Alert-QSAR. Implications for Electrophilic Theory of Chemical Carcinogenesis
Abstract
:1. Introduction
2. Alert-QSAR Method
3. Results on Genotoxic Carcinogenesis
- Physicochemical parameters: meaningful physicochemical parameters as hydrophobicity, polarizability, total energy, electronegativity, and chemical hardness should be considered in order to better interpret the derived models in terms of molecular mechanisms.
- Universal hydrophobicity: when a full molecule is identical to its structural alert, the sign of the structural alert may be flipped relative to that of the molecule for the action-reaction solubility characteristics. For instance, this can be applied to the LogP of the reagent because it is the logarithm (base 10) of the partition coefficient (P), the ratio of the compound’s organic (oil)-to-aqueous phase concentrations. Therefore, the opposite and equal values of the molecule and its identical structural alert induce a kind of “universality” in the solvation ability of the concerned toxicant. This approach may be applied to molecules with a recognized high toxicological or carcinogenic potential, and should not be overestimated in the molecular series employed. For the present trial series (Table 1), this approach was employed for molecule no. 3, acetaldehyde (ethanal, C2H4O). Such an approach is justified, because this compound’s average global production is about 106 tons/year [23]; it is a common electrophile in organic synthesis [24] (in agreement with Miller’s electrophilic theory [5,6] of genotoxic carcinogenesis: “there is sufficient evidence for the carcinogenicity of acetaldehyde (the major metabolite of ethanol) in experimental animals”) [25]; it is a probable carcinogen in humans [26], but occurs naturally in coffee, bread and ripe fruit, and is produced by plants as part of their normal metabolism; and it can be spread through air, water, land or groundwater pathways and can be absorbed through inhalation, smoking or consumption [27].
- Equal steric properties: molecules with similar carcinogenic properties may be considered to have equal optimized stericities, i.e., total energies, when their true values are in the same domains. Thus, non-carcinogenic molecules may be considered to be similar in some of their physicochemical properties, including stericity (in this case, associated with total energy). For instance, in the trial series of compounds (Table 1) molecules 8 and 10, have energies of −94064.03906 [eV] and −108827.09 [eV], respectively (as calculated with PM3 and geometry optimization). These can be considered to have the same stericities in intra-cellular binding, due to their similar energies, similar activities in rats (as given by the CPD) [28], close positions in the Gaussian graph (Figure 2) and their identical carcinogenic characteristics [29] such as damage factors, disease-specific factors, and the same uncertainty factor for the combined damage and effect factors. Consequently, the common value was set from the more carcinogenic molecule (10). However, as is the case with the above “universal hydrophobicity” adjustment, the equal stericity principle should be applied with caution (as a rule, it should be applied to less than 10% of the molecules in a series) and only to mark non-congeneric series of molecules with similarity physicochemical properties.
- those based on residual-alert QSARs:
- and those based on direct-alert QSARs:
4. OECD-QSAR Principles Discussion
4.1. Principle 1: A Defined Endpoint
- (Eco-) toxicological studies, having various end-points (such as inhibition, activation, death, sterility, irritations, etc.) yet produced by a group of similar molecules, i.e., the case of congeneric studies;
- and carcinogenic studies, having essentially the same end-point as the exacerbated apoptosis that in principle diffuses in the organism no matter what the initial point of triggering is, may be initiated by highly structurally diverse molecule, being therefore classified as non-congeneric studies.
4.2. Principle 2: An Unambiguous Algorithm
- hydrophobicity (LogP), corresponding to trans-cellular membrane diffusion and with translation motion of the molecules;
- polarizability (POL), accounts for the dipole perturbation and ionic interaction, and is associated with the vibrational motion of the molecules in organism; it further accounts for potentially electrophilic effects that triggers cancer, according with the Millers’ theory [5,6], and sustained by the recent research [20]; and
- optimal total energy (Etot), which contains steric information about the molecule’s 3D structure since it is given by the equilibrium conformation [30]; it may serve therefore as a potential for the rotational motion of the molecules when triggered by interaction with organism’s receptor.
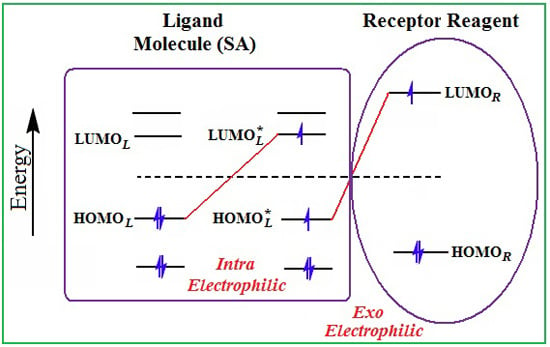
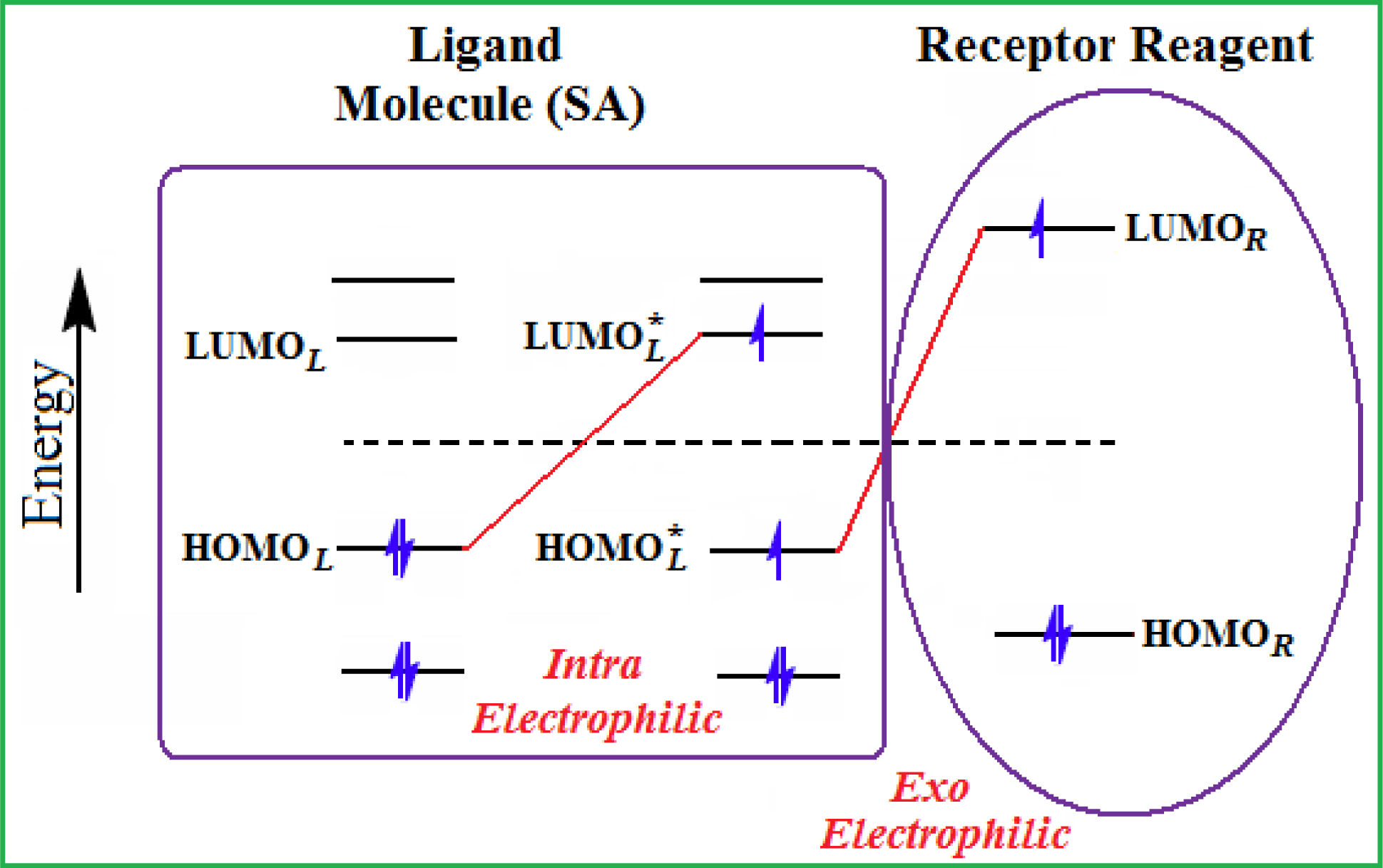
- The electronegativity equalization (EE) principle relies on the equivalence of the negative electronegativity from Equation (15) with a system’s chemical potential [41], fulfilling the Gibbs rule of phases between two molecular states. The EE principle was originally stated by Sanderson as “the molecules in their fundamental state, the electronegativities of different electronic regions in molecule–are equal” [42]. This principle was further generalized and applied to many-electron systems [43]. In this work, the principle is applied at the level of ligand-receptor binding (Figure 3). The molecular electronegativity is first equalized with that of the receptor, leading to the selection of the molecular fragment (structural alert) with electronegativity complementary to that of the receptor or adjustment of the receptor’s pocket that to fit with the ligand electronegativity. This stage corresponds to a sort of electronegativity based docking based on the fundamental quantum EE principle. The induced interaction is then stabilized through chemical hardness.
