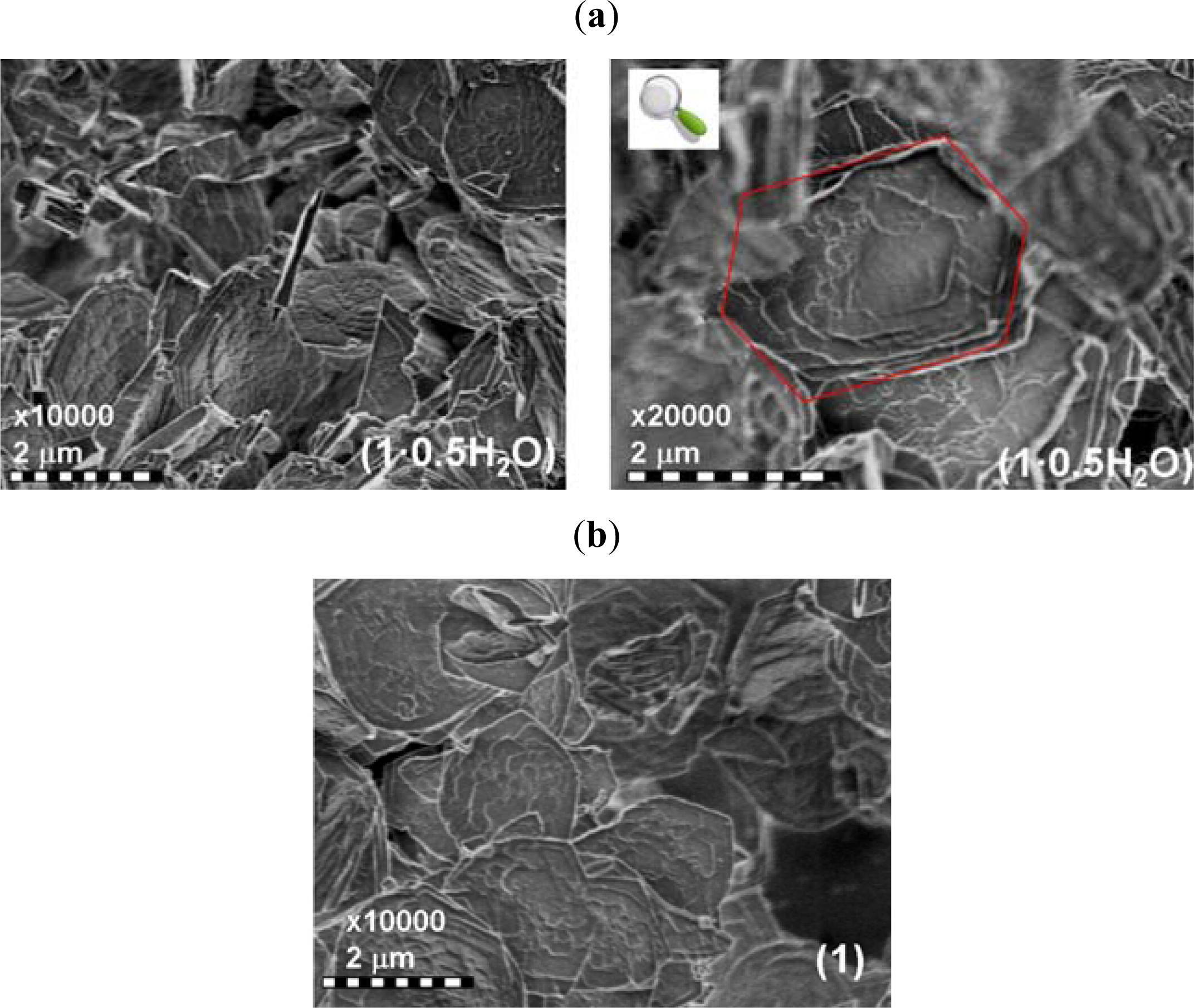
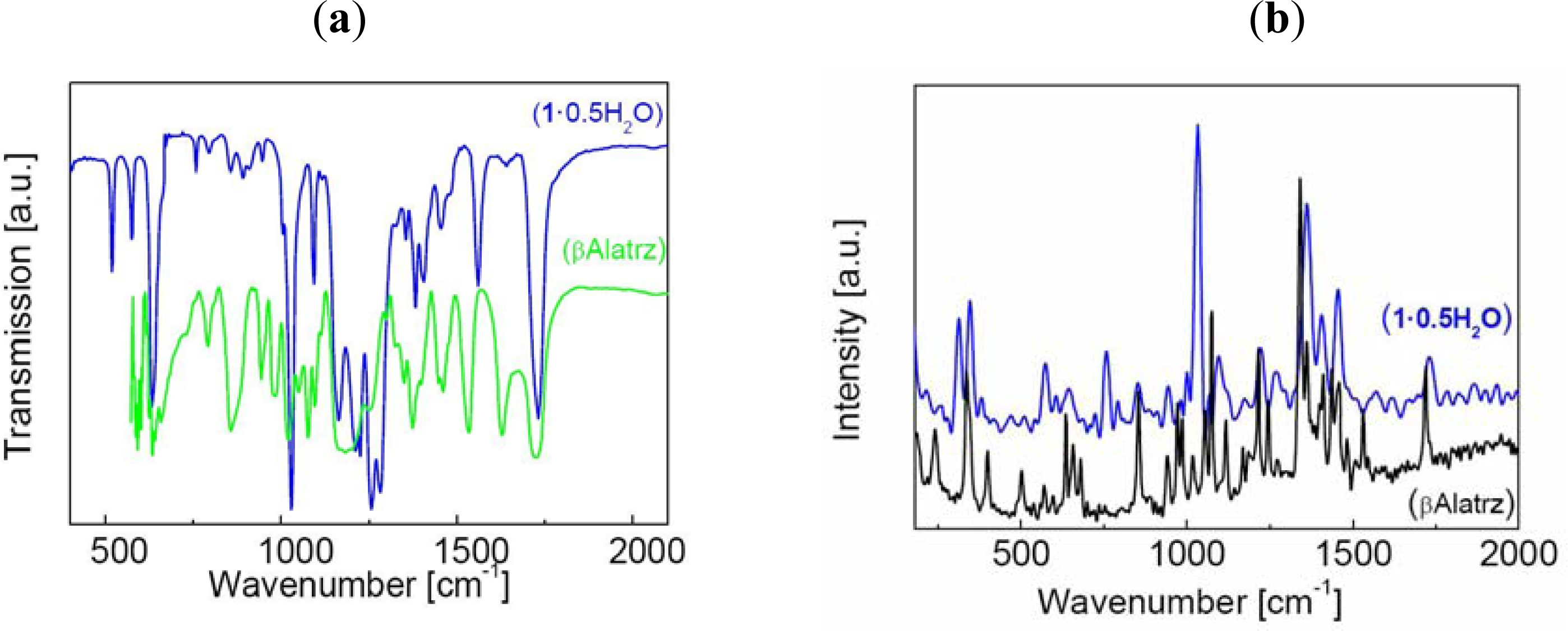
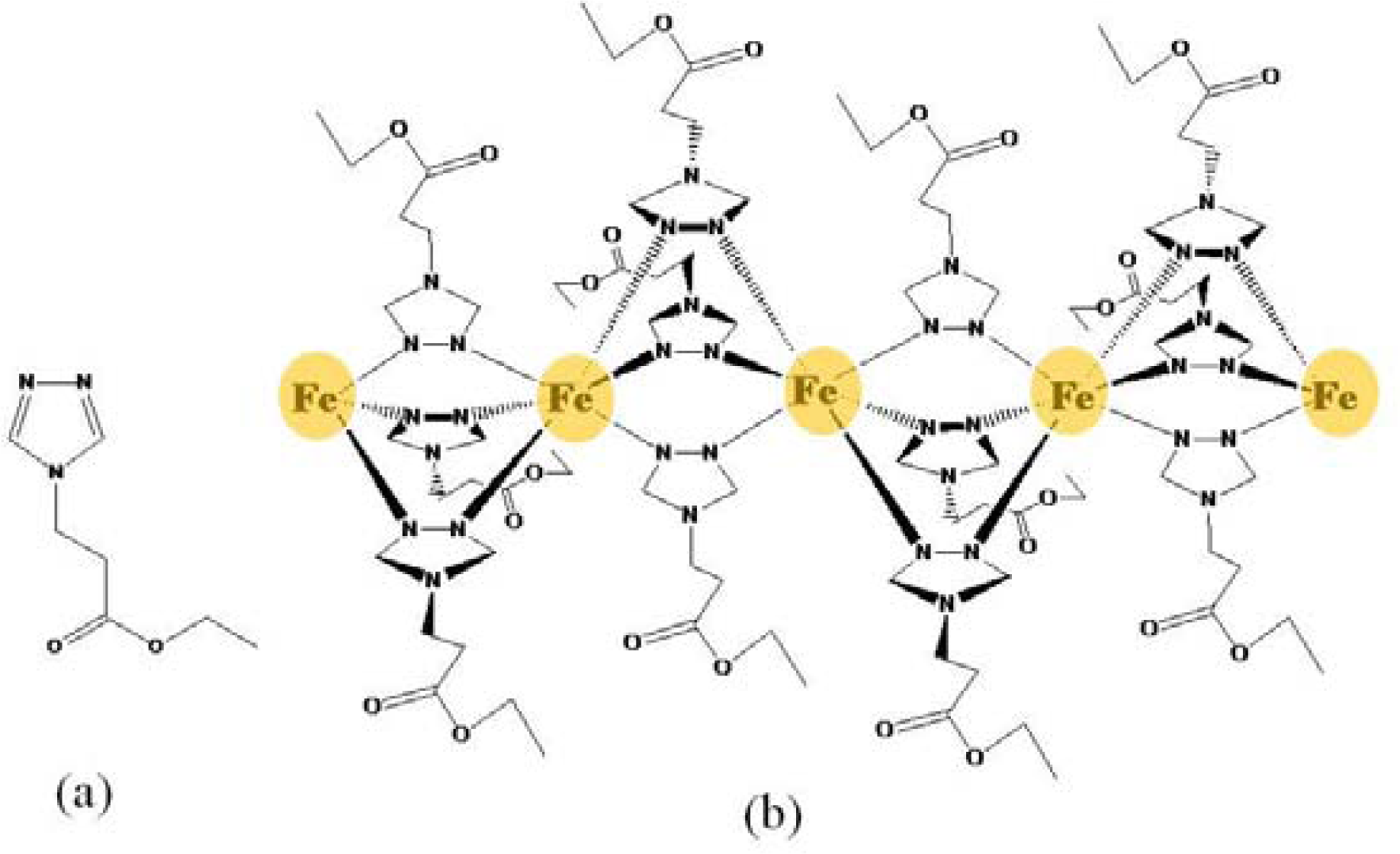
2.1. Preparation and Characterization of 1·0.5H2O and 1
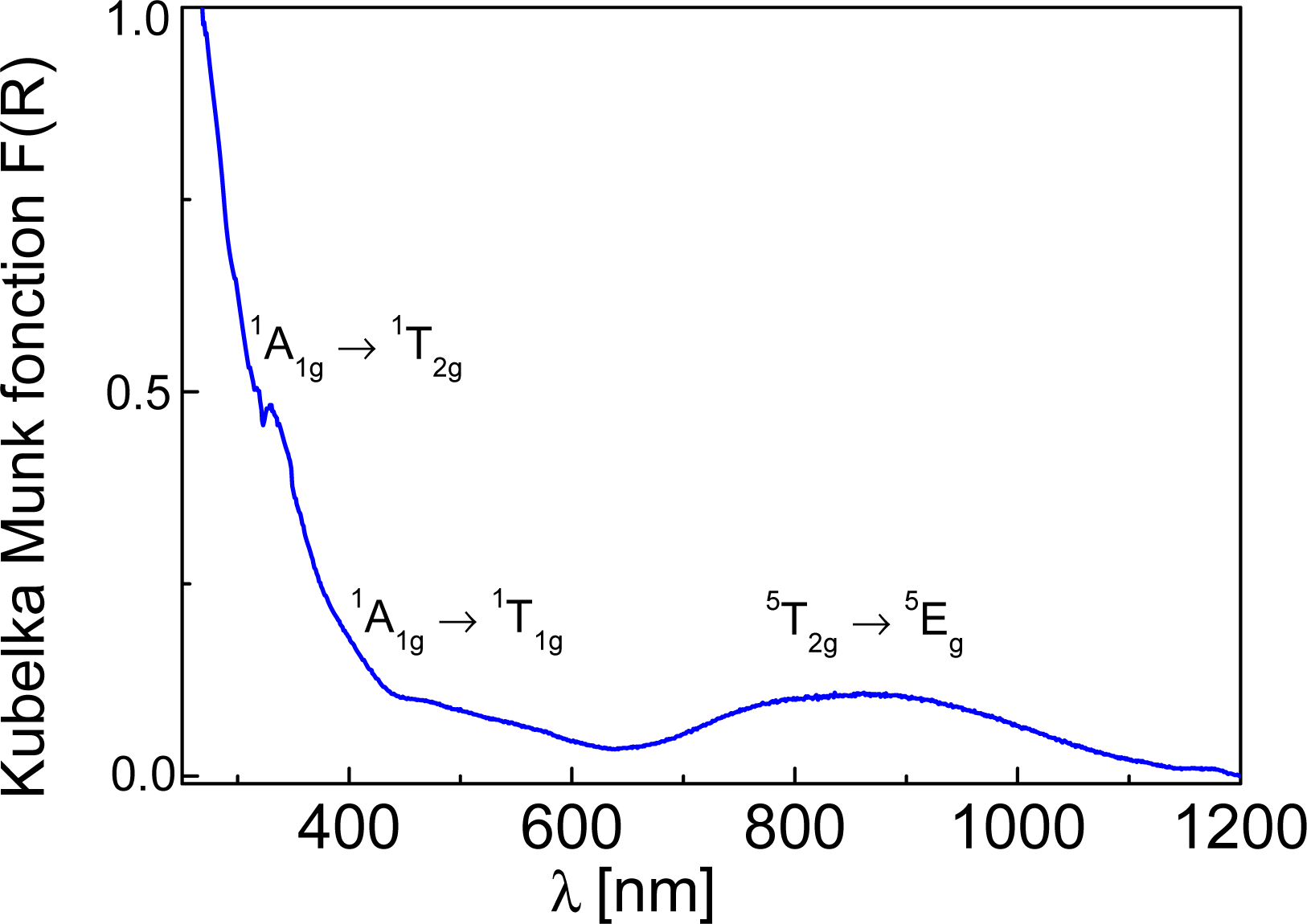
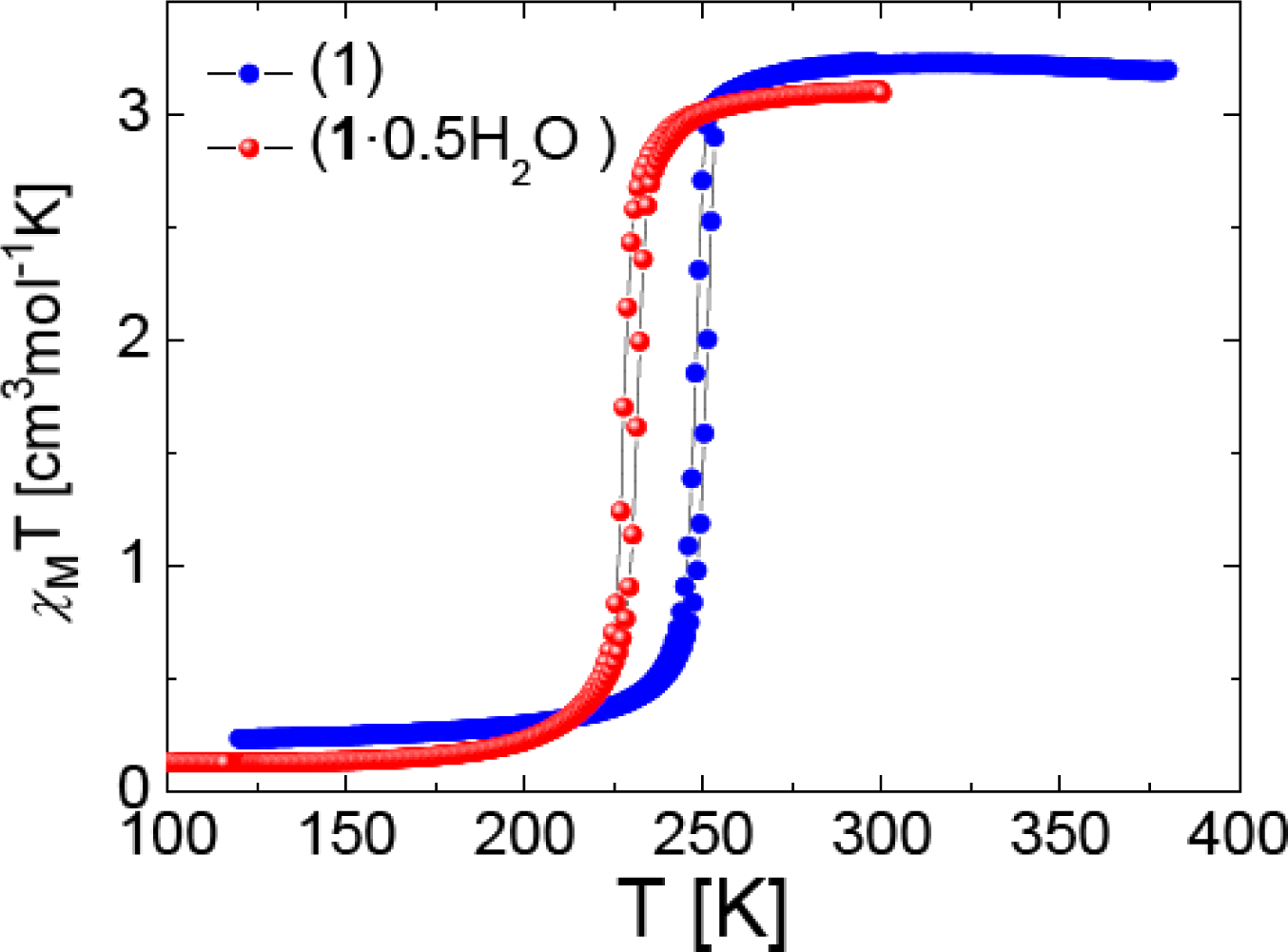
2.2. SQUID Magnetometry
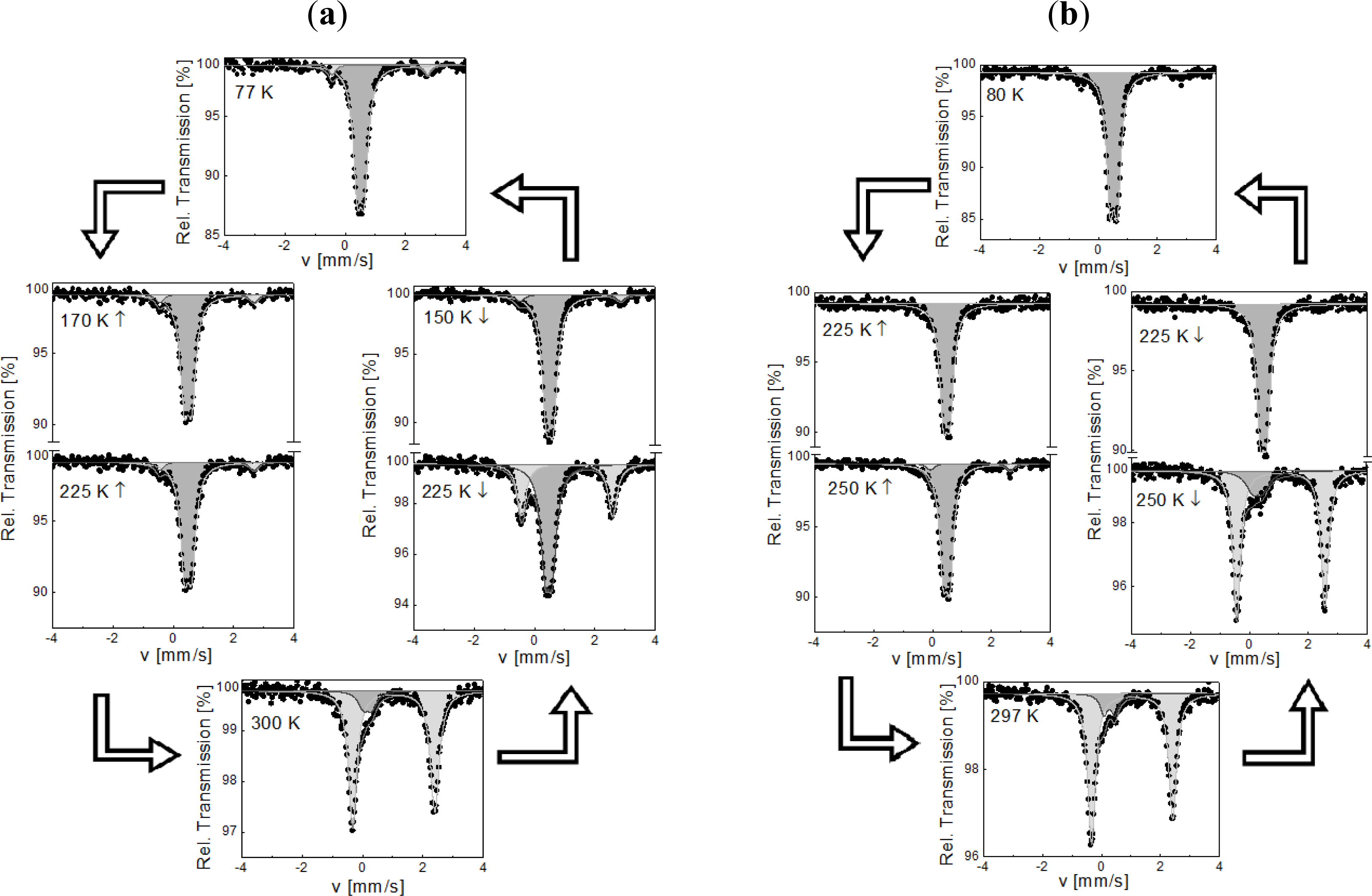
2.3. 57Fe Mössbauer Spectroscopy
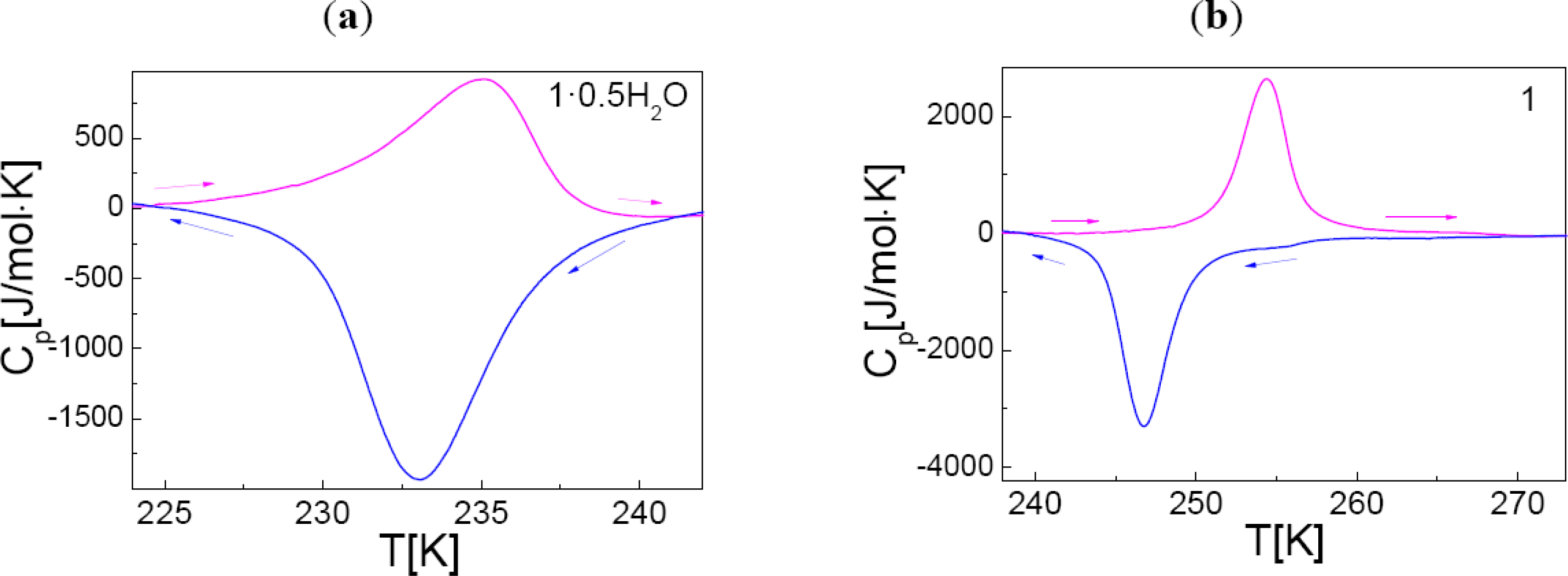
2.4. Differential Scanning Calorimetry
3. Experimental Section
3.1. Chemicals
3.2.1. Ethyl 4H-1,2,4-Triazol-4-yl-Propionate (βAlatrz)
3.2.2. [Fe(βAlatrz)3](CF3SO3)2·0.5H2O (1·0.5H2O)
3.3. Physical Measurements
4. Concluding Remarks
Acknowledgments
References
- Gütlich, P; Garcia, Y; Goodwin, HA. Spin crossover phenomena in Fe(II) complexes. Chem. Soc. Rev 2000, 29, 419–427. [Google Scholar]
- Bousseksou, A; Molnár, G; Salmon, L; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev 2011, 40, 3313–3118. [Google Scholar]
- Gütlich, P; Garcia, Y; Spiering, H. Magnetism: From Molecules to Materials, 1st ed; Miller, JS, Drillon, M, Eds.; Wiley-VCH: Hoboken, NJ, USA, 2003; Volume IV. [Google Scholar]
