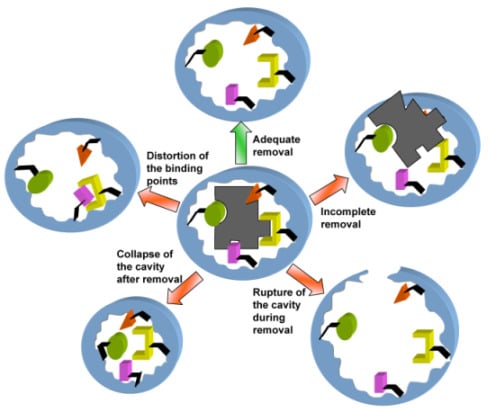
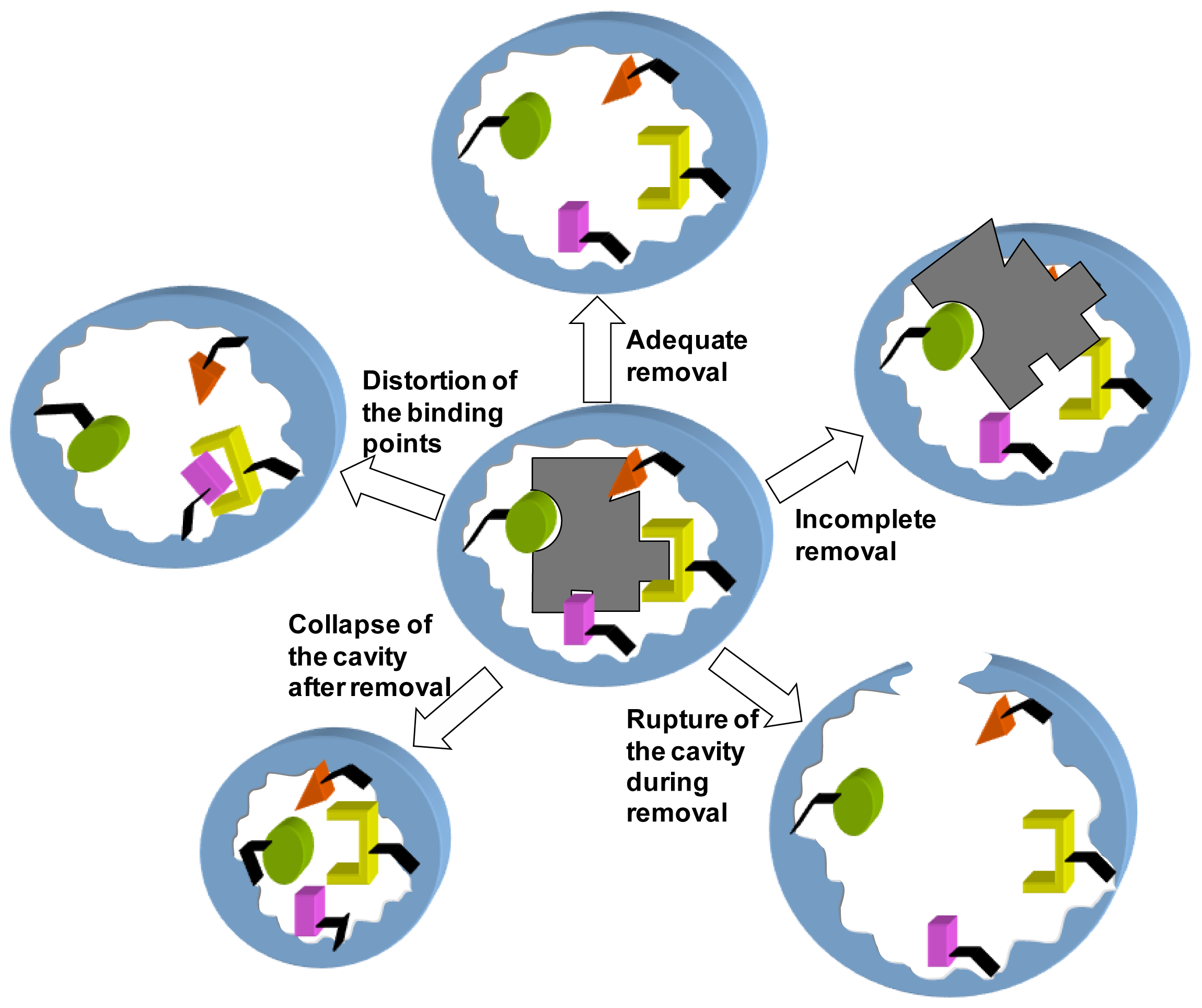
To Remove or Not to Remove? The Challenge of Extracting the Template to Make the Cavities Available in Molecularly Imprinted Polymers (MIPs)
Abstract
:1. Introduction
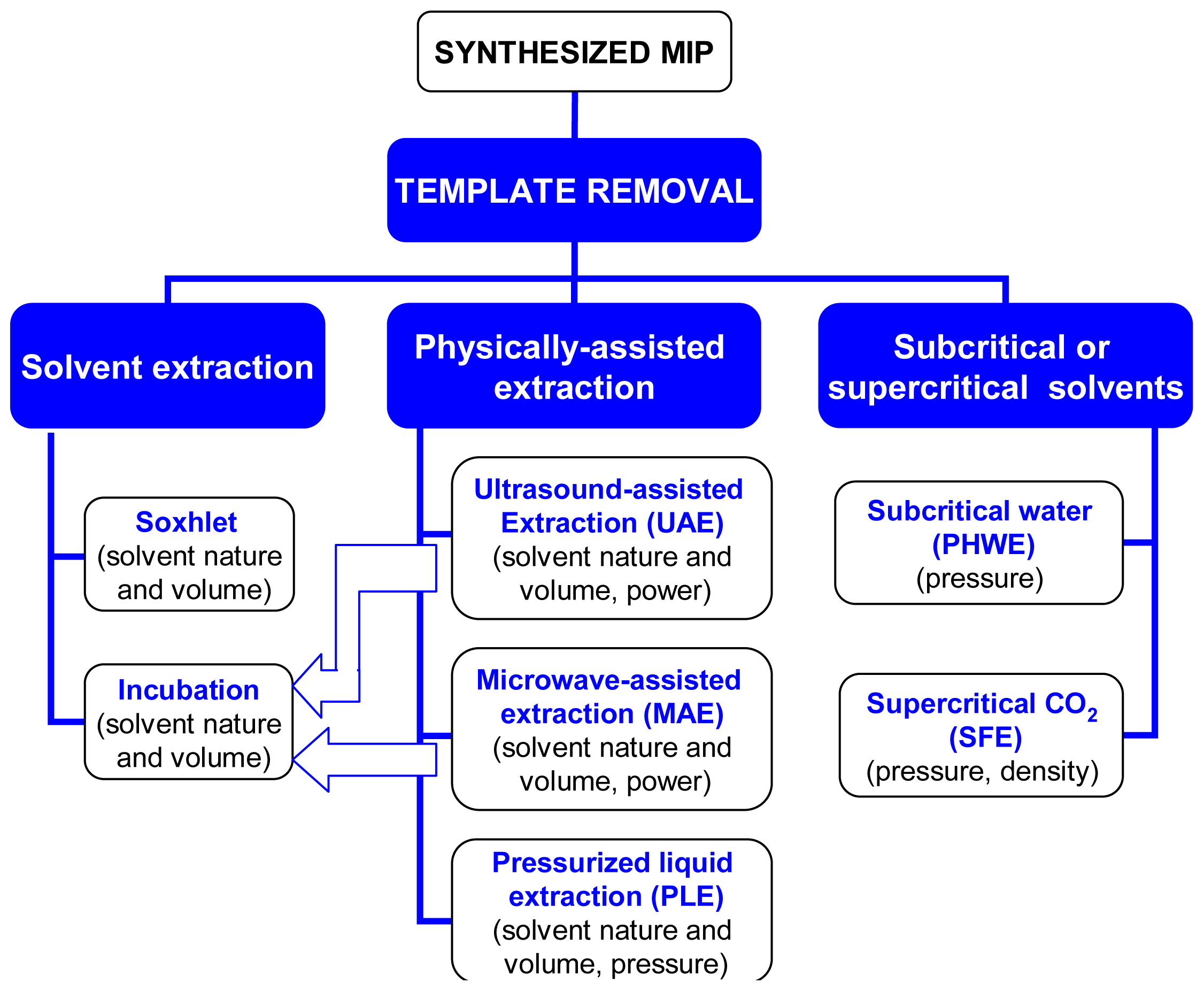
2. Extraction with Common Solvents
2.1. Conventional Soxhlet Extraction
2.2. Incubation with Solvents
3. Physically-Assisted Extraction
3.1. Microwave-Assisted Extraction (MAE)
- Pressurized MAE (PMAE), which uses closed MW-transparent vessels, filled with high tanδ solvents. The solvent absorbs the energy and the temperature raises, but boiling is prevented by the pressure inside the chamber.
- Atmospheric MAE system, in open MW-transparent vessels, which is also named Focused Microwave-assisted Soxhlet extraction (FMASE) [51]. This technique employs low tanδ solvent and, thus, only the specimen to be extracted increases its temperature. Since the extraction is carried out under mild conditions, this modality is preferred for thermolabile substances.
3.2. Ultrasound-Assisted Extraction (UAE)
3.3. Pressurized Liquid Extraction (PLE)
4. Extraction with Supercritical or Subcritical Fluids
4.1. Supercritical Fluid Extraction (SPE)
4.2. Subcritical Water Extraction (SWE)
5. Conclusions and Future Trends
Acknowledgements
References
- Alvarez-Lorenzo, C; Concheiro, A. Molecularly imprinted polymers for drug delivery. J. Chromatogr. B 2004, 804, 231–245. [Google Scholar]
- Lasakova, M; Jandera, P. Molecularly imprinted polymers and their application in solid phase extraction. J. Sep. Sci 2009, 32, 799–812. [Google Scholar]
- Lieberzeit, PA; Dickert, FL. Chemosensors in environmental monitoring: Challenges in ruggedness and selectivity. Anal. Bioanal. Chem 2009, 393, 467–472. [Google Scholar]
- Bui, BTS; Haupt, K. Molecularly imprinted polymers: Synthetic receptors in bioanalysis. Anal. Bioanal. Chem 2010, 398, 2481–2492. [Google Scholar]
- Alvarez-Lorenzo, C; Concheiro, A. Molecularly imprinted gels and nano- and microparticles. Manufacture and applications. In Smart Nano- and Microparticles; Arshady, R, Kono, K, Eds.; Kentus Books: London, UK, 2006; pp. 279–336. [Google Scholar]
- Poma, A; Turner, APF; Piletsky, SA. Advances in the manufacture of MIP nanoparticles. Trends Biotechnol 2010, 28, 629–637. [Google Scholar]
- Hu, Y; Li, Y; Liu, R; Tan, W; Li, G. Magnetic molecularly imprinted polymer beads prepared by microwave heating for selective enrichment of β-agonists in pork and pig liver samples. Talanta 2011, 84, 462–470. [Google Scholar]
- Cederfur, J; Pei, Y; Zihui, M; Kempe, M. Synthesis and screening of a molecularly imprinted polymer library targeted for penicillin G. J. Comb. Chem 2003, 5, 67–72. [Google Scholar]
- Nicholls, IA; Andersson, HS; Golker, K; Henschel, H; Karlsson, BCG; Olsson, GD; Rosengren, AM; Shoravi, S; Suriyanarayanan, S; Wiklander, JG; et al. Rational design of biomimetic molecularly imprinted materials: Theoretical and computational strategies for guiding nanoscale structured polymer development. Anal. Bioanal. Chem 2011, 400, 1771–1786. [Google Scholar]
- Ellwanger, A; Berggren, C; Bayoudh, S; Crecenzi, C; Karlsson, L; Owens, PK; Ensing, K; Cormack, P; Sherrington, D; Sellergren, B. Evaluation of methods aimed at complete removal of template from molecularly imprinted polymers. Analyst 2001, 126, 784–792. [Google Scholar]
- Luliński, P; Maciejewska, D; Bamburowicz-Klimkowska, M; Szutowski, M. Dopamine-imprinted polymers: Template-monomer interactions, analysis of template removal and application to solid phase extraction. Molecules 2007, 12, 2434–2449. [Google Scholar]