- The maximum hardness principle derives from Pearson’s observation that “there seems to be a rule of nature that molecules (or the many-electronic systems in general, n.a.) arrange themselves (in their ground or valence states, n.a.) to be as hard as possible” [44]. This principle, which has been quantitatively justified [45–48], stipulates that a maximum HOMO-LUMO gap is associated with a stabilized interaction for a molecular sample.
4.3. Principle 3: A Defined Domain of Applicability
4.4. Principle 4: Appropriate Measures of Goodness-of-Fit, Robustness and Predictivity
4.5. Principle 5: A Mechanistic Interpretation
- The first optimum paths are selected on the ergodic basis, as described in Step V of Section 3 above, by applying Equations (11) and (12) for the residual and direct alert-QSARs, respectively.
- The two classes of paths (Equations (13) and (14)) are compared on the basis of their electrophilic-docking (sub)-mechanisms identified within the unambiguous algorithm stage of the second OECD-QSAR principle. Comparison of the alpha-paths of the two alert-QSAR routes reveals that only residual-alert-QSAR correctly displays the involvement of the electronegativity in docking. As a consequence, the electrophilic-docking mechanistic interpretation of genotoxic carcinogenesis will be based only on the residual-alert-QSARs; this confirms the recent assessment of residual-QSAR as the in silico modeling technique best suited for treating chemical carcinogenesis [20]. The present approach generalizes this in two ways: by detailing the mechanistic scenario with the electronegativity-to-chemical hardness reactivity-stability influence, and by considering the structural alert information in QSAR modeling rather than working with the entire molecular structural information.
- The explicit mechanistic scenario is based on the information contained within Equations (13a)–(13c), which gives rise to a natural sequence that makes a closed loop over all three main interactions paths, given bywhich is formally represented in Figure 4.
5. Conclusions
- mutagenicity may be regarded as an electrophilic ligand-receptor interaction mechanism of covalent binding between the ligand molecule or SA and receptor;
- electronegativity and chemical hardness are crucial parameters in modeling the ligand-receptor interaction due to the electronegativity equalization and maximum chemical hardness principles, respectively;
- residual-QSAR is again shown to be reliable [20] in its treatment of genotoxic carcinogenesis, as it better incorporates electronegativity and chemical hardness principles across the optimally selected pathways of organism cells’ apoptosis;
- structural alert or molecular fragment analysis improves the residual-QSAR analysis with an enriched class of QSAR models that may be associated with molecular mechanisms of interaction in complex media;
- in general, the test performances are lower than the calibration models, but also models with considerably better behavior for test molecules compared with trials are found, especially when hydrophobicity and sometimes polarizability and/or reactivity parameters of chemical hardness and electronegativity are involved; this is not surprising since they fully support the cellular transduction process (LogP) jointly with electrophilic effects (stated by reactivity principles and polarizability).
- the mechanism of carcinogenesis, being activated by non-congeneric compounds, allows consideration of similar parameters at molecular level, by advancing the universal hydrophobicity and equal stericity transformations of about 10% of the trial compounds, but only in the situations in which the molecules display identical observed carcinogenic activities.
Acknowledgments
References
- Yamagiwa, K; Ichikawa, K. Experimental study of the pathogenesis of carcinoma. J. Cancer Res. 1918, (3), 1–29. [Google Scholar]
- Yoshida, T. Uber die serienweise verfolgung der veranderungen der leber der experimentellen hepatomerzeugung durch O-Aminoazotoluol. Trans. Jpn. Pathol. Soc. 1933, 23, 636–638. [Google Scholar]
- Hueper, WC; Wiley, FH; Wolfe, HD. Experimental production of bladder tumors in dogs by administration of beta-naphthylamine. J. Ind. Hyg. Toxicol. 1938, (20), 46–84. [Google Scholar]
- Haddow, A. Sir Ernest Laurence Kennaway FRS, 1881–1958: Chemical causation of cancer then and today. Perspect. Biol. Med. 1974, (17), 543–588. [Google Scholar]
- Miller, JA; Miller, E. Ultimate chemical carcinogens as reactive mutagenic electrophiles. In Origins of Human Cancer; Hiatt, HH, Watson, JD, Winsten, JA, Eds.; Cold Spring Harbor Laboratory: Huntington, NY, USA, 1977; pp. 605–628. [Google Scholar]
- Miller, EC; Miller, JA. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer 1981, (47), 2327–2345. [Google Scholar]
- Ames, BN. The detection of environmental mutagens and potential carcinogens. Cancer 1984, (53), 2030–2040. [Google Scholar]
- Ashby, J; Tennant, RW. Chemical structure, Salmonella mutagenicity and extent of carcinogenicity as indicators of genotoxic carcinogenesis among 222 chemicals tested by the U.S.NCI/NTP. Mutat. Res. 1988, 204, 17–115. [Google Scholar]
- Benigni, R; Netzeva, TI; Benfenati, E; Bossa, C; Franke, R; Helma, C; Hulzebos, E; Marchant, C; Richard, A; Woo, YT; et al. The expanding role of predictive toxicology: An update on the (Q)SAR models for mutagens and carcinogens. J. Environ. Sci. Health C 2007, 25, 53–97. [Google Scholar]
- Ferrari, T; Gini, G. An open source multistep model to predict mutagenicity from statistical analysis and relevant structural alerts. Chem. Cent. J. 2010, 4(Suppl 1), S2. [Google Scholar]
- Nair, PC; Sobhia, ME. Comparative QSTR studies for predicting mutagenicity of nitro compounds. J. Mol. Graphics Modell. 2008, (26), 916–934. [Google Scholar]
- Pérez-Garrido, A; Helguera, AM; Rodríguez, FG; Cordeiro, MNDS. QSAR models to predict mutagenicity of acrylates, methacrylates and α,β-unsaturated carbonyl compounds. Dent. Mater. 2010, (26), 397–415. [Google Scholar]
- Benigni, R; Bossa, C; Jeliazkova, N; Netzeva, T; Worth, A. The Benigni/Bossa Rules for Mutagenicity and CarcInogenicity—A Module of Toxtree; European Commission report EUR 23241 EN. IdeaConsult Ltd: Sofia, Bulgaria, 2008. Avalilable online: http://toxtree.sourceforge.net/carc.html (accessed on 3 August 2011).
- Price, N. Hail Caesar. Chem. Ind. 2008, (15), 18–19. [Google Scholar]
- Benfenati, E. CAESAR QSAR models for REACH. Chem. Cent. J. 2010, 4(Suppl 1), S1–S5. [Google Scholar]
- Fjodorova, N; Vračko, M; Novič, M; Roncaglioni, A; Benfenati, E. New public QSAR model for carcinogenicity. Chem. Cent. J. 2010, 4(Suppl 1), S3:1–S3:15. [Google Scholar]
- Worth, AP; Bassan, A; de Brujin, J; Gallegos Saliner, A; Netzeva, T; Patlewicz, G; Pavan, M; Tsakovska, I; Eisenreich, S. The role of the European chemicals bureau in promoting the regulatory use of (Q)SAR methods. SAR QSAR Environ. Res. 2007, (18), 111–125. [Google Scholar]
- OECD principles. Guidance Document on the Validation of (Quantitative) Structure-Activity Relationship [(q)sar] Models; OECD Environment Health and Safety Publications Series on Testing and Assessment No. 69. Organisation de Coopération et de Développement Economiques /Organization for Economic Co-operation and Development: Paris, France, 30 March 2007. Available online: http://appli1.oecd.org/olis/2007doc.nsf/linkto/env-jm-mono(2007)2 (accessed on 3 August 2011).
- Putz, MV; Putz, AM; Barou, R. Spectral-SAR realization of OECD-QSAR principles. Int. J. Chem. Model 2011, 3, 2. [Google Scholar]
- Putz, MV. Residual-QSAR. Implications for genotoxic carcinogenesis. Chem. Cent. J 2011, 5, 29. [Google Scholar] [CrossRef]
- Toplis, JG; Costello, JD. Chance correlation in structure-activity studies using multiple regression analysis. J. Med. Chem. 1972, (15), 1066–1069. [Google Scholar]
- Hansch, C; Kurup, A; Garg, R; Gao, H. Chem-bioinformatics and QSAR: A review of QSAR lacking positive hydrophobic terms. Chem. Rev. 2001, (101), 619–672. [Google Scholar]
- Eckert, M; Fleischmann, G; Jira, R; Hermann, MB; Golka, K. Acetaldehyde. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Sowin, TJ; Melcher, LM. Acetaldehyde. In Encyclopedia of Reagents for Organic Synthesis; Paquette, L, Ed.; Wiley & Sons: New York, NY, USA, 2004. [Google Scholar]
- Alcohol Drinking; World Health Organization, International Agency for Research on Cancer: Lyon, France, 1988; Available online: http://monographs.iarc.fr/ENG/Monographs/vol44/volume44.pdf (accessed on 3 August 2011).
