Abstract
Adaptation of microorganisms to low temperatures remains to be fully elucidated. It has been previously reported that peptidyl prolyl cis-trans isomerases (PPIases) are involved in cold adaptation of various microorganisms whether they are hyperthermophiles, mesophiles or phsycrophiles. The rate of cis-trans isomerization at low temperatures is much slower than that at higher temperatures and may cause problems in protein folding. However, the mechanisms by which PPIases are involved in cold adaptation remain unclear. Here we used FK506-binding protein 22, a cold shock protein from the psychrophilic bacterium Shewanella sp. SIB1 (SIB1 FKBP22) as a model protein to decipher the involvement of PPIases in cold adaptation. SIB1 FKBP22 is homodimer that assumes a V-shaped structure based on a tertiary model. Each monomer consists of an N-domain responsible for dimerization and a C-catalytic domain. SIB1 FKBP22 is a typical cold-adapted enzyme as indicated by the increase of catalytic efficiency at low temperatures, the downward shift in optimal temperature of activity and the reduction in the conformational stability. SIB1 FKBP22 is considered as foldase and chaperone based on its ability to catalyze refolding of a cis-proline containing protein and bind to a folding intermediate protein, respectively. The foldase and chaperone activites of SIB1 FKBP22 are thought to be important for cold adaptation of Shewanella sp. SIB1. These activities are also employed by other PPIases for being involved in cold adaptation of various microorganisms. Despite other biological roles of PPIases, we proposed that foldase and chaperone activities of PPIases are the main requirement for overcoming the cold-stress problem in microorganisms due to folding of proteins.
1. Introduction
Temperature has been deemed one of the most important factors for growth and modulates selection and distribution of microorganisms throughout the Earth. Microorganisms can be classified according to the range of temperatures in which they can grow: hyperthermophiles (temperature range of 80–115 °C, with optimum growth at >80 °C), thermophiles (40–110 °C, optimum ∼60 °C), mesophiles (10–50 °C, optimum ∼37 °C), psychrotrophs (0–30 °C, optimum ∼22–25 °C) and psychrophiles (0–20 °C, optimum ≤15 °C) [].
Adaptation of microorganisms that live in extreme temperatures (either high or low) is gaining particular interest among scientists, due to the importance of understanding the molecular basis of adaptation and biotechnological applications []. In contrast to thermophilic and/or hyperthermophilic microorganisms, only a few studies have been conducted so far to address the adaptation to low temperatures (cold adaptation). This might be due to commercial reasons in which thermostable microorganisms or enzymes are highly valuable for biotechnological applications [,]. However, some cold-adapted enzymes were reported to be promising and have recently been widely applied in biotechnology [–]. Therefore, understanding the adaptation of microorganisms to low temperature, including the machinery enzymes, is also important for industrial purpose. The information may be also ecologically important since approximately 70% of the earth’s biosphere has a constant temperature of 4–5 °C [,] the reason why it is populated predominantly by psychrophilic and psychrotropic bacteria. It is noted also that some pathogenic microorganisms survive in low temperature, therefore the insight on this mechanism would be useful for controlling pathogens.
Once microorganisms are exposed to low temperatures they may face a series of problems that affect their growth. Primarily, problems encountered at low temperatures are associated with membrane viscosity and permeability, synthesis of macromolecules (e.g., replication, transcription and translation), slower metabolic rates due to lower kinetics of enzymatic reactions, and the ability to sense and transmit temperature signals to the regulatory networks of cells for effective responses [].
Protein folding can be given as an example of problems associated with the synthesis of macromolecules and a slower metabolic rate. Folding of proteins, especially for cis-proline containing proteins, is suggested to be a rate-limiting step for bacterial growth in cold environments []. The conformations of folded proteins are usually compatible with only one isomer of a peptide bond, in which the trans form is preferred to the cis form due to energetic reasons []. However, in the case of peptide bonds preceding proline residues (Xaa-Pro), some of them correctly form cis peptide bonds in folded proteins []. Consequently, interconversion of cis-trans Xaa-Pro peptide bonds take place during folding processes and are termed as peptidyl prolyl cis-trans isomerizations.
However, the kinetics of cis-trans isomerization is intrinsically slow because it involves the rotation about a partial double bond. Isomerization is much slower at low temperatures, as are other chemical reactions, since it is thermodynamically linked []. Therefore, cis-trans isomerization is regarded as a rate-limiting step of protein folding. Peptidyl prolyl cis-trans isomerases (PPIases; EC 5.2.1.8) catalyze cis-trans isomerization of Xaa-Pro peptide bonds (Figure 1) and assist proteins to achieve correct native conformations. Due to this ability, PPIases are considered to be foldases, together with protein disulfide isomerases (PDI) [,]. Currently, at least three structurally unrelated families of PPIase have been indentified: FK506-binding proteins (FKBP), cyclophilins, and parvulins [,].
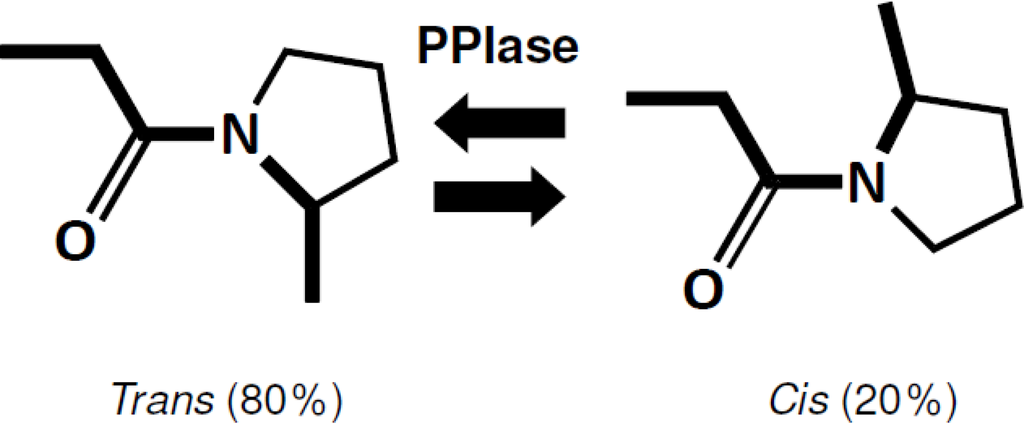
Figure 1.
The cis–trans isomerization reaction catalyzed by PPIases. In a denatured state of proteins, all peptide bonds assume the energetically stable trans conformation. Exceptions include the peptide bond preceding the proline residue (Xaa-Pro). This bond exists in an equilibrium state, in which 80% of this bond assumes a trans conformation and 20% assumes a cis conformation. PPIase does not change this equilibrium state, but equally accelerates both rates from trans to cis and from cis to trans. For proteins containing cis-proline residues in a native state, cis-trans isomerization of the proline residue has been reported to be a rate-limiting step of protein folding.
Psychrophilic and psychrothrophic bacteria produce a cold shock response, which is defined as the sum of cellular reactions to overcome the problems associated with decreases in temperature []. Cold shock responses are indicated by the expression of cold shock proteins (CSPs) or class 1 proteins, which are involved in the adaptation of microorganisms to low-temperature environments. Foldases and chaperones have been classified as CSPs, which are important for overcoming a series of problems in protein folding at low temperatures, including slow cis-trans isomerization [].
Despite PPIases being clearly classified as foldases, multidomain PPIases have also been reported to exhibit chaperone functions as indicated by their ability to bind incompletely folded proteins and prevent aggregation [–]. In addition, Wang and Tsou [] noted that foldases may also act as chaperones and vice versa. Therefore, the dual functions of PPIases would be an interesting topic for in-depth study in relation to their involvement in cold-adaptation.
However, to our knowledge, studies pertaining to this issue are limited. Despite some PPIases from cold-adapted organisms have been reported and linked to cold adaptation [,,], these reports are based on proteomic analysis without further structural and functional studies. Our group has been extensively studying FKBP22 from the psychrotrophic bacterium, Shewanella sp. SIB1 (SIB1 FKBP22). Expression of SIB1 FKBP22 was significantly increased when Shewanella sp. SIB1 was grown at low temperatures, indicating that it is involved in cold-adaptation []. SIB1 FKBP22 is thought to have dual functions, since it exhibits PPIase activity (foldase) and is able to bind to a folding intermediate protein (chaperone). This review focuses on structural and functional studies of SIB1 FKBP22 and its possible involvement in cold adaptation through its dual function as a foldase and a chaperone.
2. Shewanella sp. SIB1 as a Psychrophilic Bacterium
Shewanella sp. SIB1 was previously isolated from reservoir water at the Shibugaki petroleum processing plant (Teikoku Oil, Niigata, Japan). Physiological and morphological characterizations were examined using an API 20NE Kit (bioMérieux, Durham, NS, USA) and scanning electron microscopy, respectively []. Physiologically, strain SIB1 is able to assimilate glucose, d-mannitol, maltose and malic acid. In addition, this strain possesses cytochrome oxidase and β-glucosidase enzymes and exhibits protease activity as indicated by its ability to degrade gelatin substrates. Further biochemical characterization has shown that the SIB1 strain is consistently able to degrade a catechol (a hydroxylated aromatic hydrocarbon), although in small amounts. On the contrary, the SIB1 strain cannot degrade two other hydrocarbon compounds, namely alkenes (aliphatic carbon) and naphtalene (an aromatic hydrocarbon). Morphologically, strain SIB1 is rod-shaped with a diameter of 0.3 μm and a length of 1.0–1.3 μm (Figure 2). The 16S RNA gene of strain SIB1 shows a 99.9% homology to the 16S RNA gene of psychrotrophic Shewanella sp. AC10 [], which obviously classify strain SIB1 in the genus Shewanella.
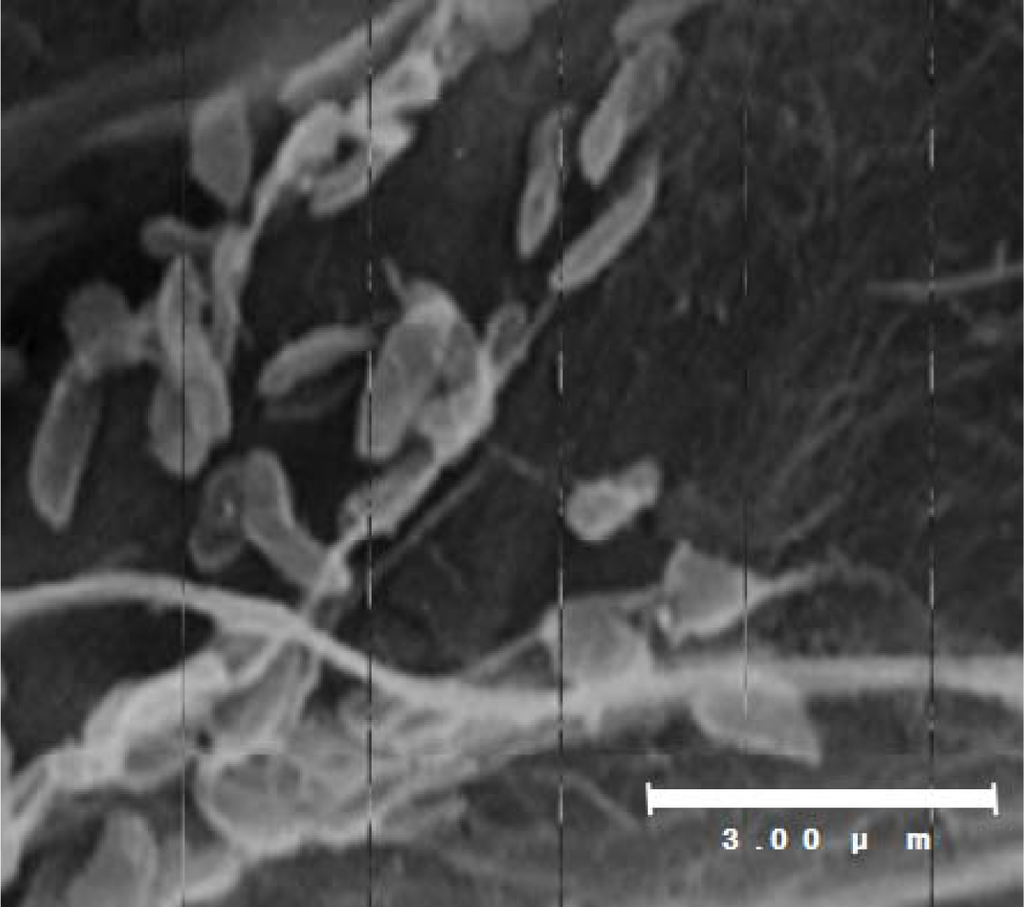
Figure 2.
