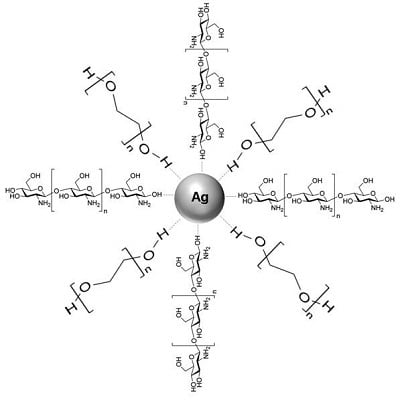
Green Synthesis and Characterization of Silver/Chitosan/Polyethylene Glycol Nanocomposites without any Reducing Agent
Abstract
:1. Introduction
2. Results and Discussion
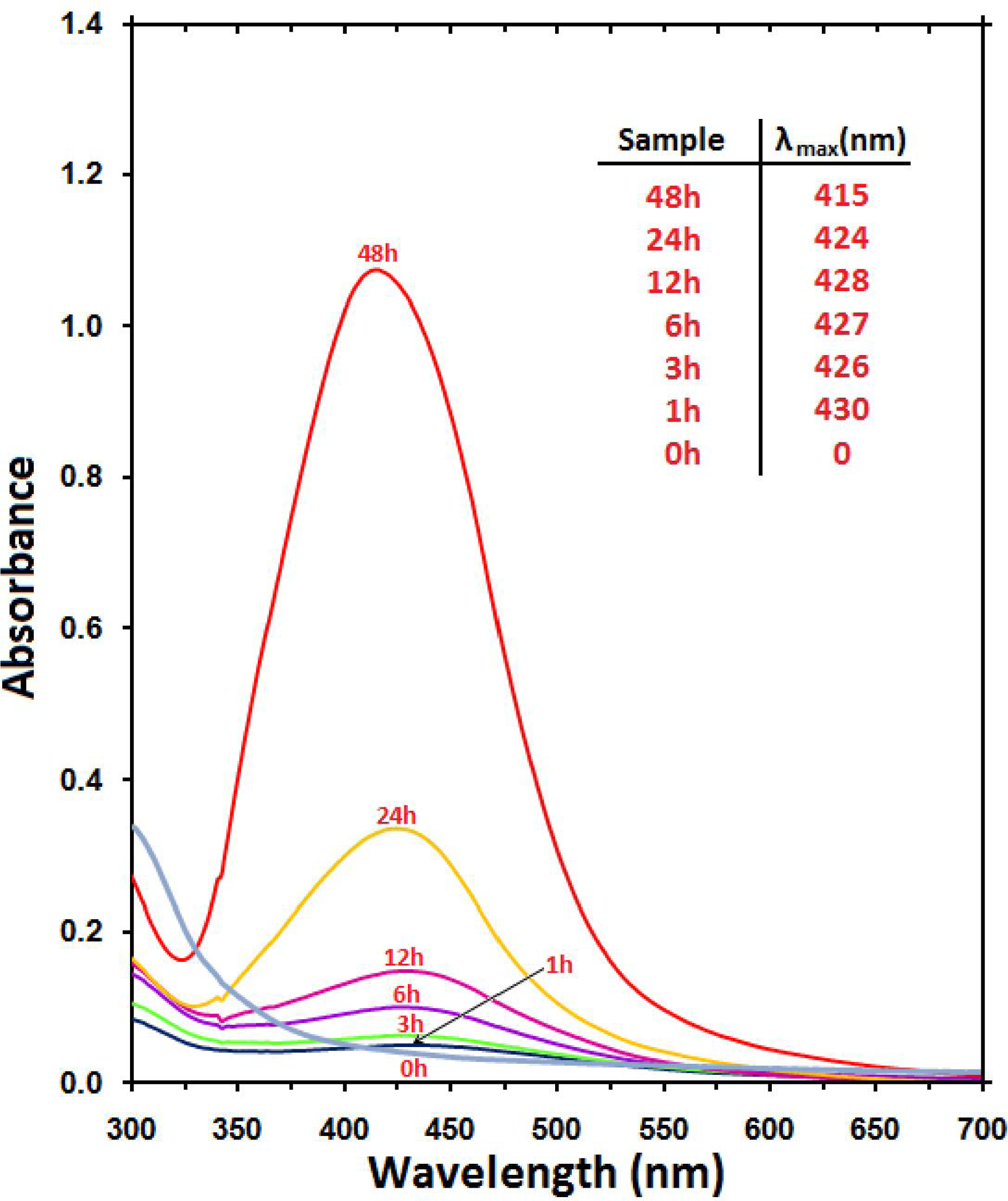
2.1. Optical Properties
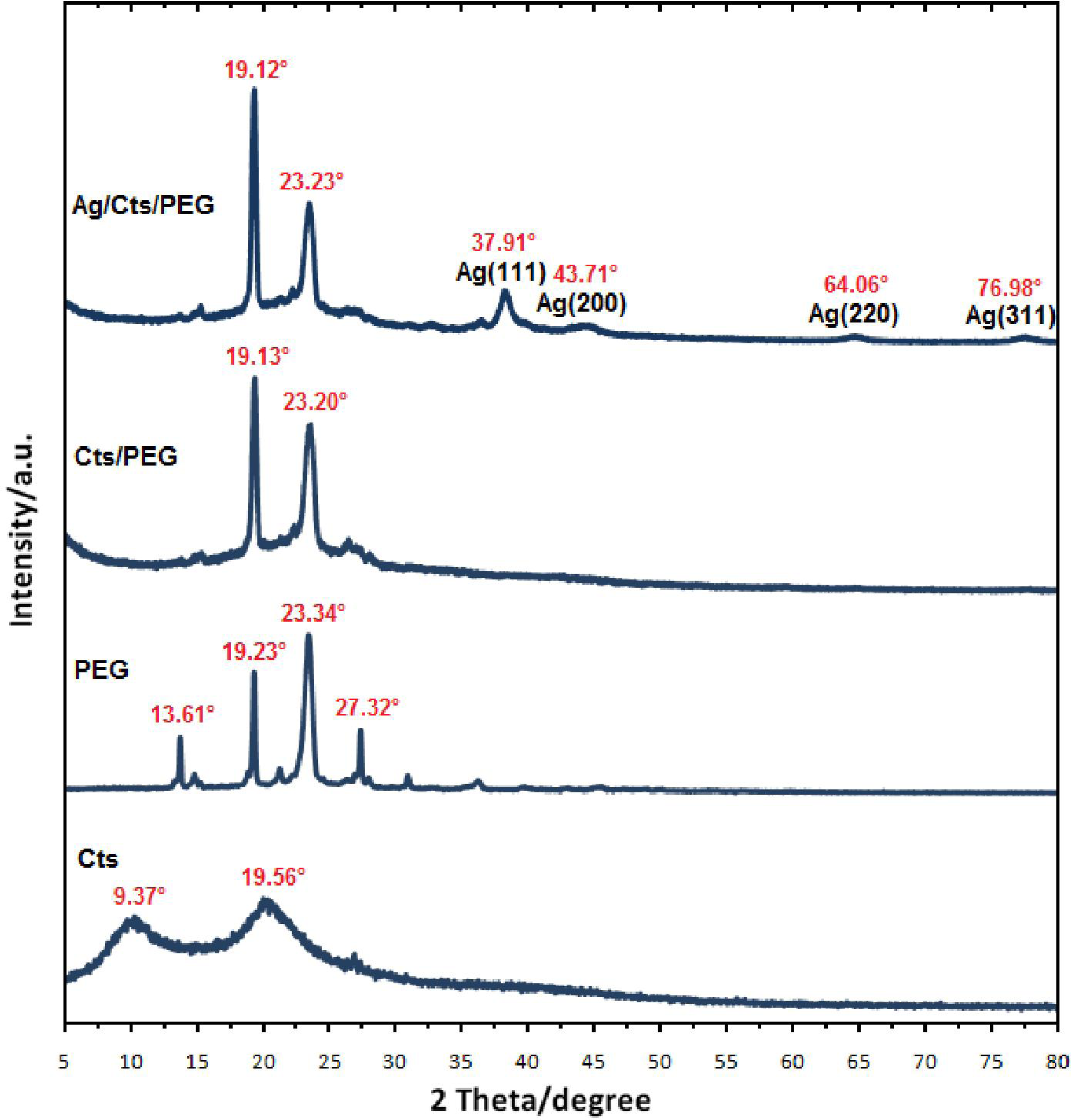
2.2. X-ray Diffraction Characterization
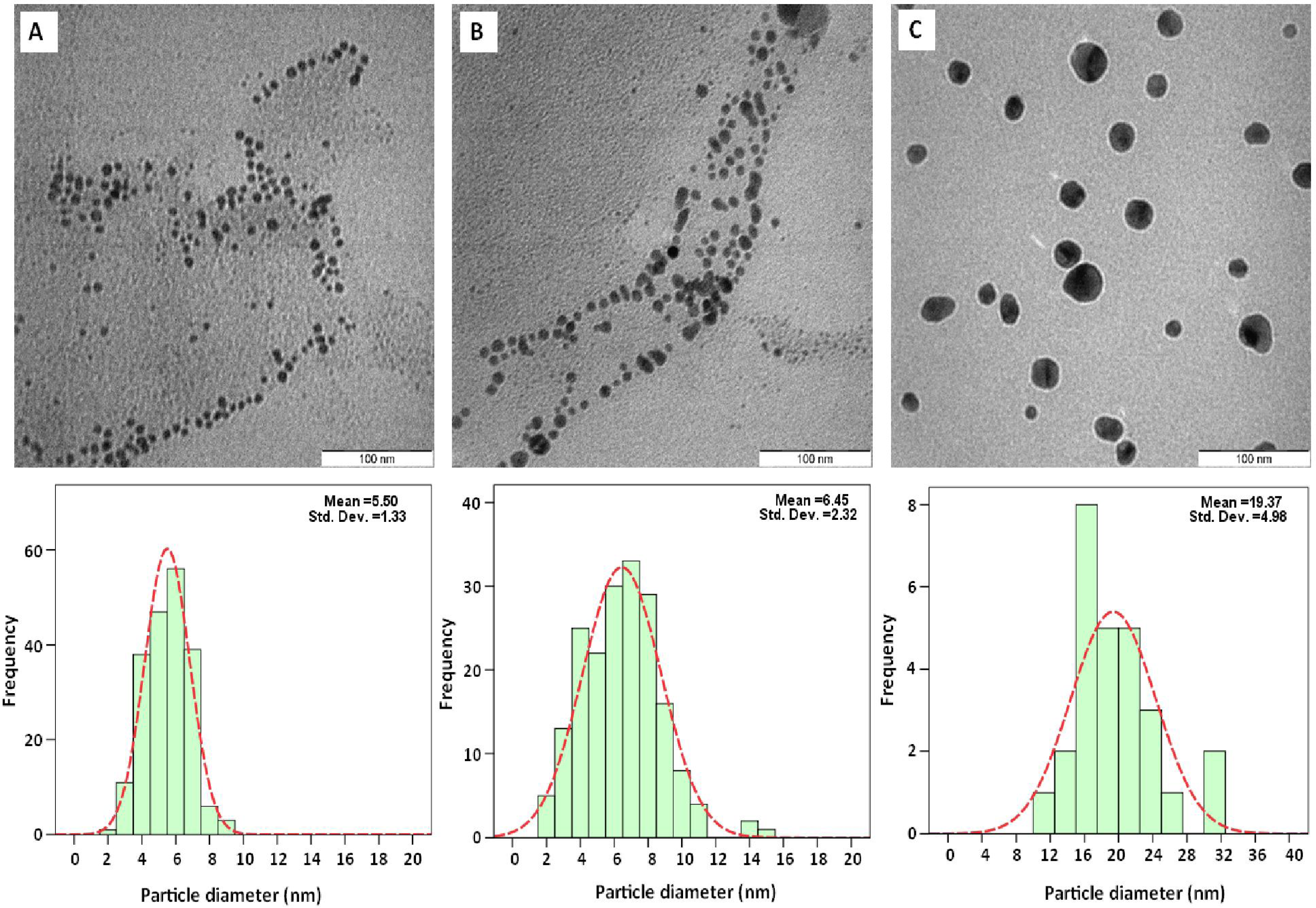
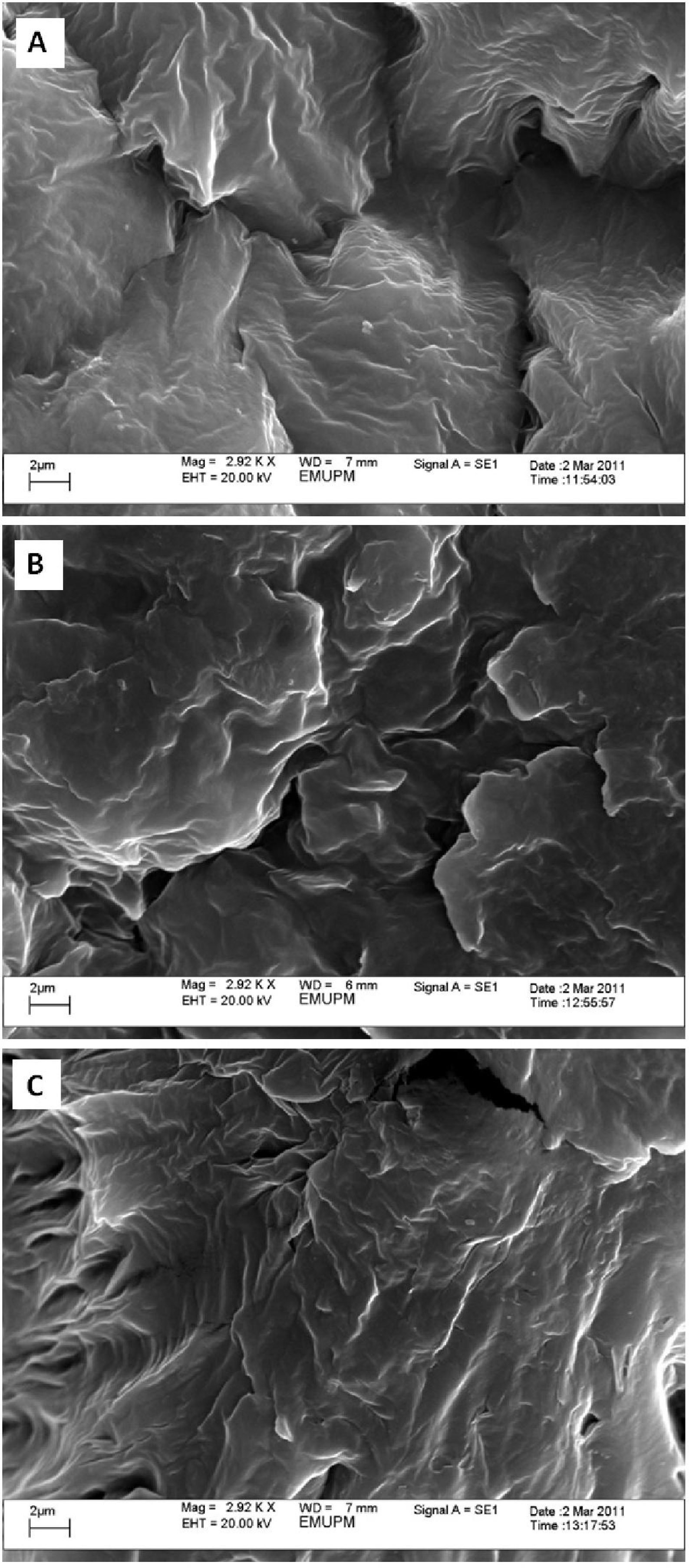
2.3. Electron Microscopic Analysis
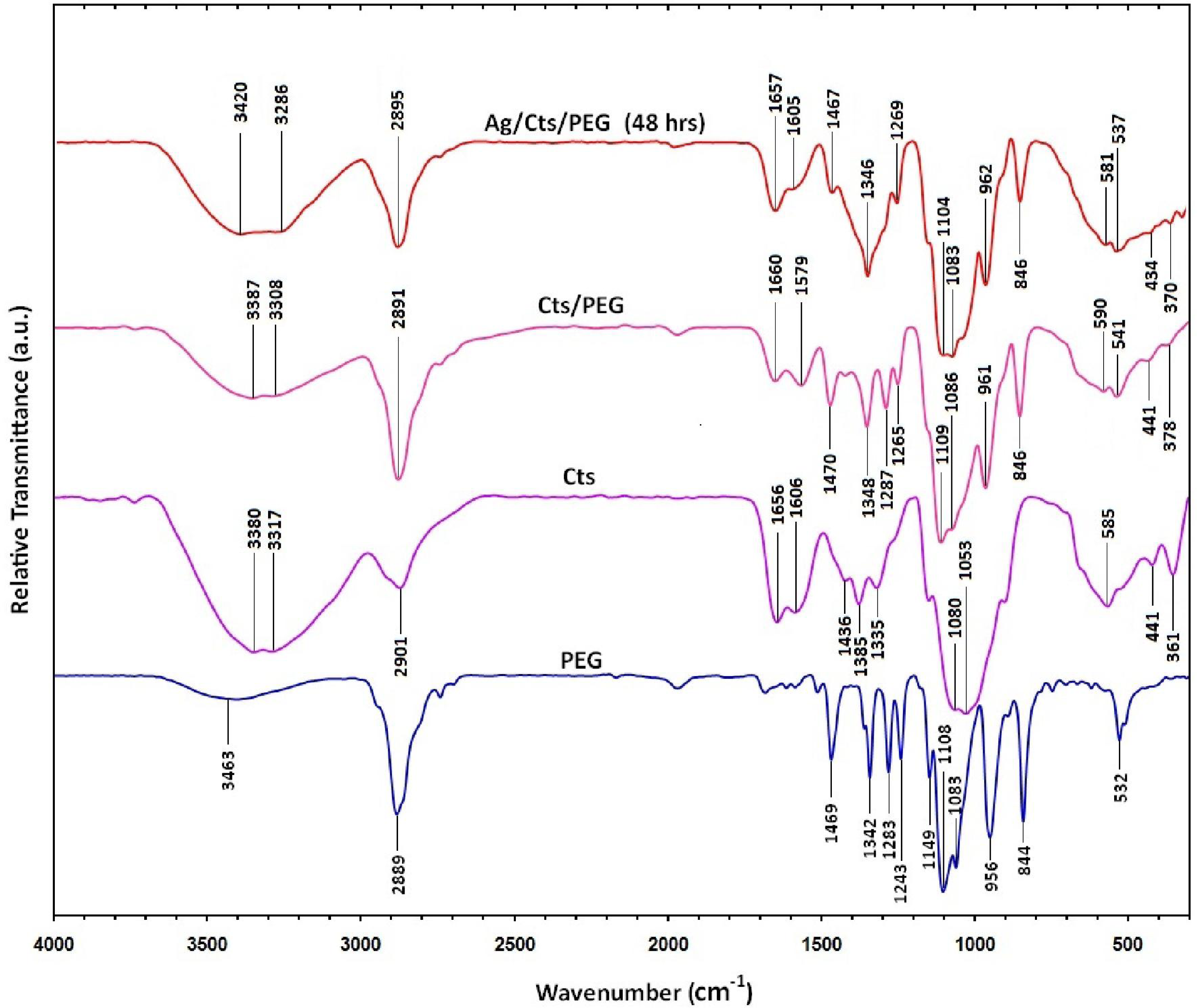
2.4. Fourier Transform Infrared Characterization
3. Experimental Section
3.1. Materials
3.2. Synthesis of Ag/Cts/PEG NCs
3.3. Ag NPs Characterizations
4. Conclusions
Acknowledgments
References
- Sharma, VK; Yngard, RA; Lin, Y. Silver nanoparticles: Green synthesis and their microbial activities. Adv. Colloid Interface Sci 2009, 145, 83–96. [Google Scholar]
- Heidarpour, F; Ghani, WAWAK; Ahmadun, FR; Sobri, S; Zargar, M; Mozafari, MR. Nano silver-coated polypropylene water filter: I. Manufacture by electron beam gun using a modified balzers 760 machine. Dig. J. Nanomater. Biostruct 2010, 5, 787–796. [Google Scholar]