- Batlokwa, BM; Mokgadi, J; Nyokong, T; Torto, N. Optimal template removal from molecularly imprinted polymers by pressurized hot water extraction. Chromatographia 2011, 73, 589–593. [Google Scholar]
- Shea, KJ; Sasaki, DY; Stoddard, GJ. Fluorescence probes for evaluating chain solvation in network polymers. An analysis of the solvatochromic shift of the dansyl probe in macroporous styrene-divinylbenzene and styrene-diisopropenylbenzene copolymers. Macromolecules 1989, 22, 1722–1730. [Google Scholar]
- Ou, SH; Wu, MC; Chou, TC; Liu, CC. Polyacrylamide gels with electrostatic functional groups for the molecular imprinting of lysozyme. Anal. Chim. Acta 2004, 504, 163–166. [Google Scholar]
- Levi, L; Srebnik, S. Simulation of protein-imprinted polymers. 1. Imprinted Pore properties. J. Phys. Chem. B 2010, 114, 107–114. [Google Scholar]
- Lanza, F; Sellergren, B. The application of molecular imprinting technology to solid phase extraction. Chromatographia 2000, 53, 599–611. [Google Scholar]
- Szumski, M; Buszewski, B. Molecularly imprinted polymers: A new tool for separation of steroid isomers. J. Sep. Sci 2004, 27, 837–842. [Google Scholar]
- Martin, P; Jones, GR; Stringer, F; Wilson, ID. Comparison of normal and reversed-phase solid phase extraction methods for extraction of β-blockers from plasma using molecularly imprinted polymers. Analyst 2003, 128, 345–350. [Google Scholar]
- Yungerman, I; Srebnik, S. Factors contributing to binding-site imperfections in imprinted polymers. Chem. Mater 2006, 18, 657–663. [Google Scholar]
- Fu, GQ; Yu, H; Zhu, J. Imprinting effect of protein-imprinted polymers composed of chitosan and polyacrylamide: A re-examination. Biomaterials 2008, 29, 2138–2142. [Google Scholar]
- Dirion, B; Lanza, F; Sellergren, B; Chassaing, C; Venn, R; Berggren, C. Selective solid phase extraction of a drug lead compound using molecularly imprinted polymers prepared by the target analogue approach. Chromatographia 2002, 56, 237–241. [Google Scholar]
- Boyd, B; Björk, H; Billing, J; Shimelis, O; Axelsson, S; Leonora, M; Yilmaz, E. Development of an improved method for trace analysis of chloramphenicol using molecularly imprinted polymers. J. Chromatogr. A 2007, 1174, 63–71. [Google Scholar]
- Schirmer, C; Meisel, H. Chromatographic evaluation of polymers imprinted with analogs of chloramphenicol and application to selective solid-phase extraction. Anal. Bioanal. Chem 2009, 394, 2249–2255. [Google Scholar]
- D’Oleo, R; Alvarez-Lorenzo, C; Sun, G. A new approach to design imprinted polymer gels without using a template. Macromolecules 2001, 34, 4965–4971. [Google Scholar]
- Moritani, T; Alvarez-Lorenzo, C. Conformational imprinting effect on stimuli-sensitive gels made with an “imprinter” monomer. Macromolecules 2001, 34, 7796–7803. [Google Scholar]
- Suryanarayanan, V; Wu, CT; Ho, KC. Molecularly imprinted electrochemical sensors. Electroanalysis 2010, 22, 1795–1811. [Google Scholar]
- Focant, JF; Pirard, C; de Pauw, E. Automated sample preparation-fractionation for the measurement of dioxins and related compounds in biological matrices: A review. Talanta 2004, 63, 1101–1113. [Google Scholar]
- Raynie, DE. Modern extraction techniques. Anal. Chem 2006, 78, 3997–4004. [Google Scholar]
- Hyötyläinen, T. Critical evaluation of sample pretreatment techniques. Anal. Bioanal. Chem 2009, 394, 743–758. [Google Scholar]