Scanning electron micrograph of Shewanella sp. SIB1. This figure was reproduced and modified from Kato et al. [].
Growth temperature profiles of strain SIB1 have been identified []. Results show that the SIB1 strain does not grow at temperatures above 30 °C, while the maximum growth rate was at 20 °C. However, maximum cell density of this strain is reached at 4 °C (Figure 3). This consideration prompted us to confidently classify this strain as a psychrophile (previously classified as psychotrophic bacterium []). Shewanella sp. SIB1 is an appropriate model to decipher the mechanisms for cold-adaptation. To achieve this goal, we have studied several proteins isolated from Shewanella sp. SIB1, including RNase HI [], FKBP22 [], alkaline phosphatase [], RNase HII [] and CutA []. However, this review focuses on SIB1 FKBP22 since, among the proteins mentioned above, only this protein is involved in cold shock responses of strain SIB1 and therefore is thought to be important for cold adaptation [].
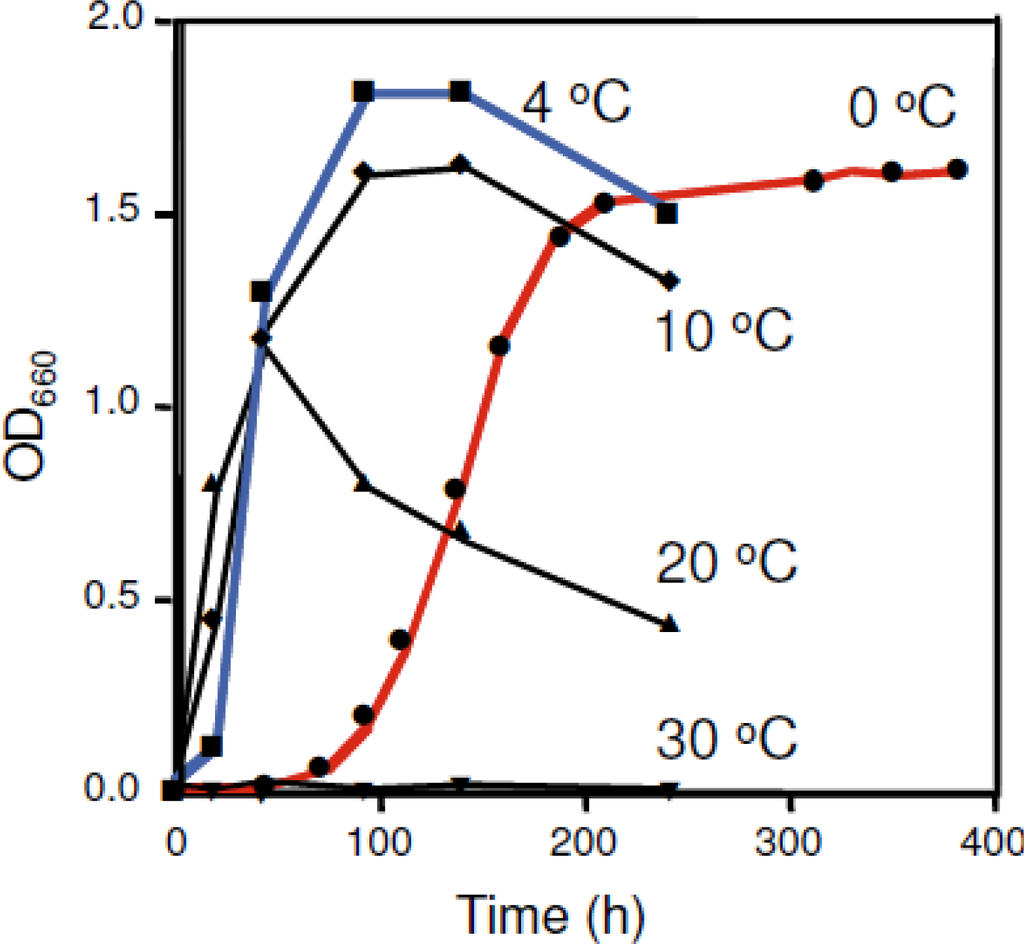
Figure 3.
Effect of temperature on the growth of Shewanella sp. SIB1. Cells were grown at 0 (closed circle), 4 (closed square), 10 (closed diamond), 20 (closed triangle), and 30 °C (upside-down-closed triangle) on the same medium that was used for isolation. This figure was reproduced and modified from Kato et al. [].
3. SIB1 FKBP22 as a Cold Shock Protein
Responses of psychrotrophic and psychrophilic bacteria to low temperatures are characterized by changes in their profile of cellular contents. Some proteins that are associated with adaptation to low temperatures are highly expressed. Hence, we have investigated proteins that are important for cold adaptation of Shewanella sp. SIB1 by employing two-dimensional electrophoresis []. As a part of proteome analysis, 2D-PAGE has been widely used in order to identify putative proteins/enzymes involved in cold adaptation of various microorganisms [,–].
The 2D-PAGE maps were obtained from extracted soluble fractions of Shewanella sp. SIB1, which were grown at 4 and 20 °C. By comparing the intensity of spots in the gel at these two temperatures, we found that the cellular content of several proteins were greatly increased at 4 °C as compared to 20 °C (Figure 4). One of these proteins is referred to as P28, which has an apparent mass of 28 kDa and an isoelectric point of 4.0. Suzuki et al. [] reported that when Shewanella sp. SIB1 is grown at 10 and 0 °C, the cellular content of P28 is increased as compared to those grown at 20 °C. However, this protein did not significantly increase at 15 °C. Collectively, P28 is thought to be greatly increased when Shewanella sp. SIB1 is grown at temperatures below 10 °C. This evidence prompted us to classify P28 as a cold shock protein of Shewanella sp. SIB1 which might be involved in cold adaptation.
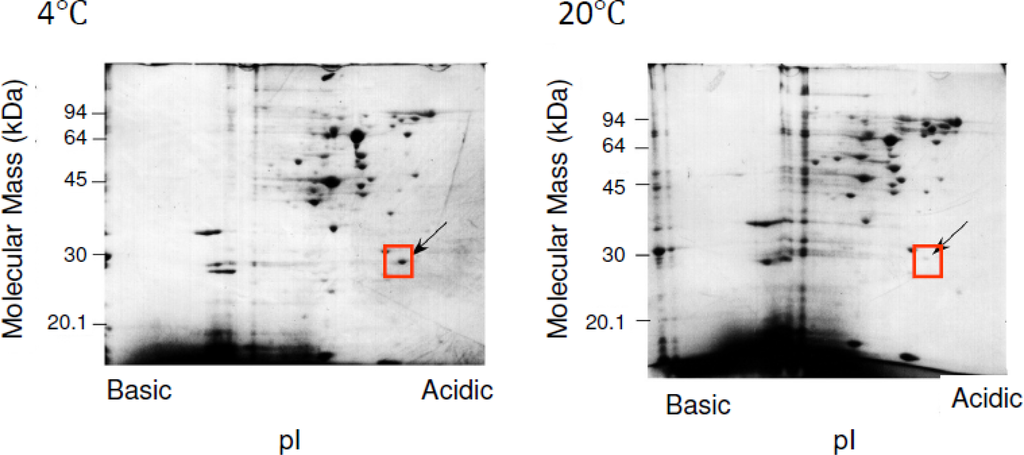
Figure 4.
2D-PAGE analysis of the proteins extracted from SIB1 cells. Soluble proteins extracted from SIB1 cells grown at 4 and 20 °C were applied to 2D-PAGE. Slab gels were stained with Coomassie Brilliant Blue. Boxes and arrows indicate the position of P28. These figures were reproduced and modified from Suzuki et al. [].
Further analysis indicates that P28 shares 56% amino acid sequence identity to Escherichia coli FKBP22 (accession no. AAC77164) [], 43% to E. coli FkpA (accession no. AAC76372) [] and 41% to Legionella pneumophila MIP (accession no. S42595) []. These proteins are members of the PPIase family which exhibit activity towards peptide (PPIasepep) and protein (PPIasepro) substrates. Electrospray ionization mass spectrometry (ESI-MS) analysis revealed that the molecular mass of P28 was 23.9 kDa, which is comparable to that calculated from the amino acid sequence. According to the aforementioned data, P28 is defined as SIB1 FKBP22, which indicates that this protein is a member of FKBP from SIB1 with a molecular mass of approximately 22 kDa.
Oligomeric state analysis by sedimentation equilibrium analytical ultracentrifugation indicated that SIB1 FKBP22 assumes a homodimeric protein of 48 kDa, about twice as large as those calculated []. L. pneumophila MIP, E. coli FkpA and E. coli FKBP22 were also reported to be homodimeric proteins [–]. Therefore, SIB1 FKBP22, as well as E. coli FkpA, was classified to the (MIP)-like FKBP protein subfamily. However, when gel filtration was employed to analyze the oligomeric state of SIB1 FKBP22, the deduced molecular mass was 3–4 times larger than calculated, indicating that SIB1 FKBP22 forms a trimeric or tetrameric structure. This discrepancy might be caused by the shape of SIB1 FKBP22, since it is apparently a non globular protein that migrates through the gel filtration column faster than other globular proteins that were used for the calibration of molecular mass.
Due to the lack of a crystal structure image of SIB1 FKBP22, a three-dimensional model of this protein has been constructed based on the crystal structure of L. pneumophila MIP []. Based on this model, these proteins are predicted to assume a non-globular V-shaped homodimeric structure, in which two monomers interact with each other at their N-domains (Figure 5). Each monomer assumes a dumbbell-like structure, in which the N-domain, consisting of α1 and α2 helices, and the C-domain, consisting of six β strands (β1–β6) and an α4 helix, are linked by an α3 helix, which is 40 residues long. The C-domains, which are located at both ends of a V-shaped structure, face each other across the cleft of this structure. The interface of two monomers, which is located at the bottom of the V-shaped structure, is stabilized by the hydrophobic interactions between the α1 helix of one monomer and the α2 helix of the other.
