Synthesis of Brushite Particles in Reverse Microemulsions of the Biosurfactant Surfactin
Abstract
:1. Introduction
2. Results and Discussion
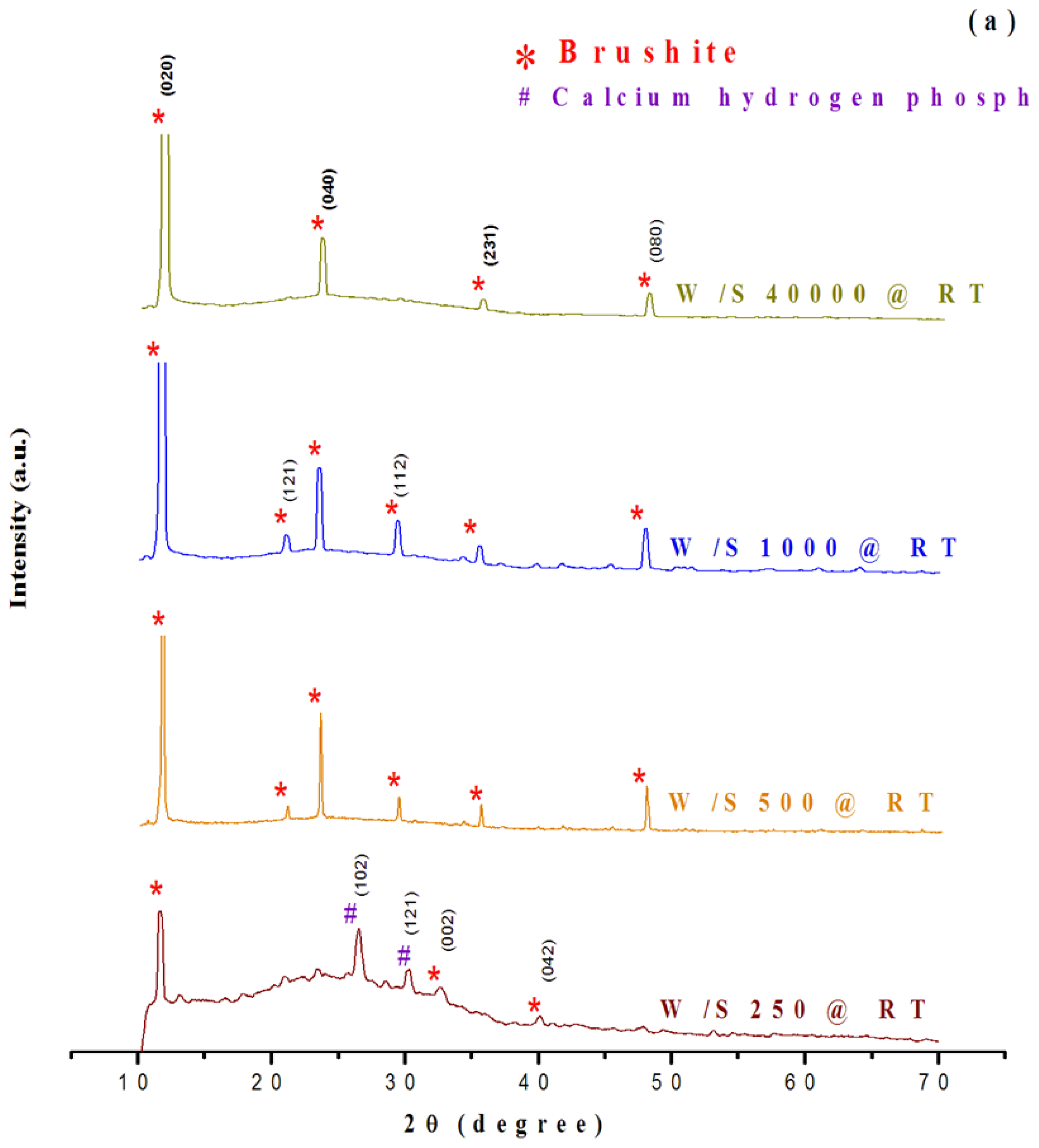
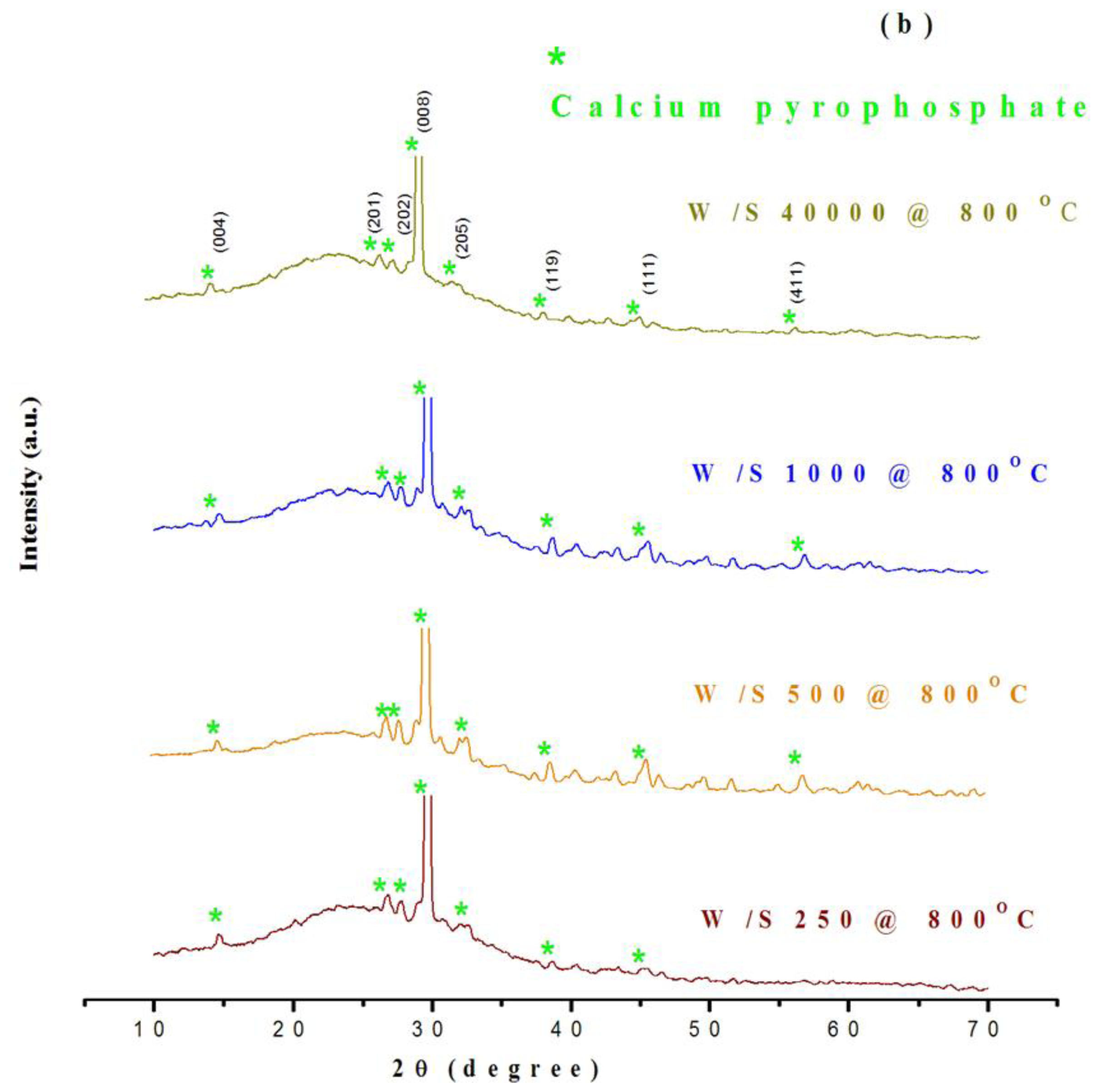
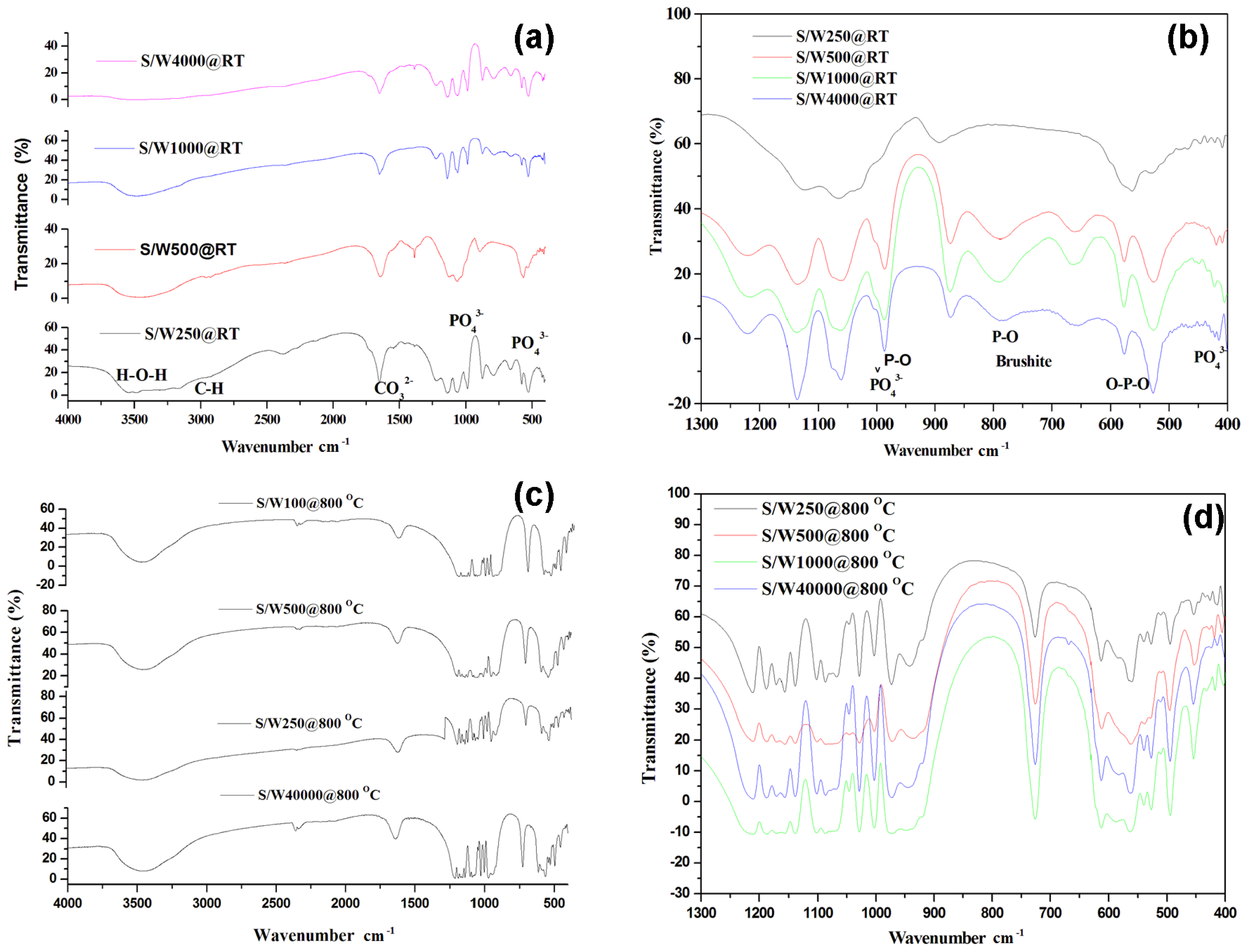
2.1. Structural Characterization of Products
2.2. Morphological and Particle Size Characterization
2.3. Mechanism of Crystallization in the Reverse Microemulsion Process
3. Experimental Section
3.1. Chemicals
3.2. Calcium Nitrate Tetrahydrate and Ammonium Phosphate Solutions
3.3. Surfactin Production
3.4. Synthesis of Nanoparticles by the Reverse Microemulsion Process
3.5. Characterization of Particles
Morphological study by TEM and SEM
Assessment of functional groups by FTIR
Assessment of phase composition and crystallinity using XRD
4. Conclusions
Acknowledgements
References
- Nilsson, M; Fernandez, E; Sarda, S; Lidgren, L; Planell, JA. Characterization of a novel calcium phosphate/sulphate bone cement. J. Biomed. Mat. Res 2002, 61, 600–607. [Google Scholar]
- Baksh, D. Davies, JE, Ed.; Design Strategies for 3-Dimensional in vitro Bone Growth in Tissue-Engineering Scaffolds. In Bone Engineering; Em Square: Toronto, Canada, 2000; pp. 488–495. [Google Scholar]