- Kronholm, J; Hartonen, K; Riekkola, ML. Analytical extractions with water at elevated temperatures and pressures. Trends Anal. Chem 2007, 26, 396–412. [Google Scholar]
- Tobiszewski, M; Mechlińska, A; Zygmunt, B; Namieśnik, J. Green analytical chemistry in sample preparation for determination of trace organic pollutants. Trends Anal. Chem 2009, 28, 943–951. [Google Scholar]
- Soxhlet, F. Die gewichtsanalytische Bestimmung des Milchfettes. Polytechnisches J. (Dingler’s) 1879, 232, 461. [Google Scholar]
- Luque de Castro, MD; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar]
- Mullett, WM; Lai, EPC. Determination of theophylline in serum by molecularly imprinted solid-phase extraction with pulsed elution. Anal. Chem 1998, 70, 3636–3641. [Google Scholar]
- Turiel, E; Martín-Esteban, A; Tadeo, JL. Molecular imprinting-based separation methods for selective analysis of fluoroquinolones in soils. J. Chromatogr. A 2007, 1172, 97–104. [Google Scholar]
- Díaz-Alvarez, M; Turiel, E; Martín-Esteban, A. Selective sample preparation for the analysis of (fluoro)quinolones in baby food: Molecularly imprinted polymers versus anion-exchange resins. Anal. Bioanal. Chem 2009, 393, 899–905. [Google Scholar]
- Turner, NW; Holdsworth, CI; Donne, SW; McCluskey, A; Bowyer, MC. Microwave induced MIP synthesis: Comparative analysis of thermal and microwave induced polymerisation of caffeine imprinted polymers. New J. Chem 2010, 34, 686–692. [Google Scholar]
- Tunc, Y; Hasirci, N; Yesilada, A; Ulubayram, K. Comonomer effects on binding performances and morphology of acrylate-based imprinted polymers. Polymer 2006, 47, 6931–6940. [Google Scholar]
- Byun, HS; Youn, YN; Yun, YH; Yoon, SD. Selective separation of aspirin using molecularly imprinted polymers. Sep. Purif. Technol 2010, 74, 144–153. [Google Scholar]
- Hillberg, AL; Brain, KR; Allender, CJ. Design and evaluation of thin and flexible theophylline imprinted polymer membrane materials. J. Mol. Recognit 2009, 22, 223–231. [Google Scholar]
- Alvarez-Lorenzo, C; Hiratani, H; Gómez-Amoza, JL; Martínez-Pacheco, R; Souto, C; Concheiro, A. Soft contact lenses capable of sustained delivery of timolol. J. Pharm. Sci 2002, 91, 2182–2192. [Google Scholar]
- Alvarez-Lorenzo, C; Yañez, F; Barreiro-Iglesias, R; Concheiro, A. Imprinted soft contact lenses as norfloxacin delivery systems. J. Control. Release 2006, 113, 236–244. [Google Scholar]
- Yanez, F; Chauhan, A; Concheiro, A; Alvarez-Lorenzo, C. Timolol-imprinted soft contact lenses: Influence of the template/functional monomer ratio and the hydrogel thickness. J Appl Polym Sci 2011. [Google Scholar] [CrossRef]
- Bolisay, LD; Culver, JN; Kofinas, P. Optimization of virus imprinting methods to improve selectivity and reduce nonspecific binding. Biomacromolecules 2007, 8, 3893–3899. [Google Scholar]
- Bolisay, LD; Culver, JN; Kofinas, P. Molecularly imprinted polymers for tobacco mosaic virus recognition. Biomaterials 2006, 27, 4165–4168. [Google Scholar]
- Nemulenzi, O; Mhaka, B; Cukrowska, E; Ramström, O; Tute, H; Chimuka, L. Potential of combining of liquid membranes and molecularly imprinted polymers in extraction of 17 β-estradiol from aqueous simples. J. Sep. Sci 2009, 32, 1941–1948. [Google Scholar]
- Madej, K. Microwave-assisted and cloud-point extraction in determination of drugs and other bioactive compounds. Trends Anal. Chem 2009, 28, 436–446. [Google Scholar]
- Cela, R; Lorenzo, RA; Casais, MC. Técnicas Analíticas de Separación en Química Analítica; Síntesis: Madrid, Spain, 2002. [Google Scholar]