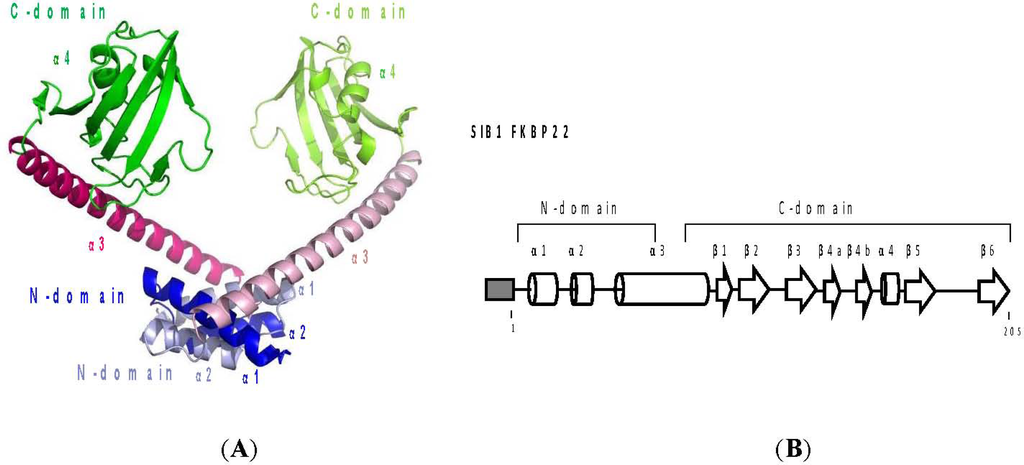
Figure 5.
(A) Three-dimensional structure model of SIB1 FKBP22. One monomer is dark-colored, while the other is light-colored. The N- and C-domains and α1–4 helices are indicated; (B) Schematic representations of the primary structure of SIB1 FKBP22. A His-tag attached to the N-termini of the proteins is represented by the shaded box. The α-helices and β-strands are represented by cylinders and arrows, respectively. These secondary structures are arranged based on tertiary models of SIB1 FKBP22. This figure was reproduced and modified from Suzuki et al. [] and Budiman et al. [].
4. Characterization of the Activity and Stability of SIB1 FKBP22 as a Cold-Adapted Enzyme
It is important to confirm whether SIB1 FKBP22 is a typical cold-adapted enzyme. In order to be involved in cold adaptation, a protein must adapt to low temperatures and remain fully functional. For this purpose, we have analyzed PPIase activity of SIB1 FKBP22 at various temperatures and determined its relationship to the stability of the protein.
4.1. PPIase Activity of SIB1 FKBP22
The PPIase activity of SIB1 FKBP22 on peptide substrates (PPIasepep activity) has been examined. N-succinyl-Ala-Leu-Pro-Phe-p-nitroanilide was used as substrate since it was identified as the best substrate for FKBP family members []. This substrate mimics the internal peptidyl-prolyl moiety of proteins containing proline.
The temperature dependence of the PPIasepep activity of SIB1 FKBP22 showed that this protein is apparently exhibited catalytic efficiency at an apparent optimum temperature of 10 °C (0.87 μM−1 s−1) (Figure 6) [], which is lower, but comparable, either to that of its mesophilic counterpart, E. coli FKBP22 (1.33 μM−1 s−1) [] or human FKBP12 (1.2 μM−1 s−1) [] when measured at 10 °C. This value is comparable to that found at 15 °C; however, it was significantly decreased at temperatures higher than 25 °C. In contrast, PPIasepep activity of E. coli FKBP22 increased as the reaction temperature increased from 4 to 25 °C [].
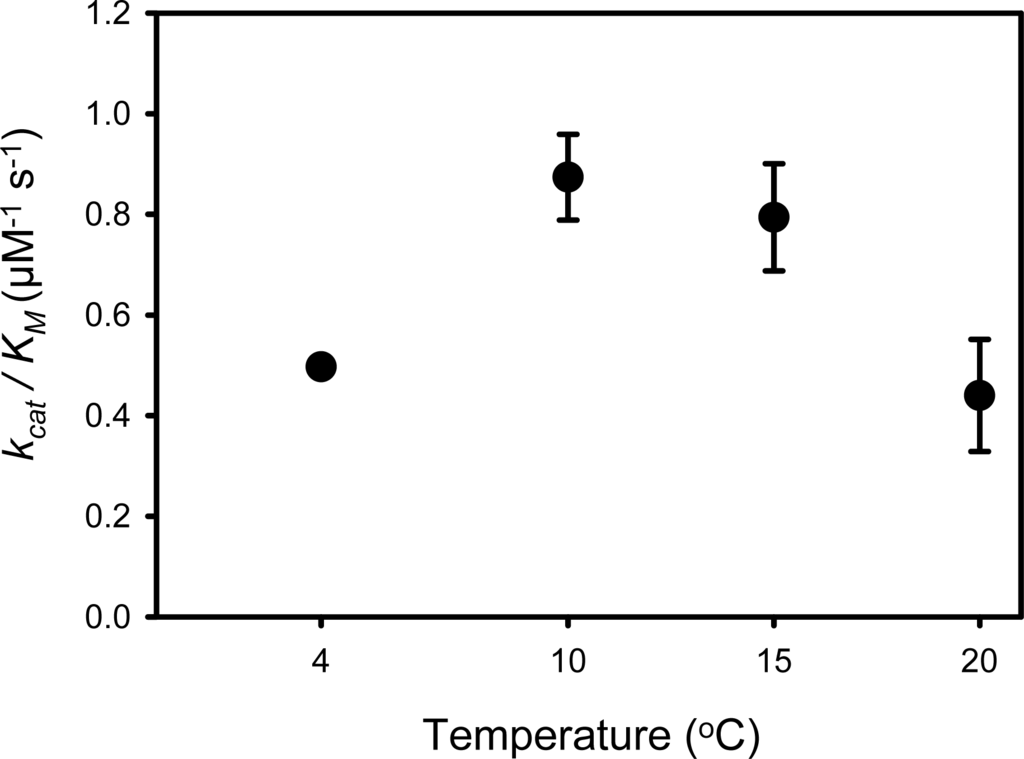
Figure 6.
The PPIasepep activity of SIB1 FKBP22 was determined by a protease coupling assay at the temperatures indicated using N-succinyl-Ala-Leu-Pro-Phe-p-nitroanilide as a substrate. The experiment was carried out in duplicate. Each plot represents the average value and standard errors of the mean are shown. This figure was reproduced and modified from Suzuki et al. [].
It has been previously reported that SIB1 FKBP22 is much less stable than E. coli FKBP22 []. However, its apparent optimal temperature for activity is significantly less than that of E. coli FKBP22. This observation follows common consensus on cold-adapted enzymes, which are specified by increases in catalytic efficiency at low temperatures, the downward shift in optimal temperatures for activity and reductions in conformational stability []. Therefore, SIB1 FKBP22 can be defined as a cold-adapted enzyme.
To fully exhibit PPIasepep activity, SIB1 FKBP22 does not require an intact molecule. This has been confirmed by our works on SIB1 FKBP22 derivative proteins: N-domain+, C-domain+, and NNC-FKBP22. The N- and C-domains+ are SIB1 FKBP22 derivatives that lack the C- and N-domains of SIB1 FKBP22, respectively, that are constructed to clarify roles of each domain for SIB1 FKBP22 []. NNC-FKBP22 is an engineered monomeric protein in which the N-domain is tandemly repeated throughout a flexible linker. NNC-FKBP22, which is unable to form a V-shaped structure, was constructed to clarify the role of the V-shaped structure of SIB1 FKBP22 []. For clarity, the three-dimensional structure models and a schematic representation of the primary structure of these SIB1 FKBP22’s derivatives are shown in Figure 7. Three dimensional models show that C-domain+ and NNC-FKBP22 are monomers, while N-domain+ is a dimer. These models are in good agreement with oligomeric states analysis conducted by using sedimentation equilibrium analytical ultracentrifugation [,].
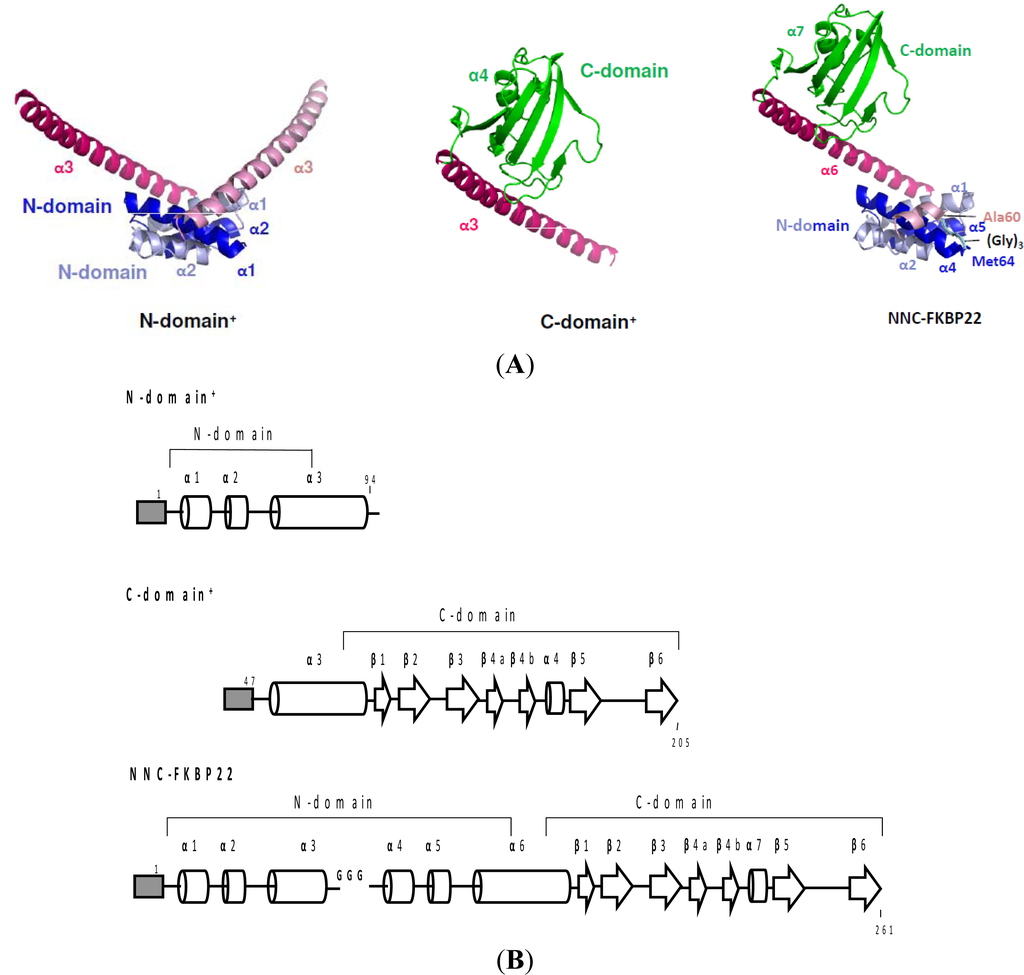
Figure 7.