- Chemical summary for acetaldehyde. US Environmental Protection Agency: Washington, DC, USA, 1994; Available online: http://www.epa.gov/chemfact/s_acetal.txt (accessed on 3 August 2011).
- Chemicals in the environment: Acetaldehyde (CAS no. 75-07-0). US Environmental Protection Agency: Washington, DC, USA, 1994; Available online: http://www.epa.gov/chemfact/f_acetal.txt (accessed on 3 August 2011).
- Gold, LS. The carcinogenic potency project. The Carcinogenic Potency Database (CPDB): Berkeley, CA, USA, 2011. Available online: http://potency.berkeley.edu/index.html (accessed on 3 August 2011).
- Huijbregts, MAJ; Rombouts, LJA; Ragas, AdMJ; van de Meent, D. Human-toxicological effect and damage factors of carcinogenic and noncarcinogenic chemicals for life cycle impact assessment. Integr. Environ. Assess. Manage. 2005, (1), 181–244. [Google Scholar]
- HyperChem 7.01, Program package and documentation. Hypercube, Inc: Gainesville, FL, USA, 2002.
- Putz, MV; Lacrămă, AM. Introducing spectral structure activity relationship (S-SAR) analysis. Application to ecotoxicology. Int. J. Mol. Sci. 2007, (8), 363–391. [Google Scholar]
- Lacrămă, AM; Putz, MV; Ostafe, V. A Spectral-SAR model for the anionic-cationic interaction in ionic liquids: Application to Vibrio fischeri ecotoxicity. Int. J. Mol. Sci. 2007, 8, 842–863. [Google Scholar]
- Chicu, SA; Putz, MV. Köln-Timişoara molecular activity combined models toward interspecies toxicity assessment. Int. J. Mol. Sci. 2009, (10), 4474–4497. [Google Scholar]
- Putz, MV; Putz, AM; Lazea, M; Ienciu, L; Chiriac, A. Quantum-SAR extension of the Spectral-SAR algorithm. Application to polyphenolic anticancer bioactivity. Int. J. Mol. Sci. 2009, (10), 1193–1214. [Google Scholar]
- Putz, MV; Putz, AM; Ostafe, V; Chiriac, A. Spectral-SAR ecotoxicology of ionic liquids-acetylcholine interaction on E. Electricus species. Int. J. Chem. Model. 2010, 2, 85–96. [Google Scholar]
- Putz, MV. QSAR & SPECTRAL-SAR in Computational Ecotoxicology; Apple Academics: Ontario, Canada, 2011; in press. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Guidelines for mutagenicity risk assessment. Fed. Regist. 1986, (51), 34006–34012. [Google Scholar]
- Koopmans, T. Uber die zuordnung von wellen funktionen und eigenwerter zu den einzelnen elektronen eines atom. Physica 1934, (1), 104–113. [Google Scholar]
- Putz, MV. Electronegativity and chemical hardness: Different patterns in quantum chemistry. Curr. Phys. Chem. 2011, (1), 111–139. [Google Scholar]
- Putz, MV. Absolute and Chemical Electronegativity and Hardness; Nova Science Publishers Inc: New York, NY, USA, 2008. [Google Scholar]
- Parr, RG; Donnelly, RA; Levy, M; Palke, WE. Electronegativity: The density functional viewpoint. J. Chem. Phys. 1978, (68), 3801–3808. [Google Scholar]
- Sanderson, RT. Principles of electronegativity. Part I. General nature. J. Chem. Educ. 1988, (65), 112–119. [Google Scholar]
- Mortier, WJ; Genechten, Kv; Gasteiger, J. Electronegativity equalization: Application and parametrization. J. Am. Chem. Soc. 1985, (107), 829–835. [Google Scholar]
- Pearson, RG. Absolute electronegativity and absolute hardness of Lewis acids and bases. J. Am. Chem. Soc. 1985, (107), 6801–6806. [Google Scholar]
- Chattaraj, PK; Lee, H; Parr, RG. Principle of maximum hardness. J. Am. Chem. Soc. 1991, (113), 1854–1855. [Google Scholar]
- Chattaraj, PK; Liu, GH; Parr, RG. The maximum hardness principle in the Gyftpoulos-Hatsopoulos three-level model for an atomic or molecular species and its positive and negative ions. Chem. Phys. Lett. 1995, (237), 171–176. [Google Scholar]
- Ayers, PW; Parr, RG. Variational principles for describing chemical reactions: The Fukui function and chemical hardness revisited. J. Am. Chem. Soc. 2000, (122), 2010–2018. [Google Scholar]
- Putz, MV. Maximum hardness index of quantum acid-base bonding. MATCH Commun. Math. Comput. Chem. 2008, (60), 845–868. [Google Scholar]
- Franke, R; Gruska, A. General introduction to QSAR. In Quantitative Structure-Activity Relationhsip (QSAR) Models of Mutagens and Carcinogens; Benigni, R, Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–40. [Google Scholar]
- Putz, MV; Lazea, M; Putz, AM; Seiman-Duda, C. Exploring Catastrophe-QSAR. Application on anti-HIV activity. Preprint. 2011; Unpublished work. [Google Scholar]
- Caspase-2 IHC Antibody; IHC WORLD, LLC: Woodstock, MD, USA, 2011; Available online: http://www.ihcworld.com/products/antibody-datasheets/Caspase2.IW-PA1113.htm (accessed on 3 August 2011).
- Parr, RG; Szentpály, LV; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, (121), 1922–1924. [Google Scholar]
- Parthasarathi, R; Subramanian, V; Roy, DR; Chattaraj, PK. Electrophilicity index as a possible descriptor of biological activity. Bioorg. Med. Chem. 2004, (12), 5533–5543. [Google Scholar]
- Pooja, M; Tripathi, V; Yadav, BS. Insilico QSAR modeling and drug development process. GERF Bull. Biosci. 2010, (1), 37–40. [Google Scholar]