- Mandal, V; Mohan, Y; Hemalatha, S. Microwave assisted extraction—An innovative and promising extraction tool for medicinal plant research. Pharmacognosy Rev 2007, 1, 7–18. [Google Scholar]
- Eskilsson, CS; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar]
- Luque-Garcia, JL; Luque de Castro, MD. Focused microwave-assisted Soxhlet extraction: Devices and applications. Talanta 2004, 64, 571–577. [Google Scholar]
- Bravo, JC; Fernández, P; Durand, JS. Flow injection fluorimetric determination of β-estradiol using a molecularly imprinted polymer. Analyst 2005, 130, 1404–1409. [Google Scholar]
- Cintas, P; Luche, JL. Green chemistry. The sonochemical approach. Green Chem 1999, 1, 115–125. [Google Scholar]
- Luque-Garcia, JL; Luque de Castro, MD. Ultrasound: A powerful tool for leaching. Trends Anal. Chem 2003, 22, 90–99. [Google Scholar]
- Tadeo, JL; Sánchez-Brunete, C; Albero, B; García-Valcárcel, AI. Application of ultrasound-assisted extraction to the determination of contaminants in food and soil samples. J. Chromatogr. A 2010, 1217, 2415–2440. [Google Scholar]
- Zheng, X; Murray, GM. Synthesis and characterisation of site-selective ion-exchange resins templated for lead (II) ion. Sep. Sci. Tech 1996, 31, 2403–2418. [Google Scholar]
- Jenkins, AL; Uy, MO; Murray, GM. Polymer based lanthanide luminescent sensors for detection of the hydrolysis product of the nerve agent Soman in water. Anal. Chem 1999, 71, 373–378. [Google Scholar]
- Svenson, J. Ultrasound-assisted preparation of molecularly imprinted polymers: Effects on polymer morphology, binding, and chromatographic behavior. Anal. Lett 2006, 39, 2749–2760. [Google Scholar]
- Zhu, H; Wang, Y; Yuan, Y; Zeng, H. Development and characterization of molecularly imprinted polymer microspheres for the selective detection of kaempferol in traditional Chinese medicines. Anal. Methods 2011, 3, 348–355. [Google Scholar]
- Hu, Y; Liu, R; Zhang, Y; Li, G. Improvement of extraction capability of magnetic molecularly imprinted polymer beads in aqueous media via dual-phase solvent system. Talanta 2009, 79, 576–582. [Google Scholar]
- Zhang, Y; Li, Y; Hu, Y; Li, G; Chen, Y. Preparation of magnetic indole-3-acetic acid imprinted polymer beads with 4-vinylpyridine and β-cyclodextrin as binary monomer via microwave heating initiated polymerization and their application to trace analysis of auxins in plant tissues. J. Chromatogr. A 2010, 1217, 7337–7344. [Google Scholar]
- Hu, Y; Li, Y; Liu, R; Tan, W; Li, G. Magnetic molecularly imprinted polymer beads prepared by microwave heating for selective enrichment of β-agonists in pork and pig liver samples. Talanta 2011, 84, 462–470. [Google Scholar]
- Peng, L; Wang, Y; Zeng, H; Yuan, Y. Molecularly imprinted polymer for solid-phase extraction of rutin in complicated traditional Chinese medicines. Analyst 2011, 136, 756–763. [Google Scholar]
- Richter, BE; Ezzell, JL; Knowles, DE; Hoefler, F; Mattulat, AKR; Scheutwinkel, M; Waddell, DS; Khurana, TGV. Extraction of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans from environmental samples using accelerated solvent extraction (ASE). Chemosphere 1997, 34, 975–987. [Google Scholar]
- Richter, BE. Extraction of hydrocarbon contamination from soils using accelerated solvent extraction. J. Chromatogr. A 2000, 2, 217–224. [Google Scholar]
- EPA Method 3545, Pressurised Fluid Extraction. In Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, 3rd ed; U.S. Government Printing Office: Washington, DC, USA, 2008; Final Update IV; EPA SW-846.