(A) Three dimensional structure models of SIB1 FKBP22 derivatives: N-domain+, C-domain+, and NNC-FKBP22. These models were generated from Figure 5A. For the N- and C-domain structures, α1–4 helices are shown. For the NNC-FKBP22 structure, the corresponding domains, helices, and side chains of the amino acid residues are indicated. A loop consisting of three glycine residues, which connects Ala60 and Met64 (corresponding to Met8 of SIB1 FKBP22), is schematically shown in cyan. The corresponding α1–4 helices of the second monomer in NNC-FKBP22 are ordered as α4–7 helices since the second monomer is covalently linked to the N-domain of the first monomer; (B) Schematic representations of the primary structures of SIB1 FKBP22 derivatives: N-domain+, C-domain+, and NNC-FKBP22. A His-tag attached to the N-termini of the proteins is represented by a shaded box. The α-helices and β-strands are represented by cylinders and arrows, respectively. These secondary structures are arranged based on tertiary models of all of SIB1 FKBP22 derivatives. These figures were reproduced and modified from Suzuki et al. [].
PPIasepep activities of SIB1 FKBP22 derivatives are shown in Figure 8. NNC-FKBP22 and the C-domain+, which are monomers, exhibited higher, but comparable, PPIasepep activities to that of SIB1 FKBP22. The temperature-dependent activities of these proteins were also identical to that of SIB1 FKBP22 [,]. The C-domain+ and NNC-FKBP22 exhibited the highest activity at 10 °C and decreased at temperatures higher than 20 °C. These results indicate that C-domain alone is sufficient to bind and catalyze the isomerization reaction on a peptide substrate. These results also suggest that a V-shaped structure is not conducive to binding a peptide substrate. In this structure, the freedom of each catalytic domain is probably restricted and therefore the probability of this domain connecting with the substrate decreases. In contrast, the monomeric structure increases the freedom of the catalytic domain and therefore the chance of this domain connecting with the substrate also increases [].
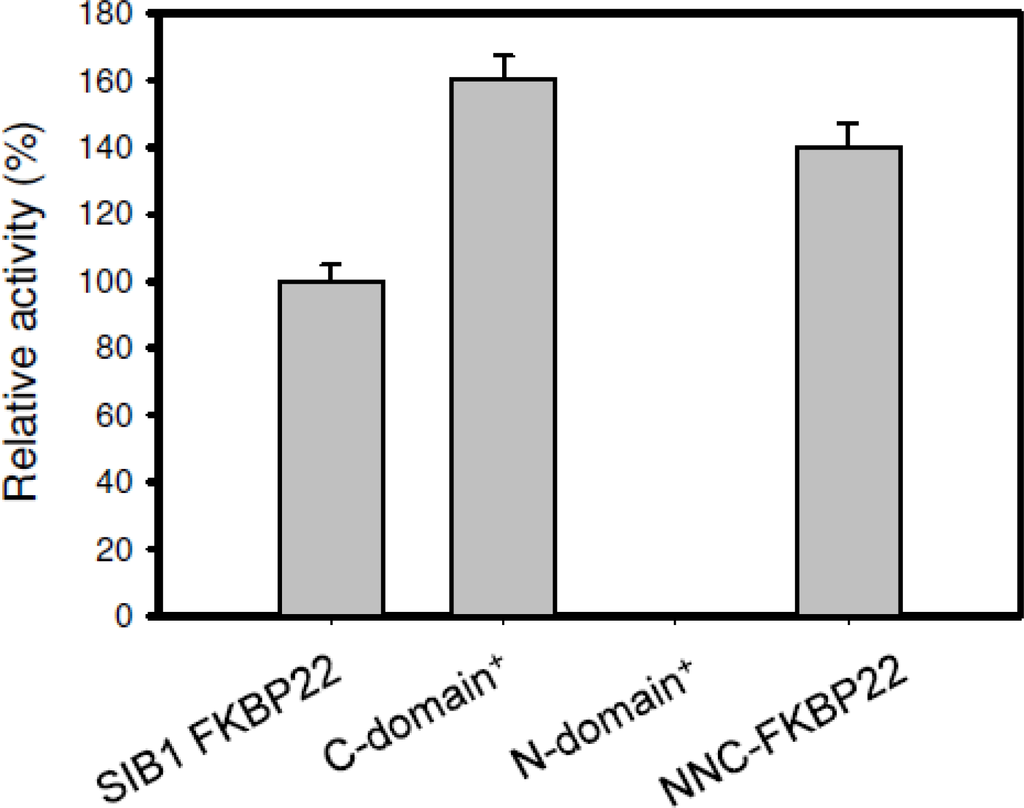
Figure 8.
PPIasepep activities of SIB1 FKBP22 and its derivatives measured at 10 °C. Relative PPIasepep activities of the SIB1 FKBP22 derivatives, which are calculated by dividing the kcat/Km value of the SIB1 FKBP22 derivatives by that of the wild-type protein (SIB1 FKBP22), are shown. The PPIasepep activity was determined by a protease coupling assay using Suc-ALPF-pNA as a substrate. The kcat/Km value of WT was 0.87 μM−1 s−1. This figure was reproduced and modified from Suzuki et al. [].
4.2. Stability of SIB1 FKBP22
The stability of SIB1 FKBP22 has been examined by differential scanning calorimetry (DSC) []. Thermal unfolding of these proteins was highly reversible as indicated by repeated thermal scans to reproduce DSC curves. The denaturation curve of SIB1 FKBP22 clearly showed two well separated transitions (Figure 9). Deconvolution of the thermogram, according to a non-two-state denaturation model, resulted in melting temperature (Tm) values of 32.5 °C and 46.6 °C for these transitions. Upon examination of the stabilities of the C- and N-domain+ (Table 1), these Tm values were nearly equal to those of the C-domain+ (35.6 °C) and N-domain+ (44.4 °C), suggesting that the thermal unfolding transitions of SIB1 FKBP22 at lower and higher temperatures represent those of its C-domain and N-domain, respectively. This result also suggested that the C-domain is less stable than the N-domain.
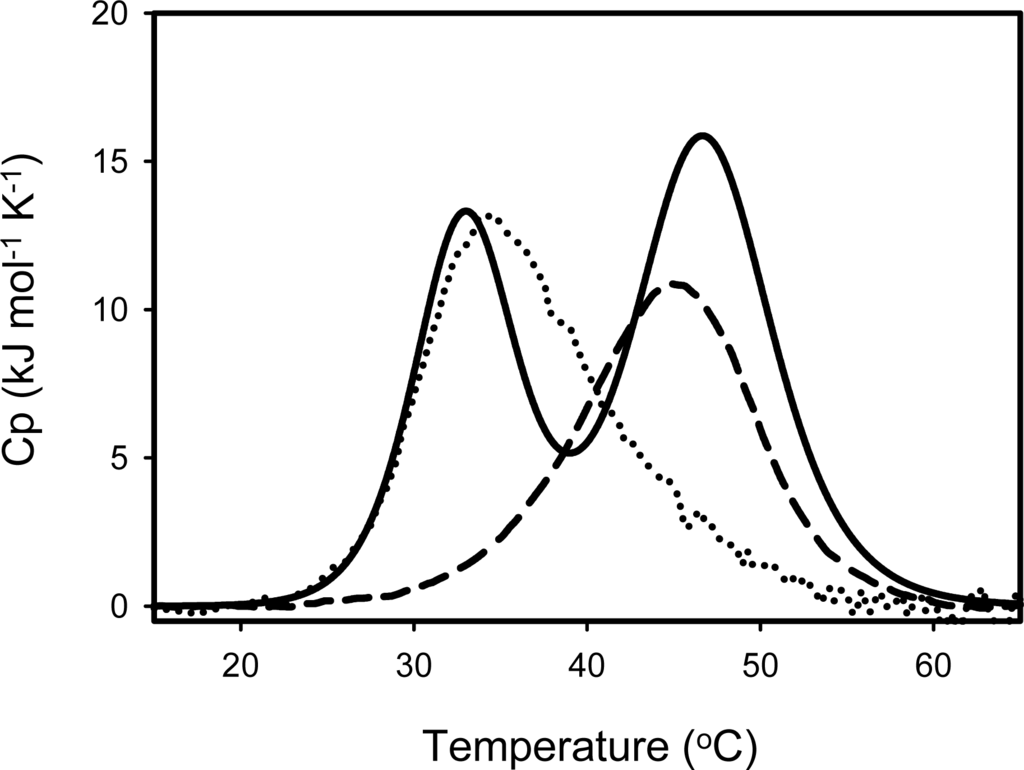
Figure 9.
DSC curves of SIB1 FKBP22 (solid line), C-domain+ (dot line) and N-domain+ (broken line). The curves were measured at a scan rate of 1 °C min−1. Proteins were dissolved in 20 mM sodium phosphate (pH 8.0) in at approximate final concentration of 1 mg mL−1. This figure was reproduced from Suzuki et al. [].

Table 1.
Thermodynamic parameters for heat induced unfolding of the protein a.
4.3. Relationship between Activity and Stability of SIB1 FKBP22
DSC data showed that unfolding of SIB1 FKBP22 initiates at a temperature higher than 25 °C. Interestingly, thermal unfolding of the C-catalytic domain is also initiated at temperatures greater than 25 °C (data not shown) []. Nevertheless, SIB1 FKBP22 and the C-domain+ exhibited maximal PPIasepep activity at 10 °C, while their activities are greatly reduced at 20 °C. Meanwhile, there is no significant difference in the CD spectrum of SIB1 FKB22 at 10 and 20 °C [], suggesting that the conformation of this protein is not significantly influenced by a temperature shift from 10 to 20 °C. However, subtle conformational changes of the active site may occur at temperatures higher than 20 °C which can promote a loss in some of the interactions between the enzyme and the substrate, leading to a decrease in the concentration of the enzyme-substrate complex, and therefore may cause a significant reduction in enzymatic activity.
Cold-adapted α-amylase and family 8 xylanase from an Antarctic bacterium also showed large differences in temperatures for enzymatic inactivation and structural unfolding [,]. The apparent optimal temperatures for enzymatic activities of these proteins are much lower than the temperatures at which any significant conformational event occurred. Different results have been observed for their mesophilic and thermophilic counterparts in which the optimal activation temperatures are closely correlated to the temperatures influencing structural transitions.
Based on the aforementioned observations, it is likely that cold adapted enzymes are characterized by large differences in the temperatures for enzymatic inactivation and structural unfolding. D’amico et al. [] proposed that this difference is caused by a cold-adaptation strategy called “localized flexibility”. Although an increase of flexibility around the active site increases kcat by reducing the energy cost of conformational change during the catalytic reaction, it should increase Km concurrently. By restricting the increase of flexibility within small regions, cold adapted enzymes prevent unfavorable increases in Km []. SIB1 FKBP22 probably adopts a similar strategy for cold adaptation.
5. Involvement of SIB1 FKBP22 in Protein Folding
The role of SIB1 FKBP22 in protein folding at low temperatures remains to be clarified. Two groups of proteins, chaperones and foldases, have been recognized as groups of accessory proteins that assist the maturation of nascent polypeptides into functional proteins within cells []. Chaperones are defined as a group of proteins that assist correct folding without covalent changes. They recognize and selectively bind nonnative proteins to prevent aggregation. In contrast, foldases catalyze necessary covalent reactions directly involved in protein folding. The foldases, therefore, differ from chaperones due to their involvement in covalent changes. Only two families of foldases have been characterized thus far: namely PDI and PPIases.