- Cao, XL; Cheng, C; Ma, YL; Zhao, CS. Preparation of silver nanoparticles with antimicrobial activities and the researches of their biocompatibilities. J. Mater. Sci. Mater. Med 2010, 21, 2861–2868. [Google Scholar]
- Mohan, YM; Lee, KJ; Premkumar, T; Geckeler, KE. Hydrogel networks as nanoreactors: A novel approach to silver nanoparticles for antibacterial applications. Polymer 2007, 48, 158–164. [Google Scholar]
- Feng, X; Qi, X; Li, J; Yang, LW; Qiu, MC; Yin, JJ; Lu, F; Zhong, JX. Preparation, structure and photo-catalytic performances of hybrid Bi2SiO5 modified Si nanowire arrays. Appl. Surf. Sci 2011, 257, 5571–5575. [Google Scholar]
- Hayward, RC; Saville, DA; Aksay, IA. Electrophoretic assembly of colloidal crystals with optically tunable micropatterns. Nature 2000, 404, 56–59. [Google Scholar]
- Narayanan, R; El-Sayed, MA. Catalysis with transition metal nanoparticles in colloidal solution: Nanoparticle shape dependence and stability. J. Phys. Chem. B 2005, 109, 12663–12676. [Google Scholar]
- Shameli, K; Ahmad, MB; Zargar, M; Yunus, WM; Ibrahim, NA; Shabanzadeh, P; Moghaddam, MG. Synthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activity. Int. J. Nanomed 2011, 6, 271–284. [Google Scholar]
- Eustis, S; El-Sayed, MA. Why gold nanoparticles are more precious than pretty gold: Noble metal surface Plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shape. Chem. Soc. Rev 2006, 35, 209–217. [Google Scholar]