- Weber, B; Bauer, W; Obel, J. An iron (II) spin-crossover complex with a 70 K wide thermal hysteresis loop. Angew. Chem. Int. Ed 2008, 47, 10098–10101. [Google Scholar]
- Garcia, Y; Ksenofontov, V; Gütlich, P. Spin transition molecular materials: New sensors. Hyperfine Interact 2002, 139–140, 543–551. [Google Scholar]
- Kahn, O; Krober, J; Jay, C. Spin Transition molecular materials for displays and data recording. Adv. Mater 1992, 4, 718–728. [Google Scholar]
- Kahn, O; Jay-Martinez, C. Spin-transition polymers: From molecular materials toward memory devices. Science 1998, 279, 44–48. [Google Scholar]
- Bodenthin, Y; Pietsch, U; Möhwald, H; Kurth, DG. Inducing spin crossover in Metallo-supramolecular polyelectrolytes through an amphiphilic phase transition. J. Am. Chem. Soc 2005, 127, 3110–3115. [Google Scholar]
- Bodenthin, Y; Pietsch, U; Grenzer, J; Geue, T; Möhwald, H; Kurth, DG. Structure and temperature behavior of metallo-supramolecular assemblies. J. Phys. Chem. B 2005, 109, 12795–12799. [Google Scholar]