Wang and Tsou [] provided interesting evidence that in some cases, foldases may act as chaperones. Likewise, chaperones may exhibit catalytic activity that is involved in folding protein. Therefore, a protein may act as foldase and chaperone to assist protein folding. In this respect, SIB1 FKBP22 may behave as a foldase and chaperone to overcome problems in protein folding at low temperatures as faced by Shewanella sp. SIB1.
5.1. SIB1 FKBP22 as a Foldase
SIB1 FKBP22 as a foldase is evidenced through its ability to accelerate folding of RNase T1 []. RNase T1 has been widely used as a protein substrate for PPIasepro activity, because of the cis-trans isomerization of two peptidyl prolyl bonds (Tyr38-Pro39 and Ser54-Pro55) of RNase T1 are rate-limiting steps for its folding [–]. RNase T1 was first unfolded using guanidine hydrochloride. Refolding was then initiated by an 80-fold dilution in the presence or absence of the enzyme. The refolding reaction can be easily monitored by measuring the increase of tryptophan fluorescence.
Acceleration of the two rate-limiting prolyl isomerizations was observed when refolding of RNase T1 is accompanied by the activities of SIB1 FKBP22 (Figure 10), suggesting that SIB1 FKBP22 catalyzes prolyl isomerization of proteins in a nonspecific manner. Temperature dependence of foldase activity was also examined by measuring activity at 10 and 20 °C []. The catalytic efficiency (kcat/Km) of SIB1 FKBP22 was greatly reduced at 20 °C (0.13 μM−1 s−1) as compared to that at 10 °C (0.50 μM−1 s−1).
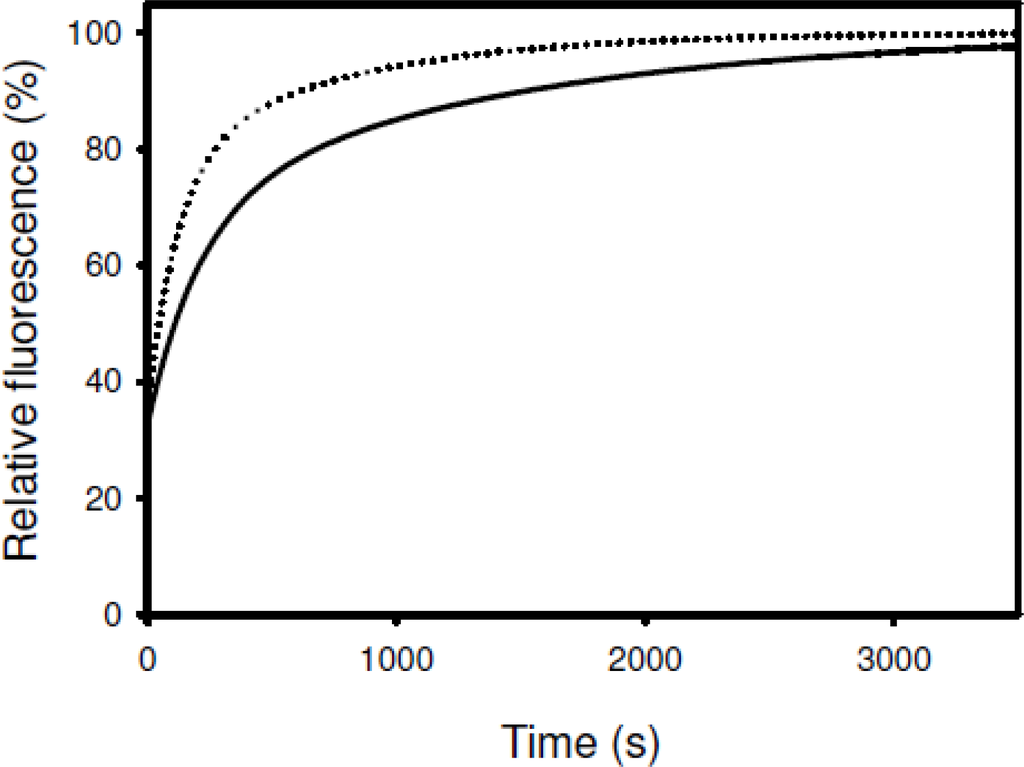
Figure 10.
Catalysis of the slow refolding reactions of RNase T1 by SIB1 FKBP22 (PPIasepro activity). The increase in tryptophan fluorescence at 323 nm during the refolding of RNase T1 (0.2 μM) is shown as a function of the refolding time. Refolding reactions were carried out at 10 °C in the absence (solid line) or presence of 8.9 nM of SIB1 FKBP22 (broken line). This figure was reproduced and modified from Suzuki et al. [].
Acceleration of this refolding reaction was also observed in the presence of E. coli FKBP22 []. However, it has been reported that the catalytic efficiency of E. coli FKBP22 increased when the temperature was increased to 20 °C. These results support previous results that found that the apparent optimum temperature of the PPIasepep activity of SIB1 FKBP22 was 10 °C. It was also found that PPIasepro activity was not detectable on single domain of FKBP (FKBP12) []. Meanwhile, multidomains of FKBPs (SlyD, FkpA, trigger factor, FKBP17, FKBP52) efficiently catalyzed folding of RNase T1 [–]. This evidence prompted us to propose that the presence of additional domains of SIB1 FKBP22, other than its catalytic domain, generates PPIasepro activity.
The importance of additional domains of SIB1 FKBP22 for PPIasepro activity has been confirmed by characterization of PPIasepro activity of the N-domain+, C-domain+, and NNC-FKBP22 [,]. As shown in Figure 11, the C-domain+, where the catalytic site is located, exhibited much less PPIasepro activity compared to that of SIB1 FKBP22. In addition, PPIasepro activity of the N-domain+ was abolished. Interestingly, NNC-FKBP22, which contains N- and C-domains, also exhibited much less PPIasepro activity compared to that of SIB1 FKBP22. This result suggests that the presence of the C-domain with full length of α3-helix is a minimum requirement for PPIasepro activity. The V-shaped structure of SIB1 FKBP22 is responsible for maximal PPIasepro activity. Since the V-shaped structure is facilitated by dimerization of the N-domain, an additional N-domain is required for maximum PPIasepro activity. Similar results have been also obtained from other multidomains of FKBPs, including E. coli FkpA and E. coli SlyD [,], in which removal of additional domains greatly reduces catalysis of protein substrates. In addition, the additional domain alone was unable to exhibit foldase activity.
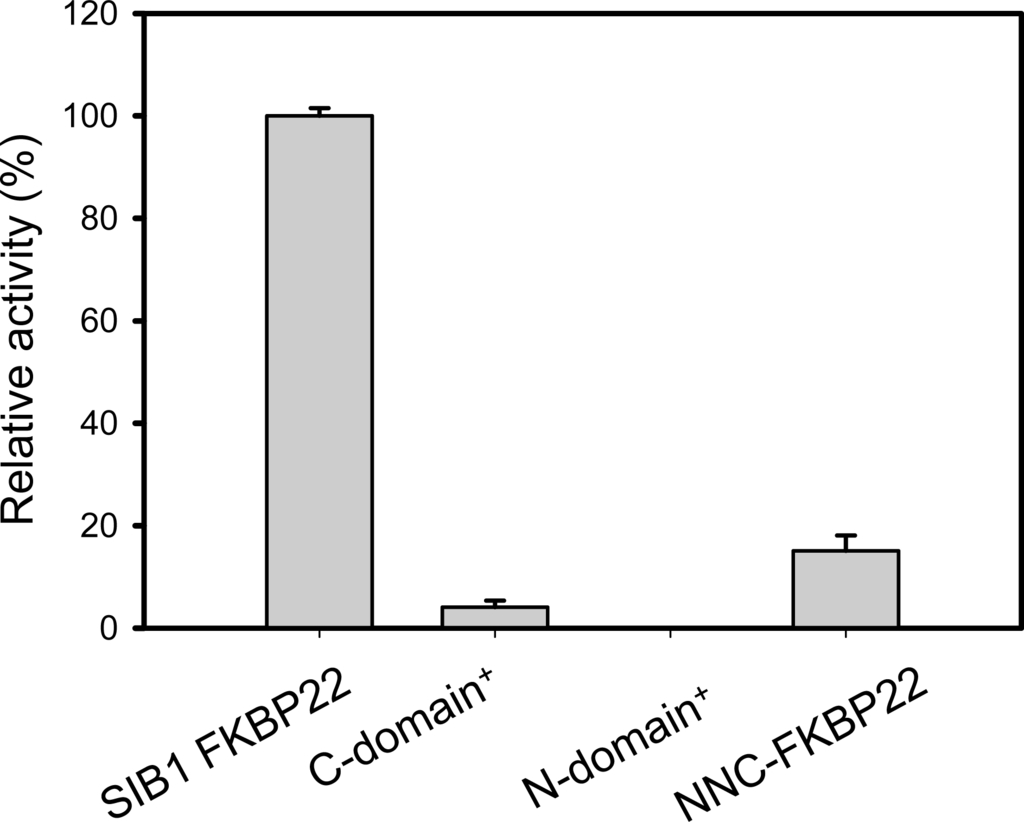
Figure 11.
PPIasepro activities of SIB1 FKBP22 and its derivatives measured at 10 °C. Relative PPIasepro activities of the SIB1 FKBP22 derivatives, which are calculated by dividing the kcat/Km value of the SIB1 FKBP22 derivatives by that of the wild-type protein (SIB1 FKBP22), are shown. The kcat/Km value of WT was 0.5 μM−1 s−1. This figure was reproduced and modified from Suzuki et al. [].
5.2. SIB1 FKBP22 as a Chaperone
Chaperones are characterized by their ability to bind unfolded protein or molten globules and thereby prevent aggregation during folding. We then examined the chaperone activity of SIB1 FKBP22 by binding to a folding intermediate of protein. Reduced and carboxymethylated (RCM) α-lactalbumin has been used as a model for a folding intermediate of a substrate. This substrate has been commonly used to analyze chaperone activity of GroEL [,] and the FKBP family of proteins [,]. α-lactalbumin is stabilized by four disulfide bonds and a single Ca2+ ion [,] and therefore reduction of these disulfide bonds produces proteins with a partially folded molten globule-like structure []. This conformational change was confirmed by measuring far-UV CD spectra of this protein in the presence and absence of 2 mM DTT, which represents reduced and non-reduced proteins, respectively []. The spectrum of the reduced protein exhibited a trough with a minimum ellipticity value of −14,000 at 205 nm, accompanied by a shoulder with an ellipticity value of −7000 at 220 nm. The spectrum of the non-reduced protein, on the other hand, exhibited a broad trough with two minimum ellipticity values of −12,500 at 208 nm and −12,000 at 220 nm.