- Tarko, L; Putz, MV. On Quantitative Structure-Toxicity Relationships (QSTR) using high chemical diversity molecules group. Preprint. 2011; Unpublished work. [Google Scholar]
- Putz, MV; Putz, AM; Lazea, M; Chiriac, A. Spectral vs. statistic approach of structure-activity relationship. Application on ecotoxicity of aliphatic amines. J. Theor. Comput. Chem. 2009, (8), 1235–1251. [Google Scholar]

| No. | Full Molecule: Chemical Structure | Full Molecule: Name Formula (CASRN) | Toxicity: TD50 Activity: A = Log[1/TD50] | Structural Alert (SA) | LogP: Full Molecules Structural Alert | POL [Ǻ3]: Full Molecules Structural Alert | Etot [kcal/mol]: Full Molecules Structural Alert | χ[eV]: Full Molecules Structural Alert | η[eV]: Full Molecules Structural Alert |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 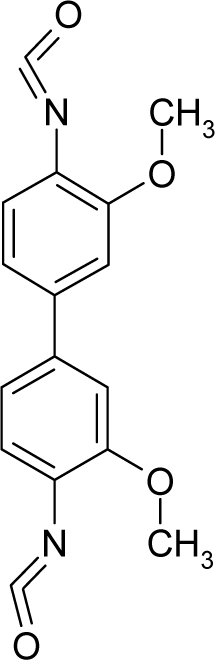 | 3,3′-Dimethoxy-4,4′-biphenylene diisocyanate (3,3′-Dimethoxybenzidine-4,4′-diisocyanate) C16H12N2O4 (91-93-0) | 1630 2.79 |  | 2.07 −0.46 | 30.03 3.27 | −82478.58594 −13584.68848 | 4.74077805 5.0497393 | 3.85074395 5.54064075 |
| 2. | 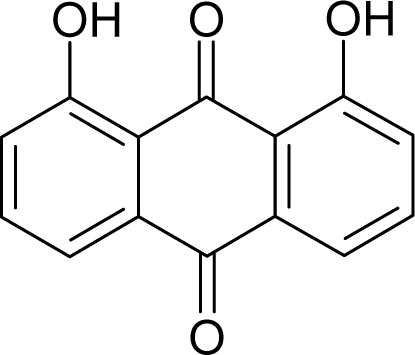 | Chrysazin (Danthron) C14H8O4 (117-10-2) | 245 3.61 | 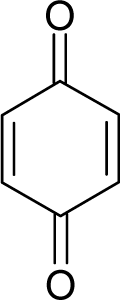 | 1.87 1.52 | 24.44 10.8 | −68162.28125 −31325.97266 | 5.4079765 6.3137325 | 3.9369375 4.6074975 |
| 3. | 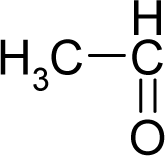 | Acetaldehyde C2H4O (75-07-0) | 153 3.82 | 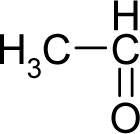 | −0.58 +0.58 | 4.53 4.53 | −13662.00781 −13662.00781 | 4.94880425 4.94880425 | 5.75505575 5.75505575 |
| 4. |  | Allyl isothiocyanate C4H5NS (57-06-7) | 96 4.02 |  | 1.17 0.19 | 11.74 6.43 | −20700.27344 −11094.80273 | 4.9388987 5.032356 | 4.2117593 4.346452 |
| 5. |  | Isobutyl nitrite C4H9NO2 (542-56-3) | 54.1 4.27 |  | 1.63 0.38 | 9.96 2.62 | −31363 −17580.39258 | 5.294418075 5.4457523 | 5.263031925 5.3349777 |
| 6. | 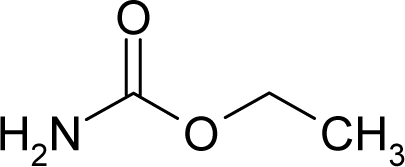 | Urethane C3H7NO2 (51-79-6) | 41.3 4.38 |  | −0.06 −0.44 | 8.35 4.68 | −27989.58203 −21103.80273 | 4.573154 4.474373 | 5.741656 5.576267 |
| 7. | 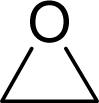 | Ethylene oxide C2H4O (75-21-8) | 21.3 4.67 | 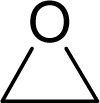 | −0.16 −0.16 | 4.31 4.31 | −13626.54297 −13626.54297 | 4.4747555 4.4747555 | 6.8617045 6.8617045 |
| 8. | 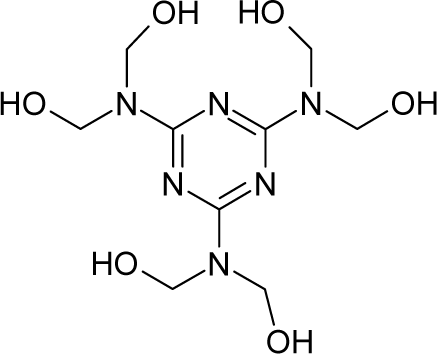 | Hexa(hydroxymethyl)mela mine C9H18N6O6 (531-18-0) | 10.2 4.99 | 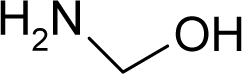 | 1.96 0.08 | 27.19 5.08 | −108827.09 −14382.44336 | 4.05956015 3.782247 | 4.50969485 7.116263 |
| 9. |  | 1,2-Dichloroethane C2H4Cl2 (107-06-2) | 8.04 5.09 | 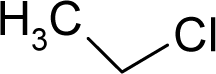 | 1.59 1.22 | 8.3 6.37 | −21506.41406 −14559.67578 | 5.0714835 4.586889 | 5.6050665 5.823301 |
| 10. |  | Tris(2,3-dibromopropyl) phosphate C9H15Br6O4P (126-72-7) | 3.83 5.42 |  | 5.37 3.73 | 35.91 25.15 | −108827.09 −82903.73 | 5.6512295 5.3925243 | 4.5231705 4.63098575 |
| 11. |  | Beta-Propiolactone C3H4O2 (57-57-8) | 1.46 5.84 | 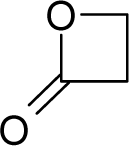 | −0.25 −0.25 | 6.23 6.23 | −23148.73047 −23148.73047 | 5.2018966 5.2018966 | 6.0842834 6.0842834 |
| 12. |  | Chlorambucil C14H19Cl2NO2 (305-03-3) | 0.896 6.048 | 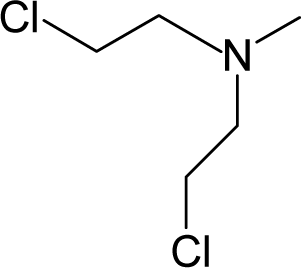 | 4.14 1.2 | 31.04 13.32 | −76933.42969 −32495.47656 | 4.350258535 4.4258064 | 4.405313465 5.31470165 |
| 13. |  | Azaserine C5H7N3O4 (115-02-6) | 0.793 6.10 | 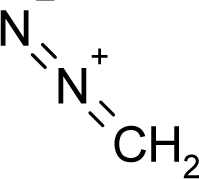 | −1.03 −0.04 | 14.25 4.11 | −54439.625 −10877.61426 | 5.2656847 4.431137 | 4.7215543 4.794258 |
| 14. |  | Dacarbazine C6H10N6O (4342-03-4) | 0.71 6.15 |  | −0.92 0.48 | 17.95 4.18 | −49126.58594 −12249.66113 | 4.9880568 4.2572947 | 4.1820822 5.06465235 |
| 15. | 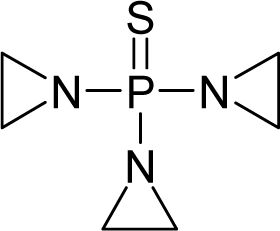 | Thiotepa (Tris(aziridinyl)-phosphine sulfide) C6H12N3PS (52-24-4) | 0.164 6.789 | 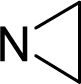 | 0.54 −0.38 | 17.63 5.02 | −38905.46484 −10956.04395 | 5.2831755 3.5910075 | 3.8071835 6.3290665 |
| 16. |  | Aflatoxin-B1 C17H12O6 (1162-65-8) | 0.0032 8.49 | 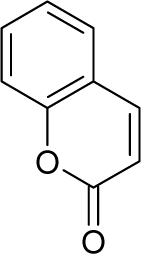 | 0.99 1.82 | 29.86 15.7 | −91307.82331 −40247.55469 | 5.3273625 5.2410253 | 3.9567405 4.2472247 |
| 17. |  | 2,3,7,8-Tetrachlorodibenzo-p-dioxin C12H4Cl4O2 (1746-01-6) | 0.0000457 10.34 | 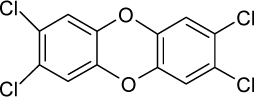 | 4.93 4.93 | 28.31 28.31 | −76933.75 −76933.75 | 4.7914412 4.7914412 | 4.0075488 4.0075488 |
| 18. | 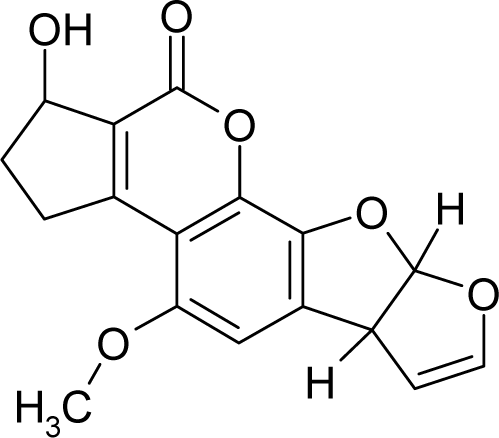 | Aflatoxicol C17H14O6 (29611-03-8) | 0.00247 8.61 | 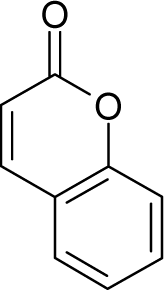 | 0.46 1.82 | 30.41 15.7 | −91979.58594 −40247.55469 | 5.140259 5.2410253 | 3.945276 4.2472247 |
| 19. | 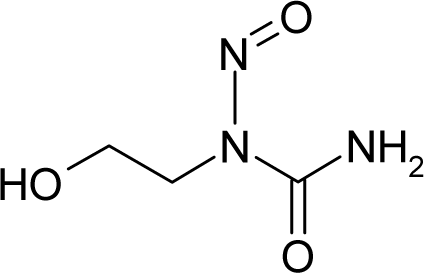 | 1-(2-Hydroxyethyl)-1-nitrosourea C3H7N3O3 (13743-07-2) | 0.244 6.61 | 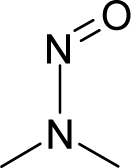 | −0.95 0.37 | 10.92 2.55 | −42184.19141 −14202.18945 | 5.42904375 5.8565512 | 5.08170625 6.2971588 |