- Ahmad, MB; Shameli, K; Darroudi, M; Yunus, WMZW; Ibrahim, NA. Synthesis and characterization of silver/clay/chitosan bionanocomposites by UV-irradiation method. Am. J. Appl. Sci 2009, 6, 2030–2035. [Google Scholar]
- Shameli, K; Ahmad, MB; Yunus, WMZW; Ibrahim, NA; Gharayebi, Y; Sedaghat, S. Synthesis of silver/montmorillonite nanocomposite using γ-irradiation. Int. J. Nanomed 2010, 5, 1067–1077. [Google Scholar]
- Navaladian, S; Viswanathan, B; Viswanath, RP; Varadarajan, TK. Thermal decomposition as route for silver nanoparticles. Nanoscale Res. Lett 2007, 2, 44–48. [Google Scholar]
- Sharma, VK; Yngard, RA; Lin, Y. Silver nanoparticles: Green synthesis and their microbial activities. Adv. Colloid Interface Sci 2009, 145, 83–96. [Google Scholar]
- Huang, HZ; Yuan, Q; Yang, XR. Preparation and characterization of metal-chitosan nanocomposites. Colloids Surf. B 2004, 39, 31–37. [Google Scholar]
- Raveendran, P; Fu, J; Wallen, SL. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc 2003, 125, 13940–13941. [Google Scholar]