The interaction between SIB1 FKBP22 with α-lactalbumin was monitored by surface plasmon resonance imaging using the Biacore X instrument (GE Health care, USA). Reduced and non-reduced α-lactalbumins (100 μM each) were injected onto the sensor chip, on which SIB1 FKBP22 was immobilized. An increase of resonance units (RU) was detected when reduced α-lactalbumin was injected, but was not detected when non-reduced α-lactalbumin was injected onto the same sensor chip (Figure 12). This result indicates that SIB1 FKBP22 does not bind to non-reduced α-lactalbumin, but will bind to reduced α-lactalbumin.
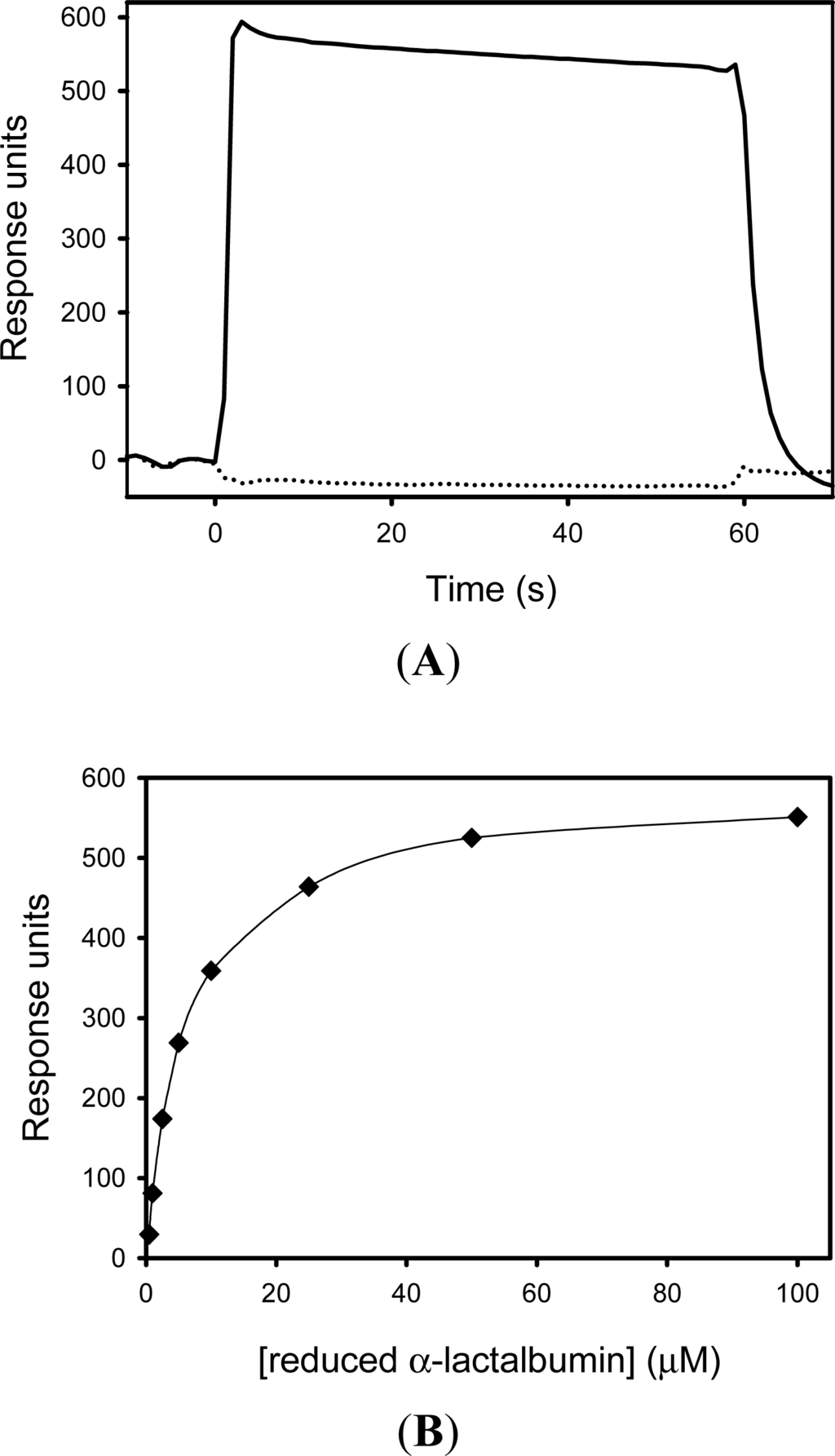
Figure 12.
Binding of reduced α-lactalbumin to SIB1 FKBP22. (A) Sensorgrams from Biacore X showing the binding of reduced α-lactalbumin (100 μM) to immobilized SIB1 FKBP22 (solid line). The sensorgram showing the binding of non-reduced α-lactalbumin (100 μM) to SIB1 FKBP22, is also shown (broken line). Injections were made from time zero for 60 s. (B) Relationships between the equilibrium binding response and concentration of reduced α-lactalbumin. The equilibrium binding responses of SIB1 FKBP22 (closed diamond) is shown as a function of concentration of reduced α-lactalbumin. The solid line represents the fitting curve of a single binding site affinity model using the BIAevaluation program. These figures were reproduced from Budiman et al. [], which is comparable to the result of Suzuki et al. [].
A KD value for the binding of reduced α-lactalbumin to SIB1 FKBP22 was determined to be 2.2 μM [], which is comparable to our latest report []. This value was determined by measuring equilibrium binding responses at various concentrations of reduced α-lactalbumin (Figure 12).
The ability of SIB1 FKBP22 to bind to a folding intermediate protein raised further questions regarding the binding site for the protein substrate in SIB1 FKBP22. To address this issue, we have analyzed the binding affinity of each domain (N- and C-domain+) and an engineered monomeric mutant, NNC-FKBP22, to a protein substrate. Results showed that the N-domain+ and NNC-FKBP22 do not bind to non-reduced α-lactalbumin, but will bind to reduced α-lactalbumin [,]. However, increasing RU values were not detected when non-reduced or reduced α-lactalbumin was injected onto a sensor chip, on which C-domain+ was immobilized, indicating that the C-domain+ does not bind to either non-reduced or reduced α-lactalbumin [].
The binding affinity of the N-domain+ was comparable to that of SIB1 FKBP22 [] (Table 2). By contrast, the KD value for binding of reduced α-lactalbumin to NNC-FKBP22 was 6.5-fold higher than that of SIB1 FKBP22, indicating that the binding affinity of NNC-FKBP22 to a folding intermediate of proteins is greatly reduced as compared to that of SIB1 FKBP22.

Table 2.
The KD values of SIB1 FKBP22 and its derivatives. The KD values of SIB1 FKBP22, the N-domain+ and C-domain+ are reproduced from Suzuki et al. []. The KD value of NNC-FKBP22 is taken from Budiman et al. [] and normalized on the basis of data from Suzuki et al. [].
Altogether, the binding site for the protein substrate is located at the N-domain, which is separated from the catalytic site of the C-domain. Our results showed that the binding site of SIB1 FKBP22 for a folding intermediate of protein is located at the N-terminal domain, which supports the proposal by Arie et al. [] and Saul et al. [] that the chaperone and PPIase activities of FkpA reside in the N- and C-terminal domains, respectively. The low affinity of NNC-FKBP22 indicated that a V-shaped structure of SIB1 FKBP22 is important for the efficient binding to a protein substrate []. This evidence may explain the low PPIasepro activity of the C-domain+ and NNC-FKBP22. These mutants lack the V-shaped structure and are therefore unable to efficiently bind to the protein substrate (RNase T1). In addition, the N-domain+ is likely able to efficiently bind to RNase T1; however, PPIasepro activity could not be detected due to the removal of the catalytic site.
However, the ability of SIB1 FKBP22 to prevent chemical or heat-induced aggregation remains to be investigated. Our latest study showed that SIB1 FKBP22 could effectively prevent DTT-induced aggregation of insulin, which indicated that SIB1 FKBP22 clearly exhibited chaperone activity. This information is now being submitted to be published elsewhere.
5.3. The Relationship between Foldase and Chaperone Activities
Chaperone activity is required for binding to a protein substrate. This binding is efficiently facilitated by the V-shaped structure through a “Mother’s arm” model, as proposed for the substrate binding mechanism of E. coli FkpA []. According to this model, two long α3 helices act as flexible “arms”, which can bend at the “elbows” (presumably located at the middle of the α3 helices). Two catalytic domains act as “hands” and the active site residues act as “fingers” for protein substrates. As a “mother” holds her “baby” by bending both of her arms, a dimeric form of E. coli FkpA holds a protein substrate by bending its two long α3 helices. A V-shaped structure of SIB1 FKBP22 may also be required to hold a protein substrate by a similar mechanism. The plasticity of the V-shaped structure may lead to a conformational flexibility to adopt various kinds of protein substrates. Once the protein is cradled by SIB1 FKBP22, the plasticity of the two α3 helices allows access to the isomerase’s catalytic site. The C-domains from each monomer of SIB1 FKBP22 attaches to the substrate and leads to the isomerization of the prolyl bonds. This idea explains the adaptability of the V-shaped structure to accelerate the folding of protein substrates through substrate binding and isomerization of the trans-cis prolyl bonds of the protein substrate.
6. A Possible Role of SIB1 FKBP22 in Cold Adaptation
SIB1 FKBP22 has been shown to be overproduced when the cells are grown at low temperatures and has shown typical features of cold-adapted enzymes. These features prompted us to propose that SIB1 FKBP22 is involved in cold adaptation of Shewanella sp. SIB1. However, it remains to be clarified exactly how FKBP22 is involved in the cold-adaptation of Shewanella sp. SIB1. According to the aforementioned results, we propose that SIB1 FKBP22 is important for folding proteins in the cells of Shewanella sp. SIB1 when grown at low temperatures. This might be due to the ability of SIB1 FKBP22 to act as a foldase and chaperone at low temperatures.
We believed that, as for other bacteria [], exposing Shewanella sp. SIB1 to low temperatures causes a series of cellular problems, including the folding of proteins. In respect to the folding of proteins containing a cis-proline residue, cis-trans isomerization becomes much slower at low temperatures, since it is thermodynamically linked []. Moreover, slow folding, which might be caused by slow cis-trans isomerization, promotes others problems, which include the misfolding or degradation of protein substrates.
To address this problem, SIB1 FKBP22 was significantly increased when Shewanella sp. SIB1 was grown at low temperatures. This evidence was obviously shown by our 2D-PAGE analysis, as previously described. Once expressed, SIB1 FKBP22 will act as foldase through its PPIasepro activity to catalyze a rate-limiting step of folding proteins containing a cis-proline residue. This response is part of strategy used for a cold adaptation process through proper folding of proteins and/or maintenance of the quaternary structure of proteins [].
The ability of SIB1 FKBP22 to act as a foldase is supported by its chaperone activity that facilitates the binding affinity to a folding intermediate protein substrate. In the absence of chaperone activity, the PPIasepro activity of SIB1 FKBP22 was significantly decreased and therefore could not act as an efficient foldase. Moreover, chaperone activity of SIB1 FKBP22 may also be important to prevent aggregation of proteins without a cis-proline residue. It has been reported that low temperature caused denaturation and aggregation of proteins []. However, the ability of SIB1 FKBP22 to prevent aggregation of proteins remains to be shown experimentally.
It is also possible for SIB1 FKBP22 to have other roles for the cold adaptation of Shewanella sp. SIB1. It has been reported that PPIase is involved in various biological processes including cell signaling, cell cycling, immune responses, and neural function []. However, the involvement of SIB1 FKBP22 in other biological functions of Shewanella sp. SIB1 and its relationship with cold adaptation remains to be clarified.