- Giergielewicz-Możajska, H; Dąbrowski, L; Namieśnik, J. Accelerated Solvent Extraction (ASE) in the analysis of environmental solid samples—some aspects of theory and practice. Crit. Rev. Anal. Chem 2001, 31, 149–165. [Google Scholar]
- de Koning, S; Janssen, HG; Brinkman, UAT. Modern methods of sample preparation for GC analysis. Chromatographia 2009, 69, S33–S78. [Google Scholar]
- Nieto, A; Borrull, F; Pocurull, E; Marce, RM. Pressurized liquid extraction: A useful technique to extract pharmaceuticals and personal-care products from sewage sludge. Trends Anal. Chem 2010, 29, 752–764. [Google Scholar]
- Pronyk, C; Mazza, G. Design and scale-up of pressurized fluid extractors for food and bioproducts. J. Food Eng 2009, 95, 215–226. [Google Scholar]
- Mendiola, JA; Herrero, M; Cifuentes, A; Ibañez, E. Use of compressed fluids for sample preparation: Food applications. J. Chromatogr. A 2007, 1152, 234–246. [Google Scholar]
- Ramos, L; Kristenson, EM; Brinkman, UAT. Current use of pressurised liquid extraction and subcritical water extraction in environmental analysis. J. Chromatogr. A 2002, 975, 3–29. [Google Scholar]
- Carabias-Martínez, R; Rodríguez-Gonzalo, E; Revilla-Ruiz, P; Hernández-Méndez, J. Pressurized liquid extraction in the analysis of food and biological samples. J. Chromatogr. A 2005, 1089, 1–17. [Google Scholar]
- Hyötyläinen, T. Critical evaluation of sample pretreatment techniques. Anal. Bioanal. Chem 2009, 394, 743–758. [Google Scholar]