- Shameli, K; Ahmad, M; Yunus, WMZW; Rustaiyan, A; Ibrahim, NA; Zargar, M; Abdollahi, Y. Green synthesis of silver/montmorillonite/chitosan bionanocomposites using the UV irradiation method and evaluation of antibacterial activity. Int. J. Nanomed 2010, 5, 875–887. [Google Scholar]
- Wei, D; Sun, W; Qian, W; Ye, Y; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res 2009, 344, 2375–2382. [Google Scholar]
- Sun, SH; Fullerton, EE; Weller, D; Murray, CB. Compositionally controlled FePt nanoparticles materials. IEEE Trans. Magn 2001, 37, 1239–1243. [Google Scholar]
- Pimpang, P; Sutham, W; Mangkorntong, N; Mangkorntong, P; Choopun, S. Effect of stabilizer on preparation of silver and gold nanoparticle using grinding method. Chiang Mai J. Sci 2008, 35, 250–257. [Google Scholar]
- Bajpai, SK; Mohan, YM; Bajpai, M; Tankhiwale, R; Thomas, V. Synthesis of polymer stabilized silver and gold nanostructures. J. Nanosci. Nanotechnol 2007, 7, 2994–3010. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci 2006, 31, 603–63. [Google Scholar]
- Illum, L. Review Chitosan and its use as a pharmaceutical excipient. Pharm. Res 1998, 15, 1326–1331. [Google Scholar]
- Huang, HZ; Yuan, Q; Yang, XR. Preparation and Characterization of metal-chitosan nanocomposites. Colloids Surf. B 2004, 39, 31–37. [Google Scholar]