- Zhao, H; He, W; Wang, Y; Zhang, X; Li, Z; Yan, S; Zhou, W; Wang, G. Biomineralization of large hydroxyapatite particles using ovalbumin as Biosurfactant. Mater. Lett 2008, 62, 3603–3605. [Google Scholar]
- Singh, S; Bhardwaj, P; Singh, V; Aggarwal, S; Mandal, UK. Synthesis of nanocrystalline calcium phosphate in microemulsion—effect of nature of surfactants. J. Colloid Interface Sci 2008, 319, 322–329. [Google Scholar]
- Kundu, B; Soundrapandian, C; Nandi, SK; Mukherjee, P; Dandapat, N; Roy, S; Datta, BK; Mandal, TK; Basu, D; Bhattacharya, RN. Development of new localized drug delivery system based on ceftriaxone-sulbactam composite drug impregnated porous hydroxylapatite: a systematic approach for in vitro and in vivo animal trial. Pharm. Res 2010, 27, 1659–1676. [Google Scholar]
- Kundu, B; Lemos, A; Soundrapandian, C; Sen, PS; Datta, S; Ferreira, JMF; Basu, D. Development of porous HAp and β-TCP scaffolds by starch consolidation with foaming method and drug-chitosan bilayered scaffold based drug delivery system. J. Mater. Sci. Mater. Med 2010, 21, 2955–2969. [Google Scholar]
- Chen, L; Tang, SQ; Wang, YJ; Wei, K. Formation of calcium phosphate nanoparticles in reverse microemulsion. Mater. Lett 2005, 59, 210–214. [Google Scholar]
- Singh, S; Singh, V; Aggarwal, S; Mandal, UK. Synthesis of brushite nanoparticles at different temperatures. Chem. Pap 2010, 64, 491–498. [Google Scholar]