- Benito-Peña, E; Martin, S; Orellana, G; Moreno-Bondi, MC. Water-compatible molecularly imprinted polymer for the selective recognition of fluoroquinolone antibiotics in biological samples. Anal. Bioanal. Chem 2009, 393, 235–245. [Google Scholar]
- Mojica, ERE; Autschbach, J; Bright, FV; Aga, DS. Tetracycline speciation during molecular imprinting in xerogels results in class-selective binding. Analyst 2011, 136, 749–755. [Google Scholar]
- Fidalgo-Used, N; Blanco-González, E; Sanz-Medel, A. Sample handling strategies for the determination of persistent trace organic contaminants from biota samples. Anal. Chim. Acta 2007, 590, 1–16. [Google Scholar]
- García-Rodríguez, D; Carro-Díaz, AM; Lorenzo-Ferreira, RA. Supercritical fluid extraction of polyhalogenated pollutants from aquaculture and marine environmental samples: A review. J. Sep. Sci 2008, 31, 1333–1345. [Google Scholar]
- Bunte, G; Hürttlen, J; Pontius, H; Hartlieb, K; Krause, H. Gas phase detection of explosives such as 2,4,6-trinitrotoluene by molecularly imprinted polymers. Anal. Chim. Acta 2007, 591, 49–56. [Google Scholar]
- Yañez, F; Martikainen, L; Braga, ME; Alvarez-Lorenzo, C; Concheiro, A; Duarte, CM; Gil, MH; Sousa, HC. Supercritical fluid-assisted preparation of imprinted contact lenses for drug delivery. Acta Biomater 2011, 7, 1019–1030. [Google Scholar]
- Herrero, M; Cifuentes, A; Ibanez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem 2006, 98, 136–148. [Google Scholar]
- Hageman, KJ; Mazeas, L; Grabanski, CB; Miller, DJ; Hawthorne, SB. Coupled subcritical water extraction with solid-phase microextraction for determining semivolatile organics in environmental solids. Anal. Chem 1996, 68, 3892–3898. [Google Scholar]