- Chen, ZT; Gao, L. A facile and novel way for the synthesis of nearly monodisperse silver nanoparticles. Mater. Res. Bull 2007, 42, 1657–1661. [Google Scholar]
- Popa, M; Pradell, T; Crespo, D; Calderon-Moreno, JM. Stable silver colloidal dispersions using short chain polyethylene glycol. Colloids Surf. A 2007, 303, 184–190. [Google Scholar]
- Shkilnyy, A; Souce, M; Dubios, P; Warmont, F; Saboungi, ML; Chourpa, I. Poly(ethylene glycol)-stabilized silver nanoparticles for bioanalytical application of SERS spectroscopy. Analyst 2009, 134, 1868–1872. [Google Scholar]
- Luo, CC; Zhang, YH; Zeng, XW; Zeng, YW; Wang, YG. The role of poly(ethylene glycol) in the formation of silver nanoparticles. J. Colloid Interface Sci 2005, 288, 444–448. [Google Scholar]
- Cao, XL; Cheng, C; Ma, YL; Zhao, CS. Preparation of silver nanoparticles with antimicrobial activities and the researches of their biocompatibilities. J. Mater. Sci. Mater. Med 2010, 21, 2861–2868. [Google Scholar]
- Huang, HZ; Yang, XR. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr. Res 2004, 339, 2627–2631. [Google Scholar]
- Shankar, R; Sahoo, U; Shahi, V. Synthesis and characterization of fluorescent polymer metal nanocomposites comprising poly(silylene-co-silyne)s and silver nanoparticles. Macromolecules 2011, 44, 3240–3249. [Google Scholar]