- Chen, L; Ying, JW; Kun, W. Nucleation kinetics of calcium phosphate nanoparticles in reverse micelle solution. Colloids Surf. A 2008, 315, 268–274. [Google Scholar]
- Higgins, RJ. An Economical Process for Manufacturing of Nano-Sized Powders Based on Microemulsion-Mediated Synthesis. Proceedings of the Joint NSF-NIST Conference on Nanoparticles, Arlington, VA, USA, May 1997.
- Rahman, KSM; Gakpe, E. Production, characterisation and applications of biosurfactants—Review. Biotechnology 2008, 7, 360–370. [Google Scholar]
- Vallet-Regi, M; Gonzalez-Calbet, JM. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem 2004, 32, 1–31. [Google Scholar]
- Lu, X; Wang, Y; Wang, J; Qu, S; Weng, J; Xin, R; Leng, Y. Calcium phosphate crystal growth under controlled environment through urea hydrolysis. J. Cryst. Growth 2006, 297, 396–402. [Google Scholar]
- Ng, SX; Guo, J; Ma, J; Loo, SCJ. Synthesis of high surface area mesostructured calcium phosphate particles. Acta Biomater 2010, 6, 3772–3781. [Google Scholar]
- Safronova, TV; Reshotka, DS; Putlyaev, VI; Lukin, ES; Ivanov, VK. Phase composition of powdered material based on calcium hydroxyapatite and sodium dihydrophosphate. Glass Ceram 2009, 66, 7–8. [Google Scholar]

| Water/Surfactant Ratio (W/S) | A | B | ||||
|---|---|---|---|---|---|---|