| 20. | 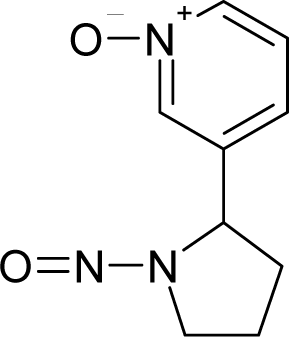 | N'-Nitrosonornicotine-1-N-oxide C9H11N3O2 (78246-24-9) | 0.876 6.06 | 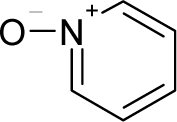 | 0.25 0.12 | 19.48 10.35 | −53174.95313 −25900.39453 | 5.04527 4.9295405 | 4.273811 4.3386305 |
| 21. |  | Benzo(a)pyrene C20H12 (50-32-8) | 0.956 6.02 |  | 5.37 5.37 | 36.04 36.04 | −58881.02734 −58881.02734 | 4.631374 4.631374 | 3.410258 3.410258 |
| 22. |  | 2-Acetylaminofluorene C15H13NO (53-96-3) | 1.22 5.91 |  | 2.61 2.61 | 26.26 26.26 | −56110.60547 −56110.60547 | 4.38615285 4.38615285 | 4.02819215 4.02819215 |
| 23. |  | 1,2-Dibromoethane C2H4Br2 (106-93-4) | 1.52 5.82 |  | 1.71 1.29 | 9.7 7.07 | −28203.0625 −15407.94336 | 6.1527065 5.5320367 | 5.0695035 5.37857335 |
| 24. |  | Michler's ketone C17H20N2O (90-94-8) | 5.64 5.25 |  | 3.8 2.31 | 19.85 15.46 | −67801.28125 −29500.11719 | 4.3453716 3.6634669 | 4.1924714 4.2943161 |
| 25. |  | Ethylene thiourea (ETU) C3H6N2S (96-45-7) | 8.13 5.09 | 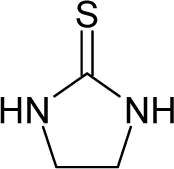 | 0.33 0.33 | 11.45 11.45 | −22095.42578 −22095.42578 | 4.40057075 4.40057075 | 4.20081425 4.20081425 |
| 26. | 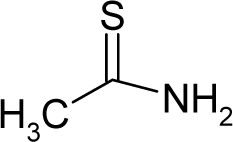 | Thioacetamide C2H5NS (62-55-5) | 11.5 4.94 | 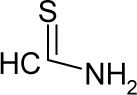 | −0.21 −0.42 | 9.04 7.21 | −15263.96289 −11813.05762 | 4.72959049 4.7550568 | 3.99513951 4.0219202 |
| 27. | 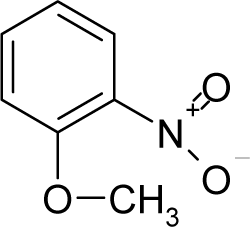 | o-Nitroanisole C7H7NO3 (91-23-6) | 15.6 4.81 | 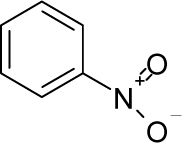 | −0.18 0.07 | 14.75 12.28 | −45613.03906 −35381.23828 | 5.5631575 5.8686355 | 4.3657605 4.7339745 |
| 28. |  | 2-Aminodipyrido[1,2-a:3`,2`-d]imidazole (Glu-P-2) C10H8N4 (67730-10-3) | 42.3 4.37 | 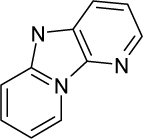 | 2.35 2.9 | 20.73 19.38 | −45103.06641 −40998.30859 | 4.5267029 4.7452532 | 3.7506371 3.87575785 |
| 29. | 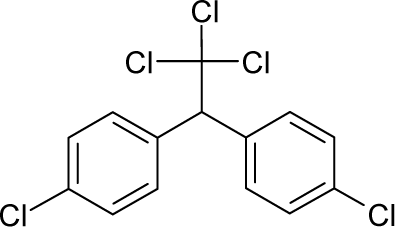 | Dichlorodiphenyltrichloroet hane (DDT) C14H9Cl5 (50-29-3) | 84.7 4.07 | 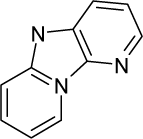 | 6.39 4.92 | 33.4 25.23 | −77956.60156 −52871.28516 | 4.95182645 5.0230205 | 4.50488155 3.2895935 |
| 30. |  | p-Cresidine C8H11NO (120-71-8) | 98 4.01 |  | 1.48 1.26 | 16.09 11.79 | −36280.75391 −22612.99212 | 3.9300665 3.7259962 | 4.3473585 4.3413348 |
| 31. |  | Ethyl 2-(4-chlorophenoxy)-2-methylpropionate (Clofibrate) C12H15Cl O3 (637-07-0) | 169 3.77 |  | 2.97 2.56 | 24.73 12.36 | −65740.6875 −25464.87109 | 4.49111609 4.6624658 | 4.53578491 4.72527225 |
| 32. |  | Vinyl acetate C4H6O2 (108-05-4) | 341 3.47 | 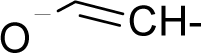 | −0.01 1.28 | 8.65 3.98 | −26598.12305 −12920.42871 | 4.6849081 4.5472153 | 5.2657279 4.91445575 |
| 33. | 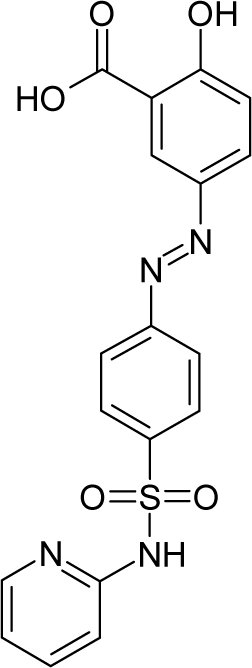 | Salicylazosulfapyridine C18H14N4O5S (599-79-1) | 1590 2.799 |  | 4.54 4.35 | 36.79 22.15 | −107222.1719 −43772.44922 | 5.209331 5.0515378 | 3.898064 4.2222632 |
| No. | Full Molecule: Chemical Structure | Full Molecule: Name Formula (CASRN) | Toxicity: TD50 Activity: A = Log[1/TD50] | Structural Alert (SA) | LogP: Full Molecules Structural Alert | POL [Ǻ3]: Full Molecules Structural Alert | Etot [kcal/mol]: Full Molecules Structural Alert | χ[eV]: Full Molecules Structural Alert | η[eV]: Full Molecules Structural Alert |
|---|---|---|---|---|---|---|---|---|---|
| 34. |  | Phenacetin C10H13NO2 (62-44-2) | 1250 2.90 | 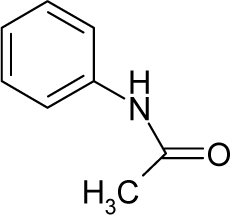 | 0.99 −0.03 | 19.85 15.73 | −49230.08203 −36279.96484 | 4.063315 4.1985829 | 4.307675 4.3648181 |
| 35. |  | Dimethylvinyl chloride (DMVC) C4H7Cl (513-37-1) | 31.8 4.498 | 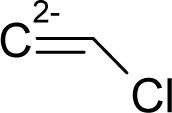 | 1.51 0.45 | 9.85 4.05 | −20725.60325 −13014.37793 | 4.32596855 5.6212095 | 4.98083445 4.2918295 |
| 36. | 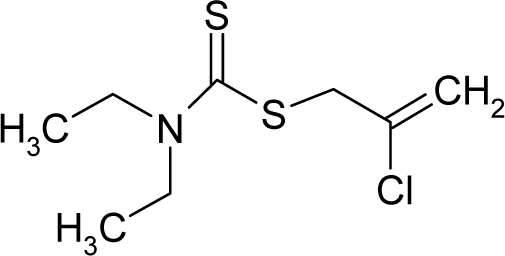 | Sulfallate C8H14ClNS2 (95-06-7) | 26.1 4.58 |  | 2.73 0.62 | 24.79 10.21 | −46435.69922 −16106.21777 | 4.8447835 5.093712 | 3.8753115 3.905288 |
| 37. | 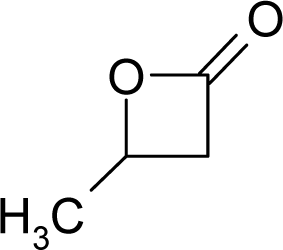 | beta-Butyrolactone C4H6O2 (3068-88-0) | 13.8 4.86 | 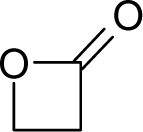 | 0.17 −0.25 | 8.06 6.23 | −26599.55273 −23148.73047 | 5.1344426 5.2020294 | 6.0826774 6.0841706 |
| 38. | 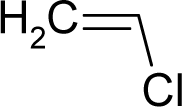 | Vinyl Chloride C2H3Cl (75-01-4) | 6.11 5.21 | 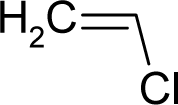 | 1.01 1.01 | 6.18 6.18 | −13820.70898 −13820.70898 | 4.56666095 4.56666095 | 5.27117005 5.27117005 |
| 39. | 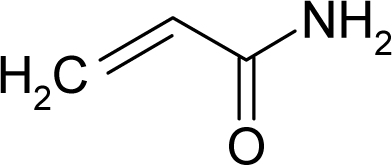 | Acrylamide C3H5NO (79-06-1) | 3.75 5.43 | 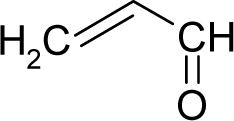 | −0.28 0.17 | 7.52 6.17 | −20478.92578 −16372.65625 | 4.77457395 5.4404992 | 4.91861805 5.25305085 |
| 40. | 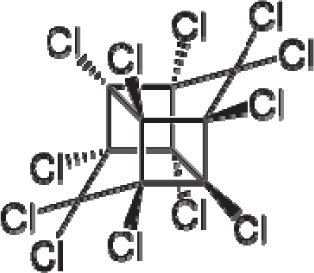 | Mirex C10Cl12 (2385-85-5) | 1.77 5.75 | 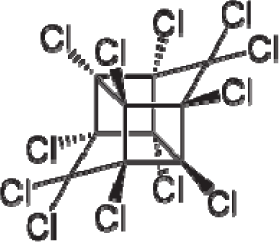 | 6.41 6.41 | 38.39 38.39 | −114919.4688 −114919.4688 | 5.27780275 5.2778028 | 5.22349725 5.22349725 |