- Kelly, KL; Coronado, E; Zhao, LL; Schatz, GC. The optical properties of metal nanoparticles: The influence of size, shape and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar]
- Kreibig, U; Vollmer, M. Optical Properties of Metal Clusters; Springer: Berlin, Germany, 1995; pp. 14–41. [Google Scholar]
- Stamplecoskie, KG; Scaiano, JC. Light emitting diode can control the morphology and optical properties of silver nanoparticles. J. Am. Chem. Soc 2010, 132, 1825–1827. [Google Scholar]
- Bhainsa, KC; D’Souza, SF. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigates. Colloids Surf. B 2006, 47, 160–164. [Google Scholar]
- Peng, S; McMahon, JM; Schatz, GC; Gray, SK; Sun, Y. Reversing the size-dependence of surface Plasmon resonances. PNAS 2010, 107, 14530–14534. [Google Scholar]
- Li, SK; Shen, YH; Xie, AJ; Yu, XR; Qiu, LG; Zhang, L; Zhang, QF. Green synthesis of silver nanoparticles using Capsicum Anuum L. extract. Green Chem 2007, 9, 852–858. [Google Scholar]
- Jiang, HJ; Moon, KS; Zhang, ZQ; Pothukuchi, S; Wong, CP. Variable frequency microwave synthesis of silver nanoparticles. J. Nanopart. Res 2006, 8, 117–124. [Google Scholar]