7. Other PPIases and Involvement in Cold Adaptation
Requirements of PPIases for cold adaptation have been observed in various organisms, regardless of whether they are hyperthermophiles, mesophiles or psychrophiles. These findings also revealed that all members of PPIases may be involved in cold adaptation with unclear mechanism.
Apart from SIB1 FKBP22, other FKBP members were also reported to be involved in cold adaptation in various organisms. FKBP18 from a hyperthermophilic archea, Thermococcus sp. KS-1, has been thought to be important at lower growth temperatures than the optimum in Thermococcus sp. KS-1 cells []. In the mesophilic bacterium, E. coli, the cold inducible trigger factor (EcTF) has been reported to be important for cold adaptation of E. coli to low temperatures. Inactivation of the gene for EcTF resulted in decreased viability of E. coli in cold temperatures []. Pyshcrophilic trigger factor of Antartic bacterium Pseudoalteromonas haloplanktis TAC125, PhTF, was also reported to be a primary chaperone for growth in the cold []. It is interesting to note that these cytoplasmic proteins exhibit both PPIase (either toward peptide and protein substrates) and chaperone activities as indicated by the ability to prevent aggregation. It is reminiscent of the ability of SIB1 FKBP22 and therefore FKBP18, EcTF and PhTF may also be involved in cold adaptation through their foldase and chaperone activities.
Other PPIases that have been previously linked to low-temperature adaptation include cell-associated PpiB of Bacillus subtilis [] and RotA of Erwinia chrysanthemi []. Based on the sequence similarities, these proteins belong to cyclophilin-like protein. However, there is no further information on the foldase and chaperone activities of these proteins. It is noted that cyclophilin is also considered as foldase based on its ability to accelerate the refolding of RNase T1 in vitro. Considering that PpiB and RotA are periplasmic proteins, it is suspected that these proteins are involved in cold adaptation, by facilitating protein folding in the periplasm, as other periplasmic PPIases do []. In addition, perisplasmic PPIase has also been reported to participate in the assembly of the outer membrane proteins []. However, it remains unclear whether this function has any relation to the cold adaptation mechanism.
CsPinA, a parvulin member of psychrophilic archaeon Cenarchaeum symbiosum, is thought to have an important role in the survival of this archaeon at low temperature []. It is commonly known that parvulin exhibits foldase and chaperone activities, which seems to be also exhibited by CsPinA. Both activities may be utilized for overcoming cellular problems that occur at low temperature. Interestingly, parvulin is unique in comparison to other PPIase members since parvulin prefers phosphorylated serine or threonine N-terminal to proline residue []. Since phosphorylated substrates are usually related to various biological processes (signal transduction, cell cycle, apoptosis), parvulin is believed to be involved in such biological processes in the cells []. However, experimental evidences are necessary to clarify the relationship between these biological roles with cold adaptation mechanism.
Noteworthy, it was found that some PPIases are secreted out of the cells, such as HP0175 of Helicobacter pylori and MIP of L. pneumophila [,]. But, these proteins have also been reported to be important for the growth of these organisms at low temperatures. Since these proteins are secreted out of the cell, the mechanism of their involvement in cold adaptation may differ to that of unsecreted PPIases, which are active inside the cell. L. pneumophila MIP is reported to contribute in facilitating protein secretion. L. pnemumophila MIP interacts with a newly secreted protein and, with its PPIase and/or chaperone activities, causes changes that convert the protein from enzymaticaly inactive to active prior or upon being secreted out the cell []. It is reported that the abundance of protein presents in wild-type culture supernatants changes upon the decrease in growth temperature of L. pneumophila []. Although there is no further information for the involvement of HPO175 in cold adaptation, it is speculated that this protein employs a similar mechanism with L. pneumophila MIP, as other secreted PPIase.
8. Conclusions
Previous studies have reported that PPIases are involved in cold adaptation of various organisms as part of their cold shock responses and adaptive mechanisms. However, there is no detailed explanation for the mechanism of involvement of PPIase in cold adaptation. In this review, we used SIB1 FKBP22 as a model protein, which was involved in the cold-shock responses of Shewanella sp. SIB1. Our extensive studies on SIB1 FKBP22 have revealed that this protein may be involved in cold adaptation through its ability to assist the folding of proteins at low temperatures, either as a foldase or a chaperone. As a foldase, SIB1 FKBP22 catalyzes the folding of proteins containing a cis-proline residue, where its folding rate is significantly restricted by cis-trans isomerizaton. As a chaperone, SIB1 FKBP22 was able to bind nonnative protein substrates, which included proteins containing cis-proline residues, during the folding reaction. Moreover, SIB1 FKBP22 may prevent the aggregation of proteins during folding through its chaperone activity. To our knowledge, SIB1 FKBP22 is the only PPIase from psychrophilic bacteria that has been extensively studied so far.
Apparently, foldase and chaperone activities are common mechanisms of PPIase which is involved in cold adaptation. However, additional mechanisms could be attributed for some periplasmic and secreted PPIases. Periplasmic PPIases are apparently not only involved in folding of various proteins in the periplasm, but also for assembling of outer membrane proteins. Meanwhile, secreted PPIases facilitate secretion of proteins that are necessary for the survival of microorganisms at low temperature. More extensive work to broaden our knowledge on these additional mechanisms of PPIases for cold adaptation is necessary.
Acknowledgments
This work was supported in part by a Grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by an Industrial Technology Research Grant Program from the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
References
- Ray, MK. Cold-stress response of low temperature adapted bacteria. Res. Signpost 2006, 37, 1–23. [Google Scholar]
- Hendrik, JP; Hartl, FU. Molecular chaperone functions of heat-shock proteins. Annu. Rev. Biochem 1993, 62, 349–384. [Google Scholar]
- Stetter, KO. Hyperthermophilic prokaryotes. FEMS Microbiol. Rev 1996, 1, 149–158. [Google Scholar]
- Cavicchioli, R; Charlton, T; Ertan, H; Omar, SM; Sidiqui, KS; Williams, TJ. Biotechnological uses of enzymes from psychrophiles. Microb. Biotechnol 2011, 4, 449–460. [Google Scholar]
- Kasana, RC; Gulati, A. Cellullase from psychrophilic microorganism: A review. J. Basic Microbiol 2001, 5, 1–8. [Google Scholar]
- Kasana, RC. Protease from psychrotrophs: An overview. Crit. Rev. Microbiol 2010, 2, 134–145. [Google Scholar]
- Joseph, B; Ramteke, PW; Thomas, G. Cold active microbial lipases: some hot issues and recent developments. Biotechnol. Adv 2008, 5, 457–470. [Google Scholar]
- Suzuki, Y; Mizutani, Y; Tsuji, T; Ohtani, N; Takano, K; Haruki, M; Morikawa, M; Kanaya, S. Gene cloning, overproduction, and characterization of thermolabile alkaline phosphatase from a psychrotrophic bacterium. Biosci. Biotechnol. Biochem 2005, 69, 364–373. [Google Scholar]
- Feller, G; Gerday, C. Psychrophylic enzymes: Hot topics in cold adaptation. Nat. Rev. Microbiol 2003, 1, 200–208. [Google Scholar]
- Piette, F; D’Amico, S; Struvay, C; Mazzucchelli, G; Renaut, J; Tutino, ML; Danchin, A; Leprince, P; Feller, G. Proteomic of life at low temperatures: trigger factor is the primary chaperone in the Antartic bacterium Pseudoalteromonas haloplanktis TAC125. Mol. Microb 2010, 76, 120–132. [Google Scholar]
- Jacob, RP; Schmid, FX. Energetic coupling between native-state prolyl isomerization and conformational protein folding. J. Mol. Biol 2008, 377, 1560–1575. [Google Scholar]
- Kay, JE. Structure-function relationships in the FK5-506-binding protein (FKBP) family of peptidylprolyl cis-trans isomerases. Biochem. J 1996, 314, 361–385. [Google Scholar]
- Wang, CC; Tsou, CL. Enzymes as chaperones and chaperones as enzymes. FEBS Lett 1998, 425, 382–384. [Google Scholar]
- Theuerkorn, M; Fischer, G; Schiene-Fischer, C. Prolyl cis/trans isomerase signaling pathway in cancer. Curr. Opin. Pharmacol 2011, 11, 1–7. [Google Scholar]
- Fanganel, J; Fischer, G. Insights into the catalytic mechanism of peptidyl prolyl cis/trans isomerases. Front. Biosci 2004, 9, 3453–3478. [Google Scholar]
- Edlich, F; Fischer, G. Pharmacological targeting of catalyzed protein folding: The example of peptide bond cis/trans isomerases. Handb. Exp. Pharmacol 2006, 172, 359–404. [Google Scholar]
- Scholz, C; Eckert, B; Hagn, F; Schaarschmidt, P; Balbach, J; Schmid, FX. SlyD proteins from different species exhibit high prolyl isomerase and chaperone activities. Biochemistry 1996, 45, 20–33. [Google Scholar]
- Weininger, U; Haupt, C; Schweimer, K; Graubner, W; Kovermann, M; Bruser, T; Szholz, C; Scaarshmidt, P; Zoldak, G; Schmid, FX; et al. NMR solution structure of SlyD from Escherichia coli: Spatial separation of prolyl isomerase and chaperone function. J. Mol. Biol 2009, 387, 295–305. [Google Scholar]
- Saul, FA; Arie, JP; Vulliez-le Normand, B; Kahn, R; Betto, NJM; Bentley, GA. Structure and functional studies of FkpA from Escherichia coli, a cis/trans peptidyl-prolyl isomerase with chaperone activity. J. Mol. Biol 2004, 335, 595–608. [Google Scholar]
- Suzuki, R; Nagata, K; Yumoto, F; Kawakami, M; Nemoto, N; Furutani, M; Adachi, K; Maruyama, T; Tanokura, M. Three-dimensional solution structure of an archaeal FKBP with a dual function of peptidyl prolyl cis-trans isomerase and chaperone-like activities. J. Mol. Biol 2003, 328, 1149–1160. [Google Scholar]