- Bodenthin, Y; Schwarz, G; Tomkowicz, Z; Geue, T; Haase, W; Pietsch, U; Kurth, DG. Liquid crystalline phase transition induces spin crossover in a polyelectrolyte amphiphile complex. J. Am. Chem. Soc 2009, 131, 2934–2941. [Google Scholar]
- Schwarz, G; Bodenthin, Y; Tomkowicz, Z; Haase, W; Geue, T; Kohlbrecher, J; Pietsch, U; Kurth, DG. Tuning the structure and the magnetic properties of metallo-supramolecular polyelectrolyte-amphiphile complexes. J. Am. Chem. Soc 2011, 133, 547–558. [Google Scholar]
- Naik, AD; Marchand-Brynaert, J; Garcia, Y. A simplified approach to N- and N,N’-linked 1,2,4-triazoles by transamination. Synthesis 2008, 1, 149–154. [Google Scholar]
- Dîrtu, MM; Naik, AD; Marchand-Brynaert, J; Garcia, Y. Room temperature hysteretic spin transition in 1D iron(II) coordination polymers. J. Phys. Conf. Ser 2010, 217, 012085. [Google Scholar]
- Naik, AD; Dîrtu, MM; Léonard, A; Tinant, B; Marchand-Brynaert, J; Su, BL; Garcia, Y. Engineering three-dimensional chains of porous nanoballs from a 1,2,4-triazole-carboxylate supramolecular synthon. Cryst. Growth Des 2010, 10, 1798–1807. [Google Scholar]