- Nerín, C; Salafranca, J; Aznar, M; Batlle, R. Critical review on recent developments in solventless techniques for extraction of analytes. Anal. Bioanal. Chem 2009, 393, 809–833. [Google Scholar]
- Teo, CC; Tan, SN; Hong Yong, JW; Hew, CS; Ong, ES. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar]
- Ong, ES; Cheong, JSH; Goh, D. Pressurized hot water extraction of bioactive or marker compounds in botanicals and medicinal plant materials. J. Chromatogr. A 2006, 1112, 92–102. [Google Scholar]
- Kubatova, A; Jansen, B; Vaudiosot, JF; Hawthorne, SB. Thermodynamic and kinetic models for the extraction of essential oil from savory and polycyclic aromatic hydrocarbons from soil with hot (subcritical) water and supercritical CO2. J. Chromatogr. A 2002, 975, 175–188. [Google Scholar]
- Crescenzi, C; Di Gorcia, A; Nazzarri, M; Samperi, R. Hot phosphate-buffered water extraction coupled on-line with liquid chromatography/mass spectrometry for analyzing contaminants in soil. Anal. Chem 2000, 72, 3050–3055. [Google Scholar]
- Batlokwa, BS; Mokgadi, J; Nyokong, T; Torto, N. Optimal template removal from molecularly imprinted polymers by pressurized hot water extraction. Chromatographia 2011, 73, 589–593. [Google Scholar]

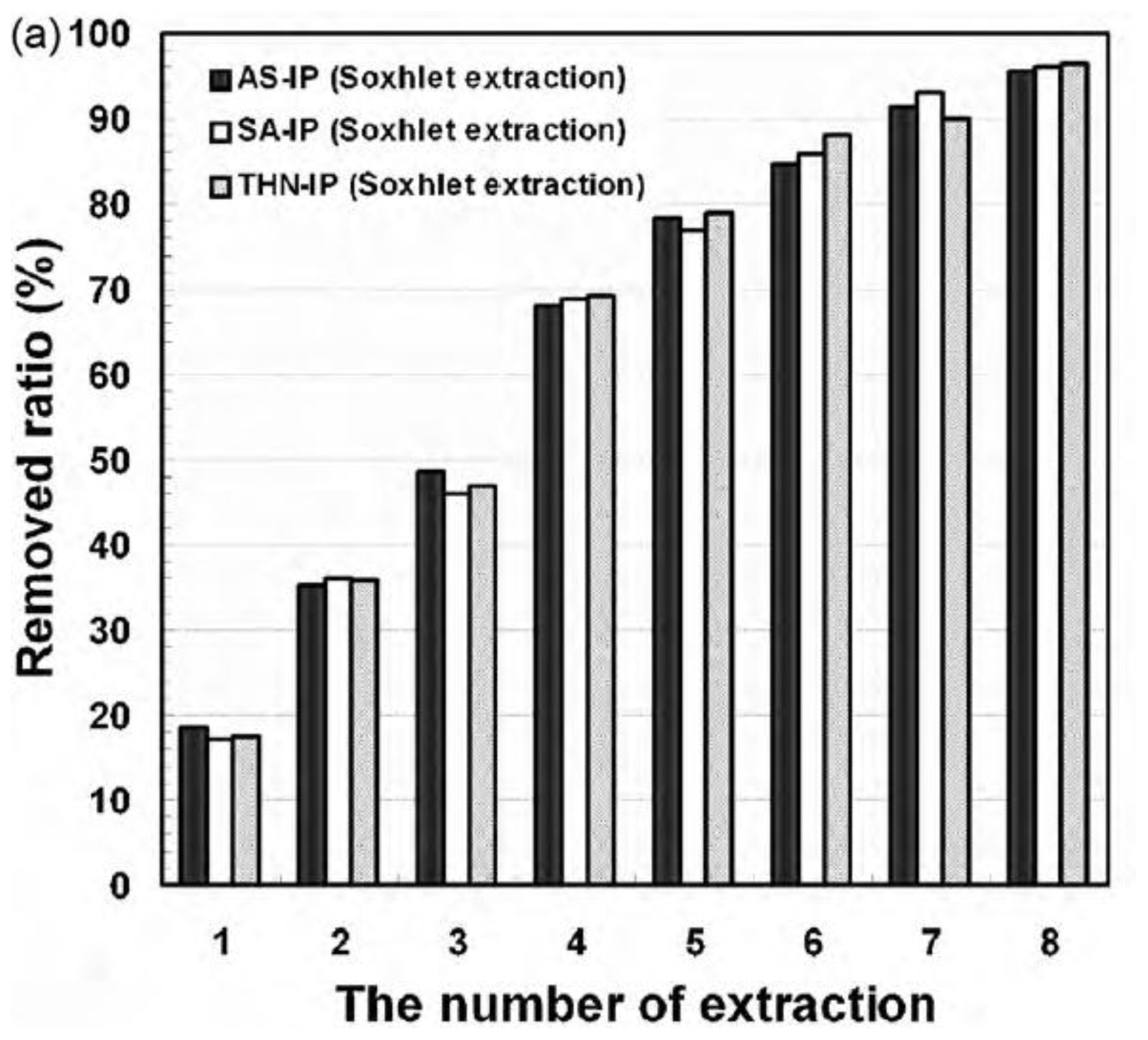
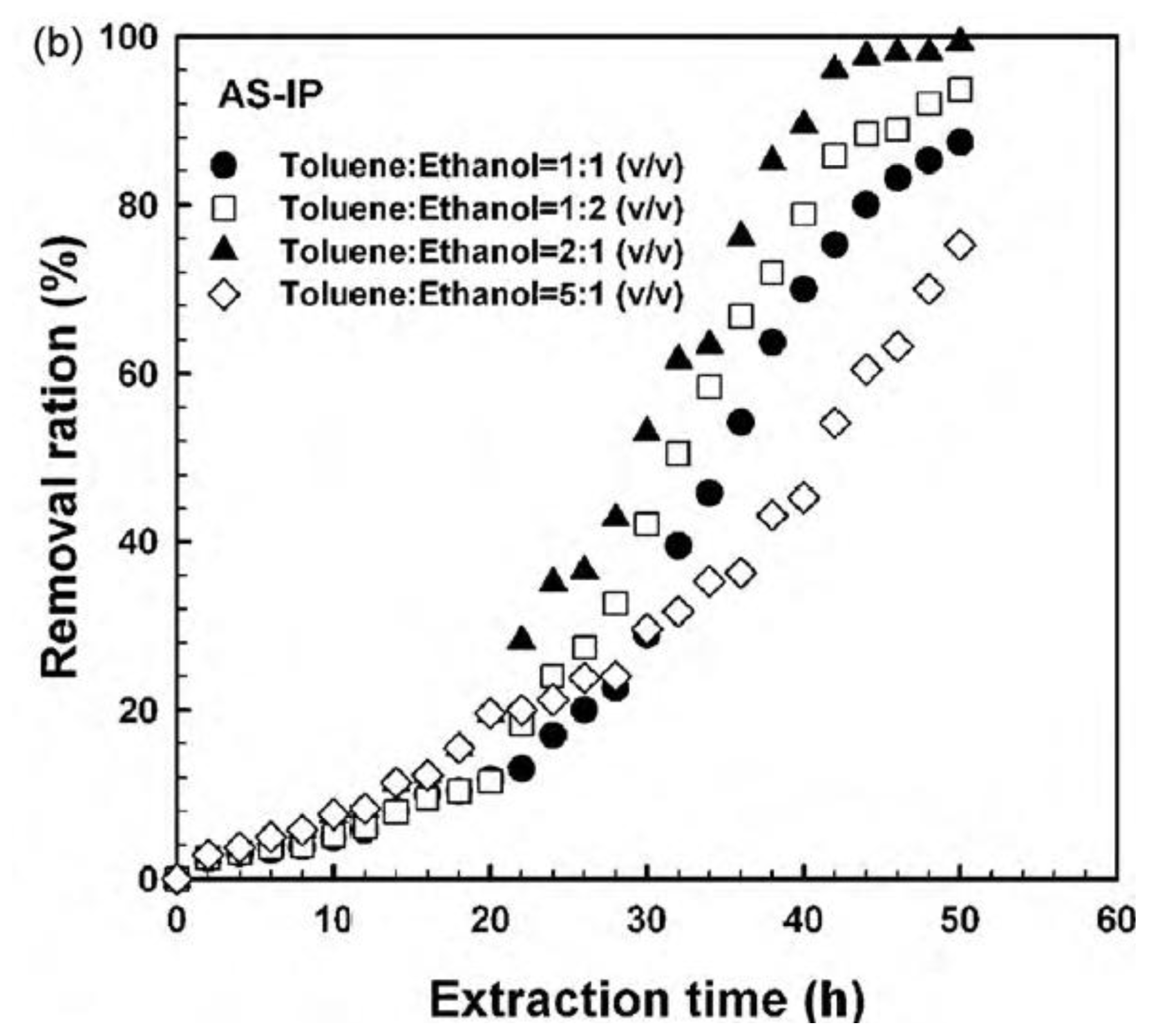
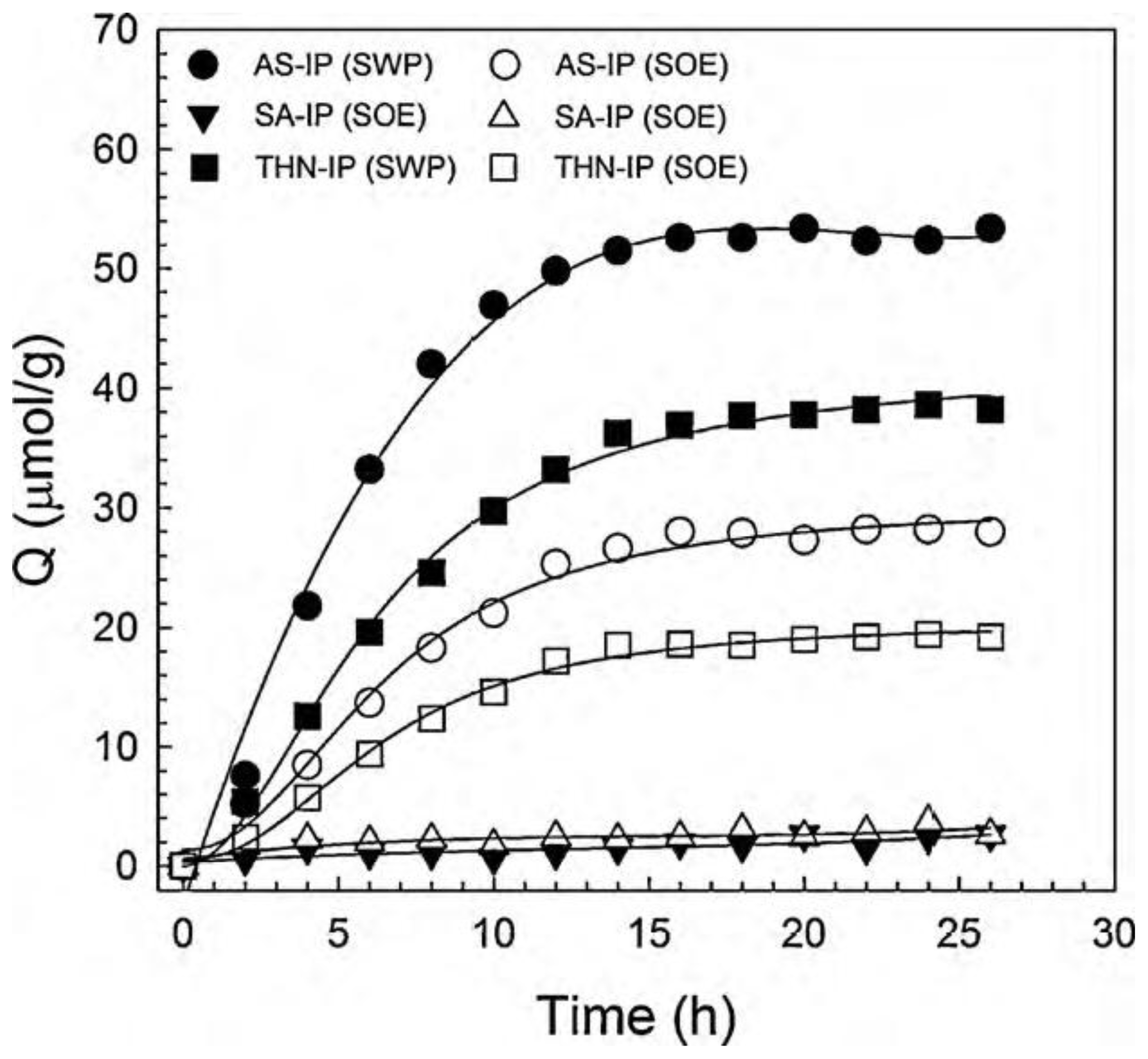
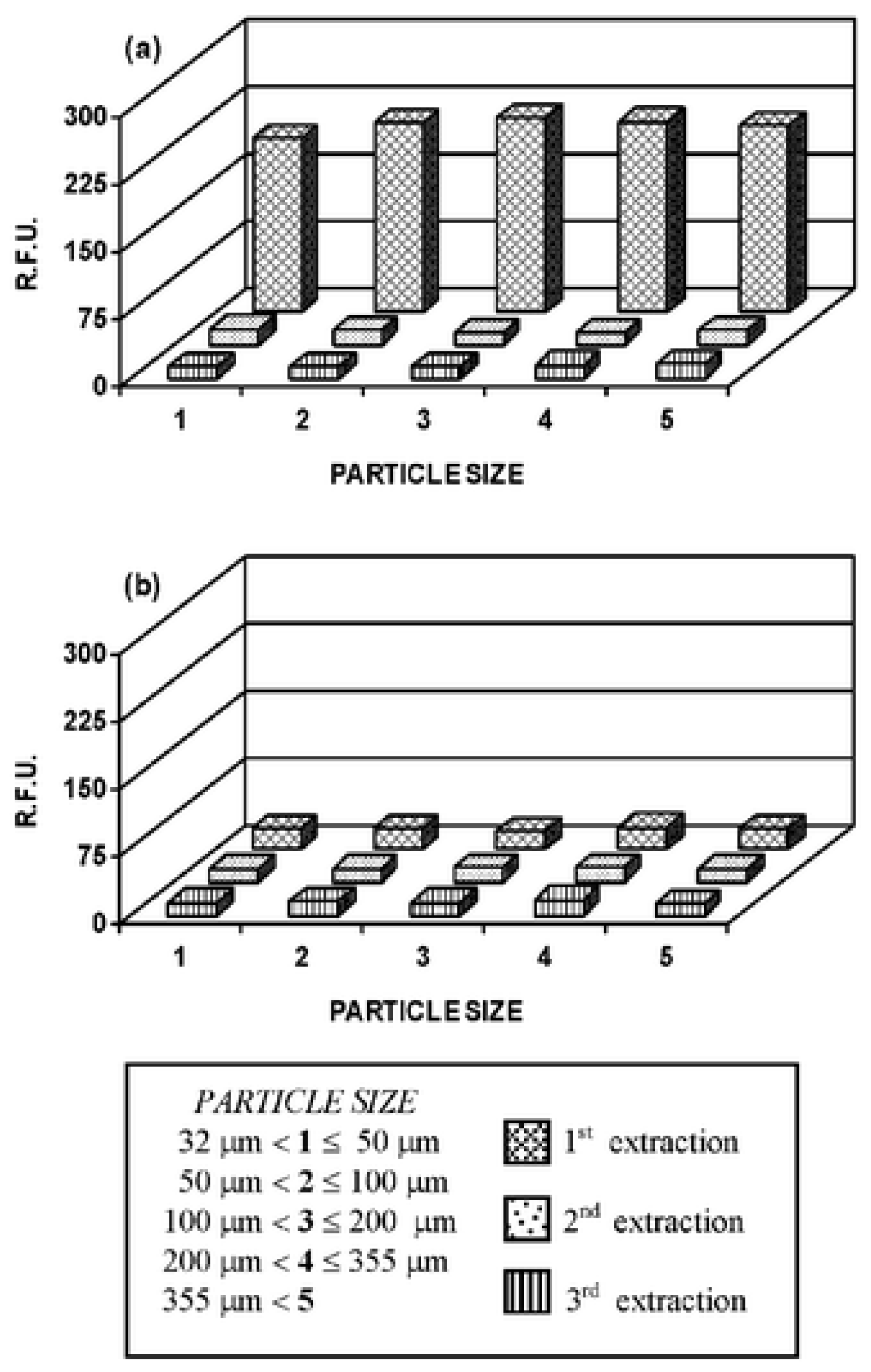