- Günister, E; Pestreli, D; Ünlü, CH; Atıcı, O; Güngör, N. Synthesis and characterization of chitosan-MMT biocomposite systems. Carbohydr. Polym 2007, 67, 358–365. [Google Scholar]
- Darder, M; Colilla, M; Ruiz-Hitzky, E. Biopolymer-clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mater 2003, 15, 3774–3780. [Google Scholar]


© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ahmad, M.B.; Tay, M.Y.; Shameli, K.; Hussein, M.Z.; Lim, J.J. Green Synthesis and Characterization of Silver/Chitosan/Polyethylene Glycol Nanocomposites without any Reducing Agent. Int. J. Mol. Sci. 2011, 12, 4872-4884. https://doi.org/10.3390/ijms12084872
Ahmad MB, Tay MY, Shameli K, Hussein MZ, Lim JJ. Green Synthesis and Characterization of Silver/Chitosan/Polyethylene Glycol Nanocomposites without any Reducing Agent. International Journal of Molecular Sciences. 2011; 12(8):4872-4884. https://doi.org/10.3390/ijms12084872
Chicago/Turabian StyleAhmad, Mansor Bin, Mei Yen Tay, Kamyar Shameli, Mohd Zobir Hussein, and Jenn Jye Lim. 2011. "Green Synthesis and Characterization of Silver/Chitosan/Polyethylene Glycol Nanocomposites without any Reducing Agent" International Journal of Molecular Sciences 12, no. 8: 4872-4884. https://doi.org/10.3390/ijms12084872