- Naik, AD; Beck, J; Dîrtu, MM; Bebrone, C; Tinant, B; Robeyns, K; Marchand-Brynaert, J; Garcia, Y. Zinc complexes with 1,2,4-triazole functionalized amino acid derivatives: Synthesis, structure and beta-lactamase assay. Inorg. Chim. Acta 2011, 368, 21–28. [Google Scholar]
- Dîrtu, MM; Neuhausen, C; Naik, AD; Léonard, A; Robert, F; Marchand-Brynaert, J; Su, BL; Garcia, Y. Superlative scaffold of 1,2,4-Triazole derivative of glycine steering linear chain to a chiral helicate. Cryst. Growth Des 2011, 11, 1375–1384. [Google Scholar]
- Hagen, KS. Iron(II) Triflate salts as convenient substitutes for perchlorate salts: Crystal structures of [Fe(H2O)6](CF3SO3)2 and Fe(MeCN)4(CF3SO3)2. Inorg. Chem 2000, 39, 5867–5869. [Google Scholar]
- Chwaleba, D; Ilczyszyn, MM; Ilczyszyn, M; Ciunik, Z. Glycine-methanesulfonic acid (1:1) and glycine-p-toluenesulfonic acid (1:1) crystals: Comparison of structures, hydrogen bonds, and vibrations. J. Mol. Struct 2007, 831, 119–134. [Google Scholar]
- Haasnoot, JG; Vos, G; Groeneveld, WL. 1,2,4-Triazole complexes, III. Complexes of transition metal(II) nitrates and fluoroborates. Z. Naturforsch. B 1977, 32, 1421–1430. [Google Scholar]