| Elution methods | Elution liquid-solid ratio (mL/g) | Elution time (h) | Elution capacity (Q, mg/g) |
|---|---|---|---|
| Soxhlet extraction | 100 | 24 | 547 |
| Ultrasonic extraction | 40 | 1 | 815 |
| Microwave-assisted extraction | 40 | 5 | 326 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lorenzo, R.A.; Carro, A.M.; Alvarez-Lorenzo, C.; Concheiro, A. To Remove or Not to Remove? The Challenge of Extracting the Template to Make the Cavities Available in Molecularly Imprinted Polymers (MIPs). Int. J. Mol. Sci. 2011, 12, 4327-4347. https://doi.org/10.3390/ijms12074327
Lorenzo RA, Carro AM, Alvarez-Lorenzo C, Concheiro A. To Remove or Not to Remove? The Challenge of Extracting the Template to Make the Cavities Available in Molecularly Imprinted Polymers (MIPs). International Journal of Molecular Sciences. 2011; 12(7):4327-4347. https://doi.org/10.3390/ijms12074327
Chicago/Turabian StyleLorenzo, Rosa A., Antonia M. Carro, Carmen Alvarez-Lorenzo, and Angel Concheiro. 2011. "To Remove or Not to Remove? The Challenge of Extracting the Template to Make the Cavities Available in Molecularly Imprinted Polymers (MIPs)" International Journal of Molecular Sciences 12, no. 7: 4327-4347. https://doi.org/10.3390/ijms12074327
APA StyleLorenzo, R. A., Carro, A. M., Alvarez-Lorenzo, C., & Concheiro, A. (2011). To Remove or Not to Remove? The Challenge of Extracting the Template to Make the Cavities Available in Molecularly Imprinted Polymers (MIPs). International Journal of Molecular Sciences, 12(7), 4327-4347. https://doi.org/10.3390/ijms12074327