| Calcium solution (mL) | n-hexane (mL) | Surfactin (g) | Phosphate solution (mL) | n-hexane (mL) | Surfactin (g) | |
| 250 | 2.25 | 10 | 1.0320 | 2.25 | 10 | 1.0320 |
| 500 | 2.25 | 10 | 0.5160 | 2.25 | 10 | 0.5160 |
| 1000 | 2.25 | 10 | 0.2580 | 2.25 | 10 | 0.2580 |
| 40,000 | 2.25 | 10 | 0.0065 | 2.25 | 10 | 0.0065 |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maity, J.P.; Lin, T.-J.; Cheng, H.P.-H.; Chen, C.-Y.; Reddy, A.S.; Atla, S.B.; Chang, Y.-F.; Chen, H.-R.; Chen, C.-C. Synthesis of Brushite Particles in Reverse Microemulsions of the Biosurfactant Surfactin. Int. J. Mol. Sci. 2011, 12, 3821-3830. https://doi.org/10.3390/ijms12063821
Maity JP, Lin T-J, Cheng HP-H, Chen C-Y, Reddy AS, Atla SB, Chang Y-F, Chen H-R, Chen C-C. Synthesis of Brushite Particles in Reverse Microemulsions of the Biosurfactant Surfactin. International Journal of Molecular Sciences. 2011; 12(6):3821-3830. https://doi.org/10.3390/ijms12063821
Chicago/Turabian StyleMaity, Jyoti Prakash, Tz-Jiun Lin, Henry Pai-Heng Cheng, Chien-Yen Chen, A. Satyanarayana Reddy, Shashi B. Atla, Young-Fo Chang, Hau-Ren Chen, and Chien-Cheng Chen. 2011. "Synthesis of Brushite Particles in Reverse Microemulsions of the Biosurfactant Surfactin" International Journal of Molecular Sciences 12, no. 6: 3821-3830. https://doi.org/10.3390/ijms12063821