| 41. | 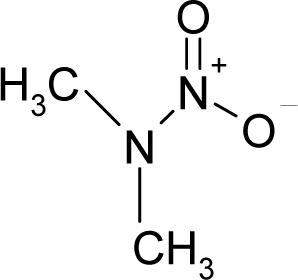 | Dimethylnitramine C2H6N2O2 (4164-28-7) | 0.547 6.26 | 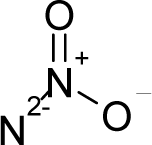 | 0.97 1.32 | 7.64 3.18 | −28551.91406 −20856.80078 | 5.288693895 7.5671675 | 5.374516105 4.9321725 |
| 42. | 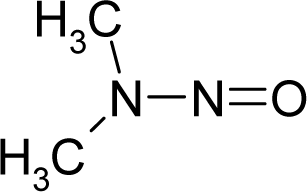 | N-Nitrosodimethylami ne C2H6N2O (62-75-9) | 0.0959 7.02 | 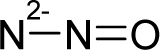 | 0.01 0.37 | 7.01 2.55 | −21802.08203 −14202.18945 | 4.6046239 5.8565512 | 5.1639551 6.2971588 |
| 43. | 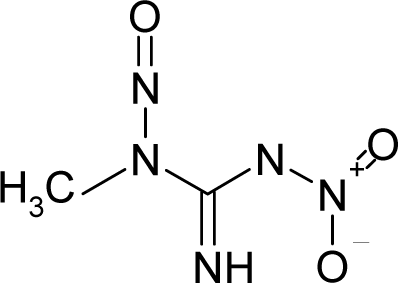 | N-Methyl-N`-nitro-N-nitrosoguanidine (1-Methyl-3-nitro-1-nitroso-guanidine) C2H5N5O3 (70-25-7) | 0.803 6.1 |  | 1.5 0.84 | 11.13 3.97 | −46112.81641 −21661.2832 | 5.475207 5.8694368 | 4.654173 5.785463245 |
| 44. |  | 1-Phenyl-3,3-dimethyltriazene C8H11N3 (7227-91-0) | 2.31 5.64 | 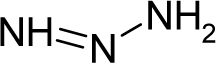 | 2.53 0.48 | 17.51 4.18 | −36944.65625 −12249.66113 | 4.65555575 4.2572947 | 4.28693125 5.06465235 |
| 45. | 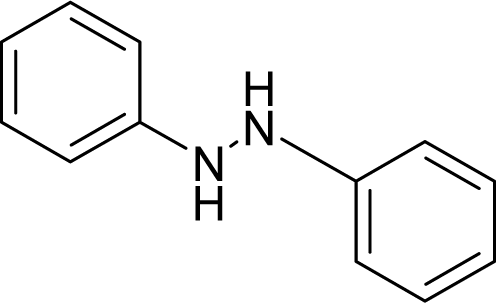 | Hydrazobenzene C12H12N2 (122-66-7) | 5.59 5.25 | 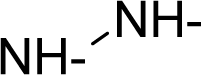 | 3.4 −0.65 | 22.8 2.83 | −44481.07422 −8164.909668 | 3.65518885 4.5593574 | 3.99645815 5.0568536 |
| 46. |  | 1'-Acetoxysafrole C12H12O4 (34627-78-6) | 25 4.6 |  | −0.11 1.28 | 22.47 3.98 | −64108.48047 −12920.42871 | 4.516422835 4.5472153 | 4.517086165 4.91445575 |
| 47. |  | o-Nitrosotoluene C7H7NO (611-23-4) | 50.7 4.29 | 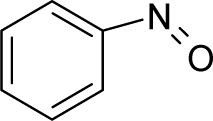 | 2.29 1.82 | 13.48 11.65 | −32074.53516 −28624.52734 | 5.20234765 5.2781928 | 4.40152935 4.43740625 |
| 48. |  | p-Nitrosodiphenyl amine C12H10N2O (156-10-5) | 201 3.7 |  | 3.07 1.82 | 22.66 11.65 | −50526.36328 −28624.52734 | 4.57337225 5.2781928 | 3.74357475 4.43740625 |
| 49. |  | 1,4-Dichlorobenzene (p-dichlorobenzene) C6H4Cl2 (106-46-7) | 644 3.19 |  | 3.08 3.08 | 14.29 14.29 | −32415.54297 −32415.54297 | 4.73892295 4.73892295 | 4.49613405 4.49613405 |
| Variable | QSAR Model | Pred. Activity | R |
|---|---|---|---|
| χSA | 5.62 − 0.07χSA | 0.026 | |
| ηSA | 6.016 − 0.149ηSA | 0.086 | |
| POLSA | 4.677 + 0.05POLSA | 0.27 | |
| LogPSA | 5.066 + 0.159LogPSA | 0.16 | |
| 4.402 − 0.00031 | 0.35 | ||
| χSA, ηSA | 6.51 − 0.09χSA − 0.156ηSA | 0.093 | |
| χSA, POLSA | 5.1 − 0.08χSA + 0.5POLSA | 0.27 | |
| χSA, LogPSA | 5.54 − 0.1χSA + 0.16LogPSA | 0.16 | |
| χSA, | 5.58 − 0.255χSA − 0.000032 | 0.371 | |
| ηSA, POLSA | 2.43 + 0.38ηSA + 0.08POLSA | 0.316 | |
| ηSA, LogPSA | 4.75 + 0.05ηSA + 0.179LogPSA | 0.167 | |
| ηSA, | 2.6 + 0.314ηSA − 0.00004 | 0.38 | |
| POLSA, LogPSA | 4.36 + 0.12POLSA − 0.44LogPSA | 0.337 | |
| POLSA, | 4.38 − 0.0625POLSA − 0.00005 | 0.382 | |
| , LogPSA | 4.09 − 0.412LogPSA − 0.00006 | 0.4305 | |
| χSA, ηSA, POLSA | 2.62 − 0.035χSA + 0.382ηSA + 0.08POLSA | 0.31 | |
| χSA, ηSA, LogPSA | 5.25 − 0.095χSA + 0.049ηSA + 0.179LogPSA | 0.17 | |
| χSA, ηSA, | 3.78 − 0.254χSA + 0.3141ηSA − 0.00004 | 0.399 | |
| χSA, POLSA, LogPSA | 4.52 − 0.0325χSA + 0.12POLSA − 0.439LogPSA | 0.337 | |
| χSA, POLSA, | 6.46 − 0.45χSA − 0.092POLSA − 0.0007 | 0.4141 | |
| χSA, LogPSA, | 5.75 − 0.36χSA − 0.45LogPSA − 0.00006 | 0.451 | |
| ηSA, POLSA, LogPSA | 2.05 + 0.39ηSA + 0.16POLSA − 0.449LogPSA | 0.373 | |
| ηSA, POLSA, | 3.09 + 0.22ηSA − 0.034POLSA − 0.00005 | 0.392 | |
| ηSA, LogPSA, | 3.23 + 0.156ηSA − 0.368LogPSA − 0.0006 | 0.436 | |
| POLSA, LogPSA, | 4.07 + 0.015POLSA − 0.45LogPSA − 0.000057 | 0.4312 | |
| Variable | QSAR Model | Pred. Activity | R |
|---|---|---|---|
| χM | 2.18 + 0.63χM | 0.187 | |
| χM, | − 65.66 + 12.1 + 1.41χM | 0.295 | |
| ηM | 7.13 − 0.405 ηM | 0.192 | |
| ηM, | 11.38 − 0731 − 0.49 ηM | 0.198 | |
| POLM | 4.71 + 0.029POLM | 0.18 | |
| POLM, | − 0.438 + 1.1 − 0.006POLM | 0.275 | |
| LogPM | 5.29 − 0.0007LogPM | 0.009 | |
| LogPM, | − 13.64 + 3.71 − 0.421LogPM | 0.325 | |
| 4.67 − 0.00001 | 0.203 | ||
| , | − 0.05 + 1.01 + 0.000001 | 0.358 | |
| χM, ηM | 3.92 + 0.68χM − 0.43ηM | 0.278 | |
| χM, ηM, | 0.015 + 0.73χM − 0.35ηM + 0.62 | 0.28 | |
| χM, POLM | 1.36 + 0.67χM + 0.031POLM | 0.27 | |
| χM, POLM, | − 6.009 + 0.86χM − 0.012POLM + 1.38 | 0.373 | |
| χM, LogPM | 2.12 + 0.639χM + 0.013LogPM | 0.188 | |
| χM, LogPM, | − 19.46 + 0.81χM − 0.44LogPM + 4.05 | 0.409 | |
| χM, | 1.64 + 0.62χM − 0.00001 | 0.27 | |
| χM, , | − 4.81 + 0.83χM + 0.00003 + 1.16 | 0.443 | |
| ηM, POLM | 6.18 − 0.264ηM + 0.015POLM | 0.204 | |
| ηM, POLM, | 1.47 − 0.64ηM − 0.05POLM + 1.48 | 0.378 | |
| ηM, LogPM | 7.66 − 0.49ηM − 0.08LogPM | 0.213 | |
| ηM, LogPM, | −12.17 − 0.52ηM − 0.531LogPM + 3.919 | 0.403 | |
| ηM, | 5.993 − 0.24ηM − 0.000008 | 0.226 | |
| ηM, , | 1.585 − 0.442ηM + 0.00001 + 1.182 | 0.426 | |
| POLM, LogPM | 4.339 + 0.72POLM − 0.279LogPM | 0.29 | |
| POLM, LogPM, | − 0.81 + 0.04POLM − 0.31LogPM + 1.08 | 0.422 | |
| POLM, | 4.67 − 0.00097POLM − 0.00001 | 0.203 | |
| POLM, , | − 0.299 − 0.0304POLM − 0.00008 + 1.091 | 0.3907 | |