- Adriaanse, JH; Askes, SHC; van Bree, Y; van Oudheusden, S; van den Bos, ED; Gunay, E; Mutikainen, I; Turpeinen, U; van Albada, GA; Haasnoot, JG; et al. Coordination chemistry of 5,6,7-trimethyl-[1,2,4]triazolo[1,5-a]pyrimidine with first-row transition-metal salts: Synthesis, spectroscopy and single-crystal structures, with counter-anion dependence of the structures. Polyhedron 2009, 28, 3143–3149. [Google Scholar]
- Sinditskii, VP; Fogelzang, AE; Dutov, MD; Sokol, VI; Serushkin, VV; Svetlov, BS; Poraikoshits, MA. Structure of complex-compounds of metal chlorides, sulfates, nitrates and perchlorates with carbohydrazine. Zh. Neorg. Khim 1987, 32, 1944–1949. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, 5th ed; Wiley-Interscience: New York, NY, USA, 1997. [Google Scholar]
- Dîrtu, MM; Rotaru, A; Gillard, D; Linares, J; Codjovi, E; Tinant, B; Garcia, Y. Prediction of the spin transition temperature in FeII one-dimensional coordination polymers: An anion based database. Inorg. Chem 2009, 48, 7838–7852. [Google Scholar]
- Dîrtu, MM; Neuhausen, C; Naik, AD; Rotaru, A; Spinu, L; Garcia, Y. Insights into the origin of cooperative effects in the spin transition of [Fe(NH2trz)3](NO3)2: The role of supramolecular interactions evidenced in the crystal structure of [Cu(NH2trz)3](NO3)2·H2O. Inorg. Chem 2010, 49, 5727–5736. [Google Scholar]
- Gütlich, P; Hauser, A; Spiering, H. Thermal and optical switching of iron(II) complexes. Angew. Chem., Int. Ed 1994, 33, 2024–2054. [Google Scholar]
- Gütlich, P; Jung, J; Goodwin, HA. Molecular Magnetism: From Molecular Assemblies to the Devices; Coronado, E, Delhaès, P, Gatteschi, D, Miller, JS, Eds.; Springer: Berlin, Germany, 1996; p. 327. [Google Scholar]
- Garcia, Y; van Koningsbruggen, PJ; Lapouyade, R; Fournès, L; Rabardel, L; Kahn, O; Ksenofontov, V; Levchenko, G; Gütlich, P. Influences of temperature, pressure, and lattice solvents on the spin transition regime of the polymeric compound [Fe(hyetrz)3]A2·3H2O (hyetrz = 4-(2′-hydroxyethyl)-1,2,4-triazole and A− = 3-nitrophenylsulfonate). Chem. Mater 1998, 10, 2426–2433. [Google Scholar]
- Garcia, Y; Niel, V; Muñoz, MC; Real, JA. Spin crossover in 1D, 2D and 3D polymeric Fe(II) networks; Spin crossover in transition metal compounds. Top. Curr. Chem 2004, 233, 229–257. [Google Scholar]
