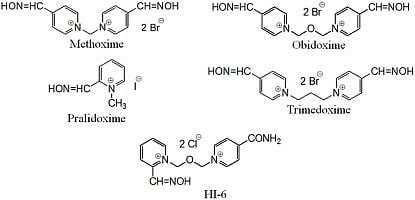
In Vitro Ability of Currently Available Oximes to Reactivate Organophosphate Pesticide-Inhibited Human Acetylcholinesterase and Butyrylcholinesterase
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
4. Conclusions
Acknowledgments
References
- Costa, LG. Current issues in organophosphate toxicology. Clin. Chim. Acta 2006, 366, 1–13. [Google Scholar]
- Maxwell, DM; Brecht, KM; Koplovitz, I; Sweeney, RE. Acetylcholinesterase inhibition: Does it explain the toxicity of organophosphorus compounds? Arch. Toxicol 2006, 80, 756–760. [Google Scholar]
- Worek, F; Koller, M; Thiermann, H; Szinicz, L. Diagnostic aspects of organophosphate poisoning. Toxicology 2005, 214, 182–189. [Google Scholar]
- Elhanany, E; Ordentlich, A; Dgany, O; Kaplan, D; Segall, Y; Barak, R; Velan, B; Shafferman, A. Resolving pathways of interaction of covalent inhibitors with the active site of acetylcholinesterases: MALDI-TOF/MS analysis of various nerve agent phosphyl adducts. Chem. Res. Toxicol 2001, 14, 912–918. [Google Scholar]
- Barak, D; Ordentlich, A; Kaplan, D; Barak, R; Mizrahi, D; Kronman, C; Segall, Y; Velan, B; Shafferman, A. Evidence for P-N bond scission in phosphoroamidate nerve agent adducts of human acetylcholinesterase. Biochemistry 2000, 39, 1156–1161. [Google Scholar]
- Millard, CB; Kryger, G; Ordentlich, A; Greenblatt, HM; Harel, M; Raves, ML; Segall, Y; Barak, D; Shafferman, A; Silman, I; Sussman, JL. Crystal structures of aged phosphonylated acetylcholinesterase: Nerve agent reaction products at the atomic level. Biochemistry 1999, 38, 7032–7039. [Google Scholar]
- Eyer, P. The role of oximes in the management of organophosphorus pesticide poisoning. Toxicol. Rev 2003, 22, 165–190. [Google Scholar]
- Worek, F; Backer, M; Thiermann, H; Szinicz, L; Mast, U; Klimmek, R; Eyer, P. Reappraisal of indications and limitations of oxime therapy in organophosphate poisoning. Hum. Exp. Toxicol 1997, 16, 466–472. [Google Scholar]
- Thompson, DF; Thompson, GD; Greenwood, RB; Trammel, HL. Therapeutic dosing of pralidoxime chloride. Drug Intell. Clin. Pharm 1987, 21, 590–593. [Google Scholar]
- Eddleston, M; Singh, S; Buckley, N. Acute organophosphorus poisoning. 2003, 1542–1553. [Google Scholar]
- Bajgar, J; Fusek, J; Kuca, K; Bartosova, L; Jun, D. Treatment of organophosphate intoxication using cholinesterase reactivators: Facts and fiction. Mini. Rev. Med. Chem 2007, 7, 461–466. [Google Scholar]
- Saxena, A; Sun, W; Luo, C; Myers, TM; Koplovitz, I; Lenz, DE; Doctor, BP. Bioscavenger for protection from toxicity of organophosphorus compounds. J. Mol. Neurosci 2006, 30, 145–148. [Google Scholar]
- Maxwell, DM; Brecht, KM; Doctor, BP; Wolfe, AD. Comparison of antidote protection against soman by pyridostigmine, HI-6 and acetylcholinesterase. J. Pharmacol. Exp. Ther 1993, 264, 1085–1089. [Google Scholar]
- Kolarich, D; Weber, A; Pabst, M; Stadlmann, J; Teschner, W; Ehrlich, H; Schwarz, HP; Altmann, F. Glycoproteomic characterization of butyrylcholinesterase from human plasma. Proteomics 2008, 8, 254–263. [Google Scholar]
- Lenz, DE; Yeung, D; Smith, JR; Sweeney, RE; Lumley, LA; Cerasoli, DM. Stoichiometric and catalytic scavengers as protection against nerve agent toxicity: A mini review. Toxicology 2007, 233, 31–39. [Google Scholar]
- Jun, D; Musilova, L; Link, M; Loiodice, M; Nachon, F; Rochu, D; Renault, F; Masson, P. Preparation and characterization of methoxy polyethylene glycol-conjugated phosphotriesterase as a potential catalytic bioscavenger against organophosphate poisoning. Chem. Biol.Inter 2010, 187, 380–383. [Google Scholar]