| , LogPM | 4.578 − 0.162LogPM − 0.000018 | 0.26 | |
| , LogPM, | − 0.603 − 0.2337LogPM − 0.00009 + 1.093 | 0.488 | |
| χM, ηM, POLM | 2.89 + 0.686χM − 0.28ηM + 0.016POLM | 0.288 | |
| χM, ηM, POLM, | − 3.19 + 0.86χM − 0.696ηM − 0.06POLM + 1.62 | 0.452 | |
| χM, ηM, LogPM | 4.458 + 0.65χM − 0.5ηM − 0.06LogPM | 0.287 | |
| χM, ηM, LogPM, | − 16.899 + 0.794χM − 0.49ηM − 0.52LogPM + 4.054 | 0.462 | |
| χM, ηM, | 3.016 + 0.65χM − 0.293ηM − 0.00007 | 0.298 | |
| χM, ηM, , | − 2.689 + 0.841χM − 0.473ηM + 0.00001 + 1.256 | 0.497 | |
| χM, POLM, LogPM | 1.481 + 0.58 χM + 0.07POLM − 0.2512LogPM | 0.338 | |
| χM, POLM, LogPM, | − 5.593 + 0.81χM + 0.037POLM − 0.281LogPM + 1.254 | 0.483 | |
| χM, POLM, | 1.503 + 0.64χM + 0.012POLM − 0.000007 | 0.276 | |
| χM, POLM, , | − 4.009 + 0.725χM −0.008POLM + 0.00001 + 1.123 | 0.469 | |
| χM, LogPM, | 1.961 + 0.538χM − 0.134LogPM − 0.000017 | 0.304 | |
| χM, LogPM, , | − 4.251 + 0.68χM − 0.219LogPM − 0.00007 + 1.166 | 0.552 | |
| ηM, POLM, LogPM | 4.87 − 0.093ηM + 0.066POLM − 0.269LogPM | 0.294 | |
| ηM, POLM, LogPM, | − 0.253 − 0.153ηM + 0.033POLM − 0.321LogPM + 1.156 | 0.466 | |
| ηM, POLM, | 6.84 − 0.39 ηM − 0.368POLM − 0.00018 | 0.239 | |
| ηM, POLM, , | 3.415 − 1.08ηM − 0.13POLM − 0.00002 + 1.598 | 0.507 | |
| ηM, LogPM, | 6.176 − 0.303ηM − 0.177LogPM − 0.000015 | 0.287 | |
| ηM, LogPM, , | 1.433 − 0.449ηM − 0.261LogPM − 0.00003 + 1.172 | 0.525 | |
| POLM, LogPM, | 4.341 + 0.06POLM − 0.273LogPM − 0.000002 | 0.298 | |
| POLM, LogPM, , | − 0.598 + 0.031POLM − 0.288LogPM − 0.000002 + 1.065 | 0.495 | |
| Variable | QSAR Model | Pred. Activity | R |
|---|---|---|---|
| 5.2856 + | 0.999 | ||
| 5.2856 + | 0.996 | ||
| 5.2856 + | 0.961 | ||
| 5.2856 + | 0.986 | ||
| 5.2856 + | 0.933 | ||
| , | 5.2856 + 0.886 + 0.114 | 0.999 | |
| , | 5.2856 + 0.987 + 0.013 | 0.999 | |
| , | 5.2856 + 0.963 + 0.037 | 0.999 | |
| , | 5.2856 + 0.98 + 0.022 | 0.999 | |
| , | 5.2856 + 1.194 − 0.206 | 0.997 | |
| , | 5.2856 + 1.101 − 0.103 | 0.996 | |
| , | 5.2856 + 1.105 − 0.118 | 0.996 | |
| , | 5.2856 − 0.709 + 1.684 | 0.991 | |
| , | 5.2856 + 1.642 − 0.670 | 0.966 | |
| , | 5.2856 + 1.395 − 0.430 | 0.991 | |
| , , | 5.2856 + 0.852 + 0.176 − 0.030 | 0.999 | |
| , , | 5.2856 + 0.884 + 0.123 − 0.007 | 0.999 | |
| , , | 5.2856 + 0.892 + 0.104 + 0.004 | 0.999 | |
| , , | 5.2856 + 0.941 − 0.045 + 0.102 | 0.999 | |
| , , | 5.2856 + 1.005 − 0.081 + 0.080 | 0.999 | |
| , , | 5.2856 + 0.983 − 0.003 + 0.023 | 0.999 | |
| , , | 5.2856 + 0.951 − 0.418 + 0.450 | 0.997 | |
| , , | 5.2856 + 1.212 − 0.290 + 0.067 | 0.997 | |
| , , | 5.2856 + 0.955 + 0.213 − 0.185 | 0.997 | |
| , , | 5.2856 − 0.423 + 1.617 − 0.227 | 0.991 | |
| , , , | 5.2856 + 0.815 + 0.170 − 0.082 + 0.093 | 0.999 | |
| , , , | 5.2856 + 0.966 − 0.121 + 0.083 + 0.074 | 0.999 | |
| , , , | 5.2856 + 0.966 − 0.461 + 0.422 + 0.038 | 0.999 | |
| , , , | 5.2856 + 0.902 + 0.117 − 0.034 + 0.016 | 0.999 | |
| , , , | 5.2856 + 0.858 + 0.193 − 0.138 + 0.088 | 0.999 | |
| , , , , | 5.2856 + 0.830 + 0.188 − 0.171 + 0.070 + 0.083 | 0.999 | |
| 5.2856 + | 0.99 | ||
| 5.2856 + | 0.96 | ||
| 5.2856 + | 0.985 | ||
| 5.2856 + | 0.928 | ||
| 5.2856 + | 0.985 | ||
| 5.2856 + | 0.985 | ||
| 5.2856 + | 0.92 | ||
| 5.2856 + | 0.93 | ||
| 5.2856 + | 0.923 | ||
| 5.2856 + | 0.902 | ||
| 5.2856 + 1.200 − 0.212 | 0.996 | ||
| 5.2856 + 1.0003 − 0.0003 | 0.995 | ||
| 5.2856 + 0.9991 + 0.0009 | 0.995 | ||
| 5.2856 − 0.490 + 1.471 | 0.988 | ||
| 5.2856 + 1.033 − 0.036 | 0.96 | ||
| 5.2856 + 0.945 + 0.062 | 0.961 | ||
| 5.2856 + 1.319 − 0.339 | 0.987 | ||
| 5.2856 + 1.148 − 0.167 | 0.986 | ||
| 5.2856 + 1.086 − 0.097 | 0.985 | ||
| 5.2856 − 0.084 + 1.081 | 0.948 | ||
| 5.2856 − 0.388 + 1.353 | 0.99 | ||
| 5.2856 + 0.308 + 0.706 | 0.944 | ||
| 5.2856 + 0.870 + 0.145 | 0.949 | ||
| 5.2856 + 1.155 − 0.173 | 0.987 | ||
| 5.2856 + 0.342 + 0.685 | 0.947 | ||
| 5.2856 + | 0.948 | ||
| 5.2856 + | 0.985 | ||
| 5.2856 + | 0.916 | ||
| 5.2856 + | 0.941 | ||
| 5.2856 + | 0.91 | ||
| 5.2856 + | 0.89 | ||
| 5.2856 + | 0.927 | ||
| 5.2856 + | 0.919 | ||
| 5.2856 + | 0.899 | ||
| 5.2856 + | 0.902 | ||
| No. Crt. | Variabile | QSAR Model | Rtrial | Rtest |
|---|---|---|---|---|
| Ia | χSA, | ARASA = 158.7 − 34χSA − 0.003 | 0.368 | 0.168 |
| ASA = 5.58 − 0.255χSA − 0.000032 | 0.371 | 0.127 | ||
| Ib | ηSA, POLSA | ARASA = −77.866 + 14.75ηSA + 0.833POLSA | 0.078 | 0.505 |
| ASA = 2.43 + 0.38ηSA + 0.08POLSA | 0.316 | 0.043 | ||
| Ic | ηSA, LogPSA | ARASA = −408.2 + 82ηSA + 8LogPSA | 0.063 | 0.725 |
| ASA = 4.75 + 0.05ηSA + 0.179LogPSA | 0.167 | 0.052 | ||
| Id | ηSA, | ARASA = −64.769 + 12.615ηSA − 0.0023 | 0.384 | 0.087 |
| ASA = 2.6 + 0.314ηSA − 0.00004 | 0.38 | 0.131 | ||
| Ie | POLSA, LogPSA | ARASA = 2.76 + 1.4POLSA − 10.68LogPSA | 0.040 | 0.222 |
| ASA = 4.36 + 0.12POLSA−0.44LogPSA | 0.337 | 0.357 | ||
| If | POLSA, | ARASA = 19.821 − 2.928POLSA − 0.0071 | 0.369 | 0.015 |
| ASA = 4.38 − 0.0625POLSA − 0.00005 | 0.382 | 0.132 | ||