- Garcia, Y; van Koningsbruggen, PJ; Lapouyade, R; Rabardel, L; Kahn, O; Wieczorek, M; Bronisz, R; Ciunik, Z; Rudolf, MF. Synthesis and spin-crossover characteristics of polynuclear 4-(2′-hydroxyethyl)-1,2,4-triazole Fe(II) molecular materials. C R Acad Sci 1998, IIc, 523–532. [Google Scholar]
- Dîrtu, MM; Garcia, Y; Nica, M; Rotaru, A; Linares, J; Varret, F. Iron(II) spin transition 1,2,4-triazole chain compounds with novel inorganic fluorinated counteranions. Polyhedron 2007, 26, 2259–2263. [Google Scholar]
- Garcia, Y; Ksenofontov, V; Mentior, S; Dîrtu, MM; Gieck, C; Bhatthacharjee, A; Gütlich, P. Rapid cooling experiments and use of an anionic nuclear probe to sense the spin transition of the 1D coordination polymers [Fe(NH2trz)3]SnF6·nH2O (NH2trz = 4-amino-1,2,4-triazole). Chem. Eur. J 2008, 14, 3745–3758. [Google Scholar]
- Recoil, Mössbauer Spectral Analysis Software for Windows, Version 1.0; Department of Physics, University of Ottawa: Ottawa, ON, Canada, 1998.
- Breuer, KH; Eysel, W. The calorimetric calibration of differential scanning calorimetry cells. Thermochim. Acta 1982, 57, 317–329. [Google Scholar]