- Li, ZY; Liu, CP; Zhu, LQ; Jing, GZ; Zhou, JM. The chaperone activity of trigger factor is distinct from its isomerase activity during co-expression with adenylate kinase in Escherichia coli. FEBS Lett 2001, 506, 108–112. [Google Scholar]
- Pirkl, F; Buchner, J. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J. Mol. Biol 2001, 308, 795–806. [Google Scholar]
- Martinez-Heckert, E; Hendrickson, WA. Structural analysis of protein folding by the long-chain archeal chaperone FKBP26. J. Mol. Biol 2011, 3, 450–464. [Google Scholar]
- Budde, I; Steil, L; Scharf, C; Volker, U; Bremer, E. Adaptation of Bacillus subtilis to growth at low temperature: A combined transcriptomic and proteomic appraisal. Microbiology 2006, 3, 831–853. [Google Scholar]
- Jaremko, L; Jaremko, M; Elfaki, I; Mueller, JW; Ejchart, A; Bayer, P; Zhukov, I. Structure and dynamics of the first archaeal parvulin reveal a new functionally important loop in parvulin-type. J. Biol. Chem 2011, 8, 6554–6565. [Google Scholar]
- Suzuki, Y; Haruki, M; Takano, K; Morikawa, M; Kanaya, S. Possible involvement of an FKBP family member protein from a psychrotrophic bacterium Shewanella sp. SIB1 in cold-adaptation. Eur. J. Biochem 2004, 271, 1372–1381. [Google Scholar]
- Kato, T; Haruki, M; Imanaka, T; Morikawa, M; Kanaya, S. Isolation and characterization of psychotrophic bacteria from oil-reservoir water and oil sands. Appl Microbiol Biotechnol 2001, 55, 794–800. [Google Scholar]
- Kulakova, L; Galkin, A; Kurihara, T; Yoshimura, T; Esaki, N. Cold-active serine alkaline protease from the psychrotrophic bacterium Shewanella strain Ac10: Gene cloning and enzyme purification and characterization. Appl. Environ. Microbiol 1999, 65, 611–617. [Google Scholar]
- Ohtani, N; Haruki, M; Morikawa, M; Kanaya, S. Heat labile ribonuclease HI from a psychrotrophic bacterium: gene cloning, characterization, and site-directed mutagenesis. Protein Eng 2001, 14, 975–982. [Google Scholar]
- Chon, H; Tadokoro, T; Ohtani, N; Koga, Y; Takano, K; Kanaya, S. Identification of RNase HII from psychrotrophic bacterium, Shewanella sp. SIB1 as a high-activity type RNase H. FEBS J 2006, 273, 2264–2275. [Google Scholar]
- Sato, A; Yokotani, S; Tadokoro, T; Tanaka, S; Angkawidjaja, C; Koga, Y; Takano, K; Kanaya, S. Crystal structure of stable protein CutA1 from psychrotrophic bacterium Shewanella sp. SIB1. J. Synchrotron Radiat 2011, 18, 6–10. [Google Scholar]
- Soni, KA; Nannapaneni, R; Tasara, T. The contribution of transcriptomic and proteomic analysis in elucidating stress adaptation responses of Listeria monocytogenes. Foodborne Pathog. Dis 2011, 8, 843–852. [Google Scholar]
- Tasara, T; Stephan, R. Cold stress tolerance of Listeria monocytogenes: A review of molecular adaptive mechanisms and food safety implications. J. Food. Prot 2006, 6, 1473–1484. [Google Scholar]
- Garnier, M; Matamoros, S; Chevret, D; Pilet, MF; Leori, F; Tresse, O. Adaptation to cold and proteomic responses of the psychrotrophic biopreservative Lactococcus piscium strain CNCM I-4031. Appl. Environ. Microbiol 2010, 24, 8011–8018. [Google Scholar]
- Ting, L; Williams, TJ; Cowley, MJ; Lauro, FM; Guilhaus, M; Raftery, MJ; Cavicchioli, R. Cold adaptation in the marine bacterium, Sphingopyxis alaskensis, assesed using quantitative proteomic. Environ. Microbiol 2010, 10, 2658–2676. [Google Scholar]
- Rahfeld, JU; Rucknagel, KP; Stoller, G; Horne, SM; Schierhorn, A; Young, KD; Fischer, G. Isolation and amino acid sequence of a new 22-kDa FKBP-like peptidyl-prolyl cis/transisomerase of Escherichia coli Similarity to Mip-like proteins of pathogenic bacteria. J. Biol. Chem 1996, 271, 22130–22138. [Google Scholar]
- Horne, SM; Young, KD. Escherichia coli and other species of the Enterobacteriaceae encode a protein similar to the family of Mip-like FK506-binding proteins. Arch. Microbiol 1995, 165, 357–365. [Google Scholar]
- Engleberg, NC; Carter, C; Weber, DR; Cianciotto, NP; Eisenstein, BI. DNA sequence of mip, a Legionella pneumophila gene associated with macrophage infectivity. Infect. Immun 1989, 57, 1263–1270. [Google Scholar]
- Suzuki, Y; Takano, K; Kanaya, S. Stabilities and activities of the N- and C-domains of FKBP22 from a psychrotrophic bacterium overproduced in E. coli. FEBS J 2005, 272, 632–642. [Google Scholar]
- Budiman, C; Bando, K; Angkawidjaja, C; Koga, Y; Takano, K; Kanaya, S. Engineering of monomeric FK506-binding protein 22 with peptidyl prolyl cis-trans isomerase: Importance of V-shaped dimeric structure for binding to protein substrate. FEBS J 2009, 276, 4091–4101. [Google Scholar]
- Tradler, T; Stoller, G; Rucknagel, KP; Schierhorn, A; Rahfeld, JU; Fischer, G. Comparative mutational analysis of peptidyl prolyl cis/trans isomerases: active sites of Escherichia coli trigger factor and human FKBP12. FEBS Lett 1997, 40, 184–190. [Google Scholar]
- D’Amico, S; Marx, JC; Gerday, C; Feller, G. Activity-stability relationships in extremophilic enzymes. J. Biol. Chem 2003, 278, 7891–7896. [Google Scholar]
- Collins, T; Meuwis, MA; Gerday, C; Feller, G. Activity, stability and flexibility in glycosidases adapted to extreme thermal environments. J Mol Biol 2003, 328, 419–428. [Google Scholar]
- Fields, PA; Somero, GN. Hot spots in cold adaptation: localized increases in conformational flexibility in lactate dehydrogenase A4 orthologs of Antarctic notothenioid fishes. Proc Natl Acad Sci USA 1998, 95, 11476–11481. [Google Scholar]
- Kiefhaber, T; Quaas, R; Hahn, U; Schmid, FX. Folding of ribonuclease T1. 1. Existence of multiple unfolded states created by proline isomerization. Biochemistry 1990, 29, 3051–3061. [Google Scholar]
- Kiefhaber, T; Quaas, R; Hahn, U; Schmid, FX. Folding of ribonuclease T1. 2. Kinetic models for the folding and unfolding reactions. Biochemistry 1990, 29, 3061–3070. [Google Scholar]
- Schindler, T; Mayr, LM; Landt, O; Hahn, U; Schmid, FX. The role of a trans-proline in the folding mechanism of ribonuclease T1. Eur. J. Biochem 1996, 241, 516–524. [Google Scholar]
- Knappe, TA; Eckert, B; Schaarschmidt, P; Scholz, C; Schmid, FX. Insertion of a chaperone domain converts FKBP12 into a powerful catalyst of protein folding. J. Mol. Biol 2007, 368, 1458–1468. [Google Scholar]
- Hayer-Hartl, MK; Ewbank, JJ; Creighton, TE; Hartl, FU. Conformational specificity of the chaperonin GroEL for the compact folding intermediates of -lactalbumin. EMBO J 1994, 13, 3192–3202. [Google Scholar]
- Okazaki, A; Ikura, T; Nikaido, K; Kuwajima, K. The chaperonin GroEL does not recognize apo-α-lactalbumin in the molten globule state. Nat. Struct. Biol 1994, 1, 439–446. [Google Scholar]
- Scholz, C; Stoller, G; Zarnt, T; Fischer, G; Schmid, FX. Cooperation of enzymatic and chaperone functions of trigger factor in the catalysis of protein folding. EMBO J 1997, 16, 54–58. [Google Scholar]
- Ramm, K; Pluckthun, A. High enzymatic activity and chaperone function are mechanistically related features to the dimeric E. coli peptidyl-prolyl-isomerase FkpA. J. Mol. Biol 2001, 310, 485–498. [Google Scholar]
- Acharya, KR; Stuart, DI; Walker, NP; Lewis, M; Philips, DC. Refined structure of baboon -lactalbumin at 1.7 Å resolution. Comparison with C-type lysozyme. J. Mol. Biol 1989, 1, 99–127. [Google Scholar]
- Acharya, KR; Ren, JS; Sturart, DI; Philips, D; Fenna, RE. Crystal structure of human -lactalbumin at 1.7 Å resolution. J. Mol. Biol 1991, 2, 571–581. [Google Scholar]
- Kuwajima, K. The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins 1989, 6, 87–103. [Google Scholar]
- Suzuki, Y; Win, OY; Koga, Y; Takano, K; Kanaya, S. Binding analysis of a psychrotrophic FKBP22 to a folding intermediate of protein using surface plasmon resonance. FEBS Lett 2005, 579, 5781–5784. [Google Scholar]
- Arie, JP; Sassoon, N; Betton, JM. Chaperone function of FkpA, a heat shock prolyl isomerase, in the periplasm of Escherichia coli. Mol Microbiol 2001, 39, 199–210. [Google Scholar]
- Hu, K; Galius, V; Pervushin, K. Structural plasticity of peptidyl−prolyl isomerase sFkpA is a key to its chaperone function as revealed by solution NMR. Biochemistry 2006, 45, 11983–11991. [Google Scholar]
- Strocchi, M; Ferrer, M; Timmis, KN; Golyshin, PN. Low temperature-induced system failure in Escherichia coli: insight from rescue by cold-adapted chaperones. Proteomics 2006, 6, 193–206. [Google Scholar]
- Lu, KP; Finn, G; Lee, TH; Nicholson, LL. Prolyl cis-trans isomerization as molecular timer. Nat. Chem. Biol 2007, 3, 619–629. [Google Scholar]
- Ideno, A; Yoshida, T; Iida, T; Furutani, M; Maruyama, T. FK506-binding protein of hyperthermophilic archeum, Thermococcus sp. KS-1, a cold-shock-inducible peptidyl-prolyl cis-trans isomerase with activities to trap and refold denatured proteins. Biochem. J 2001, 357, 465–471. [Google Scholar]
- Kandror, O; Goldberg, AL. Trigger factor is induced upon cold shock and enhances viability of Escherichia coli at low temperatures. Proc. Natl. Acad. Sci. USA 1997, 94, 4978–4981. [Google Scholar]
- Pissavin, C; Hugouvieux-Cotte-Pattat, N. Characterization of a periplasmic peptidyl-prolyl cis-trans isomerase in Erwinia chrysanthemi. FEMS Microbiol. Lett 1997, 157, 59–65. [Google Scholar]
- Missiakas, D; Raina, S. Protein folding in the bacterial periplasm. J. Bacteriol 1997, 179, 2465–2471. [Google Scholar]
- Rouviere, PE; Gross, CA. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev 1996, 10, 3170–3182. [Google Scholar]
- Basak, C; Pathak, SK; Bhattacharyya, A; Pathak, S; Basu, J; Kundu, M. The secreted peptidyl prolyl cis,trans-isomerase HP0175 of Helicobacter pylori induces apoptosis of gastric epithelial cells in a TLR4- and apoptosis signal-regulating kinase 1-dependent manner. J. Immunol 2005, 174, 5672–5680. [Google Scholar]
- DebRoy, S; Aragon, V; Kurtz, S; Cianciotto, NP. Legionella pneumophila Mip, a surface-exposed peptidylproline cis-trans-isomerase, promotes the presence of phospholipase C-like activity in culture supernatants. Infect. Immun 2006, 74, 5152–5160. [Google Scholar]
- Soderberg, MA; Rossier, O; Cianciotto, NP. The type II protein secretion system of Legionella pneumophila promotes growth at low temperatures. J. Bacteriol 2004, 186, 3712–3720. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).