| Ig | LogPSA, | ARASA = 3.142 − 6.314LogPSA − 0.0028 | 0.386 | 0.016 |
| ASA = 4.09 − 0.412LogPSA − 0.00006 | 0.430 | 0.007 | ||
| χSA, POLSA, | ARASA = 84 − 17.5χSA − POLSA − 0.005 | 0.373 | 0.056 | |
| ASA = 6.46 − 0.45χSA − 0.092POLSA − 0.0007 | 0.414 | 0.018 | ||
| χSA, LogPSA, | ARASA = 108 − 22.66χSA − 0.133LogPSA − 0.0023 | 0.371 | 0.149 | |
| ASA = 5.75 − 0.36χSA − 0.45LogPSA − 0.00006 | 0.451 | 0.136 | ||
| χSA, ηSA, POLSA | ARASA = −210 + 29.5χSA + 13ηSA + 0.5POLSA | 0.018 | 0.592 | |
| ASA = 2.62 − 0.035χSA + 0.382ηSA + 0.08POLSA | 0.31 | 0.027 | ||
| ηSA, POLSA, LogPSA | ARASA = −44.705 + 8.294ηSA + 1.176POLSA − 4.467LogPSA | 0.122 | 0.286 | |
| ASA = 2.05 + 0.39ηSA + 0.16POLSA − 0.449LogPSA | 0.373 | 0.112 | ||
| ηSA, POLSA, | ARASA = −85.72 + 16.363ηSA + 1.272POLSA + 0.0018 | 0.304 | 0.178 | |
| ASA = 3.09 + 0.22ηSA − 0.034POLSA − 0.00005 | 0.392 | 0.152 | ||
| ηSA, LogPSA, | ARASA = −42.58 + 8.352ηSA − 1.941LogPSA − 0.0029 | 0.382 | 0.039 | |
| ASA = 3.23 + 0.156ηSA − 0.368LogPSA − 0.0006 | 0.436 | 0.012 | ||
| χSA, POLSA, LogPSA | ARASA = −154 + 32.5χSA + POLSA − 8LogPSA | 0.019 | 0.399 | |
| ASA = 4.52 − 0.0325χSA + 0.12POLSA − 0.439LogPSA | 0.337 | 0.370 | ||
| POLSA, LogPSA, | ARASA = 2.151 + 0.636POLSA − 7.787LogP − 0.0021 | 0.394 | 0.038 | |
| ASA = 4.07 + 0.015POLSA − 0.45LogPSA − 0.000057 | 0.431 | 0.034 | ||
| III1 | χSA, ηSA, POLSA, LogPSA | ARASA = 42 − 2.052χSA − 3.842ηSA + 12.894POLSA − 13.842LogPSA | 0.289 | 0.277 |
| ASA = 1.921 + 0.24χSA + 0.4ηSA + 0.16POLSA − 0.45LogPSA | 0.373 | 0.007 | ||
| III2 | χSA, POLSA, LogPSA, | ARASA = −34.928 − 5.571χSA + 6POLSA − 22.142LogPSA − 0.0006 | 0.372 | 0.296 |
| ASA = 5.894 − 0.39χSA − 0.016POLSA − 0.41LogPSA − 0.00007 | 0.452 | 0.106 | ||
| III3 | ηSA, POLSA, LogPSA, | ARASA = −19.228 + 4.085ηSA + 0.657POLSA − 1.914LogPSA + 0.0002 | 0.21 | 0.184 |
| ASA = 2.72 + 0.23ηSA + 0.04POLSA − 0.45LogPSA − 0.00005 | 0.441 | 0.033 | ||
| III4 | χSA, ηSA, LogPSA, | ARASA = 384 − 63χSA − 17ηSA − 5LogPSA − 0.004 | 0.348 | 0.300 |
| ASA = 4.94 − 0.35χSA + 0.13ηSA − 0.41LogPSA − 0.00006 | 0.455 | 0.066 | ||
| III5 | χSA, ηSA, POLSA, | ARASA = 36.66 + 27.33χSA + 3.666ηSA − 172POLSA − 0.0003 | 0.274 | 0.225 |
| ASA = 5.587 − 0.41χSA + 0.12ηSA − 0.07POLSA − 0.00006 | 0.416 | 0.021 | ||
| Endpoint Paths | ΔRARASA | ΔRASA | ||||
|---|---|---|---|---|---|---|
| Trial | Test | Average | Trial | Test | Average | |
| 0.005099 | 0.264847 | 0.134973 | 0.057384 | 0.140089 | 0.098736 | |
| 0.003162 | 0.148222 | 0.075692α | 0.080006 | 0.031320 | 0.055663β | |
| 0.023194 | 0.152190 | 0.087692 | 0.080099 | 0.070576 | 0.075337 | |
| 0.098386 | 0.241588 | 0.169987 | 0.088729 | 0.104890 | 0.09681 | |
| 0.244769 | 0.327055 | 0.285912 | 0.090426 | 0.161375 | 0.125901 | |
| 0.105948 | 0.450693 | 0.278321 | 0.216933 | 0.099201 | 0.158067 | |
| 0.177115 | 0.439092 | 0.308104 | 0.206000 | 0.120933 | 0.163467 | |
| 0.362415 | 0.701156 | 0.531786 | 0.269046 | 0.045177 | 0.157112 | |
| 0.320806 | 0.733973 | 0.52739 | 0.269670 | 0.067201 | 0.168436 | |
| 0.123434 | 0.091197 | 0.107316 | 0.050447 | 0.120838 | 0.085643 | |
| 0.085440 | 0.102420 | 0.09393β | 0.026832 | 0.132672 | 0.079752γ | |
| 0.172011 | 0.152738 | 0.162375 | 0.056222 | 0.120838 | 0.08853 | |
| 0.034058 | 0.265377 | 0.149718 | 0.059135 | 0.130678 | 0.094907 | |
| 0.120282 | 0.120415 | 0.120349γ | 0.077369 | 0.257421 | 0.167395 | |
| 0.431226 | 0.187882 | 0.309554 | 0.094530 | 0.323154 | 0.208842 | |
| 0.114284 | 0.163110 | 0.138697 | 0.050009 | 0.120668 | 0.085339 | |
| 0.185690 | 0.147800 | 0.166745 | 0.050009 | 0.098005 | 0.074007 | |
| 0.027459 | 0.201221 | 0.11434 | 0.021377 | 0.146768 | 0.084073 | |
| 0.172046 | 0.146812 | 0.159429 | 0.007810 | 0.021587 | 0.014699α | |
| 0.034234 | 0.262011 | 0.148123 | 0.019924 | 0.054230 | 0.037077 | |
| 0.184173 | 0.147648 | 0.165911 | 0.010049 | 0.027018 | 0.018534 | |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Putz, M.V.; Ionaşcu, C.; Putz, A.-M.; Ostafe, V. Alert-QSAR. Implications for Electrophilic Theory of Chemical Carcinogenesis. Int. J. Mol. Sci. 2011, 12, 5098-5134. https://doi.org/10.3390/ijms12085098
Putz MV, Ionaşcu C, Putz A-M, Ostafe V. Alert-QSAR. Implications for Electrophilic Theory of Chemical Carcinogenesis. International Journal of Molecular Sciences. 2011; 12(8):5098-5134. https://doi.org/10.3390/ijms12085098
Chicago/Turabian StylePutz, Mihai V., Cosmin Ionaşcu, Ana-Maria Putz, and Vasile Ostafe. 2011. "Alert-QSAR. Implications for Electrophilic Theory of Chemical Carcinogenesis" International Journal of Molecular Sciences 12, no. 8: 5098-5134. https://doi.org/10.3390/ijms12085098
APA StylePutz, M. V., Ionaşcu, C., Putz, A.-M., & Ostafe, V. (2011). Alert-QSAR. Implications for Electrophilic Theory of Chemical Carcinogenesis. International Journal of Molecular Sciences, 12(8), 5098-5134. https://doi.org/10.3390/ijms12085098