- Rotaru, A; Dîrtu, MM; Enachescu, C; Tanasa, R; Linares, J; Stancu, A; Garcia, Y. Calorimetric measurements of diluted spin crossover complexes [FexM1−x(btr)2(NCS)2]·H2O with MII = Zn and Ni. Polyhedron 2009, 28, 2531–2536. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dîrtu, M.M.; Schmit, F.; Naik, A.D.; Rotaru, A.; Marchand-Brynaert, J.; Garcia, Y. Spin Transition Sensors Based on β-Amino-Acid 1,2,4-Triazole Derivative. Int. J. Mol. Sci. 2011, 12, 5339-5351. https://doi.org/10.3390/ijms12085339
Dîrtu MM, Schmit F, Naik AD, Rotaru A, Marchand-Brynaert J, Garcia Y. Spin Transition Sensors Based on β-Amino-Acid 1,2,4-Triazole Derivative. International Journal of Molecular Sciences. 2011; 12(8):5339-5351. https://doi.org/10.3390/ijms12085339
Chicago/Turabian StyleDîrtu, Marinela M., France Schmit, Anil D. Naik, Aurelian Rotaru, J. Marchand-Brynaert, and Yann Garcia. 2011. "Spin Transition Sensors Based on β-Amino-Acid 1,2,4-Triazole Derivative" International Journal of Molecular Sciences 12, no. 8: 5339-5351. https://doi.org/10.3390/ijms12085339