- Jun, D; Musilova, L; Kuca, K; Kassa, J; Bajgar, J. Potency of several oximes to reactivate human acetylcholinesterase and butyrylcholinesterase inhibited by paraoxon in vitro. Chem. Biol. Inter 2008, 175, 421–424. [Google Scholar]
- Bajgar, EJ. Central and Peripheral Nervous System: Effects of Highly Toxic Organophosphates and Their Antidotes; Research Signpost: Kerala, India, 2009. [Google Scholar]
- Jun, D; Musilova, L; Pohanka, M; Jung, YS; Bostik, P; Kuca, K. Reactivation of human acetylcholinesterase and butyrylcholinesterase inhibited by leptophos-oxon with different oxime reactivators in vitro. Int. J.Mol. Sci 2010, 11, 2856–2863. [Google Scholar]
- Bajgar, J. Organophosphates/nerve agent poisoning: Mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem 2004, 38, 151–216. [Google Scholar]
- Lorke, DE; Nurulain, SM; Hasan, MY; Kuca, K; Musilek, K; Petroianu, GA. Eight new bispyridinium oximes in comparison with the conventional oximes pralidoxime and obidoxime: In vivo efficacy to protect from diisopropylfluorophosphate toxicity. J. Appl. Toxicol 2008, 28, 920–928. [Google Scholar]
- Musilek, K; Kuca, K; Jun, D; Dohnal, V; Dolezal, M. Synthesis of a novel series of bispyridinium compounds bearing a xylene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase. J. Enzyme Inhib. Med. Chem 2005, 20, 409–415. [Google Scholar]
- Kuca, K; Cabal, J; Jun, D; Hrabinova, M. In vitro evaluation of acetylcholinesterase reactivators as potential antidotes against tabun nerve agent poisonings. Drug Chem. Toxicol 2006, 29, 443–449. [Google Scholar]
- Kuca, K; Kassa, J. A comparison of the ability of a new bispyridinium oxime-1-(4-hydroxyiminomethylpyridinium)-4-(4-carbamoylpyridinium)butane dibromide and currently used oximes to reactivate nerve agent-inhibited rat brain acetylcholinesterase by in vitro methods. J. Enzyme Inhib. Med. Chem 2003, 18, 529–535. [Google Scholar]
- Kuca, K; Musilova, L; Palecek, J; Cirkva, V; Paar, M; Musilek, K; Hrabinova, M; Pohanka, M; Karasova, JZ; Jun, D. Novel bisquaternary oximes—Reactivation of acetylcholinesterase and butyrylcholinesterase inhibited by paraoxon. Molecules 2009, 14, 4915–4921. [Google Scholar]
- Stenzel, J; Worek, F; Eyer, P. Preparation and characterization of dialkylphosphoryl-obidoxime conjugates, potent anticholinesterase derivatives that are quickly hydrolyzed by human paraoxonase (PON1192Q). Biochem. Pharmacol 2007, 74, 1390–1400. [Google Scholar]
- Worek, F; Diepold, C; Eyer, P. Dimethylphosphoryl-inhibited human cholinesterases: Inhibition, reactivation, and aging kinetics. Arch. Toxicol 1999, 73, 7–14. [Google Scholar]
- Kiderlen, D; Worek, F; Klimmek, R; Eyer, P. The phosphoryl oxime-destroying activity of human plasma. Arch. Toxicol 2000, 74, 27–32. [Google Scholar]
- Aurbek, N; Thiermann, H; Eyer, F; Eyer, P; Worek, F. Suitability of human butyrylcholinesterase as therapeutic marker and pseudo catalytic scavenger in organophosphate poisoning: A kinetic analysis. Toxicology 2009, 259, 133–139. [Google Scholar]
- Ellman, GL; Courtney, KD; Andres, V, Jr; Feather-Stone, RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol 1961, 7, 88–95. [Google Scholar]
- Musilova, L; Jun, D; Palecek, J; Cirkva, V; Musilek, K; Paar, M; Hrabinova, M; Pohanka, M; Kuca, K. Novel nucleophilic compounds with oxime group as reactivators of paraoxon-inhibited cholinesterases. Lett. Drug Design Disc 2010, 7, 260–264. [Google Scholar]
- Musilova, L; Kuca, K; Jung, YS; Jun, D. In vitro oxime-assisted reactivation of paraoxon-inhibited human acetylcholinesterase and butyrylcholinesterase. Clin. Toxicol. (Phila) 2009, 47, 545–550. [Google Scholar]

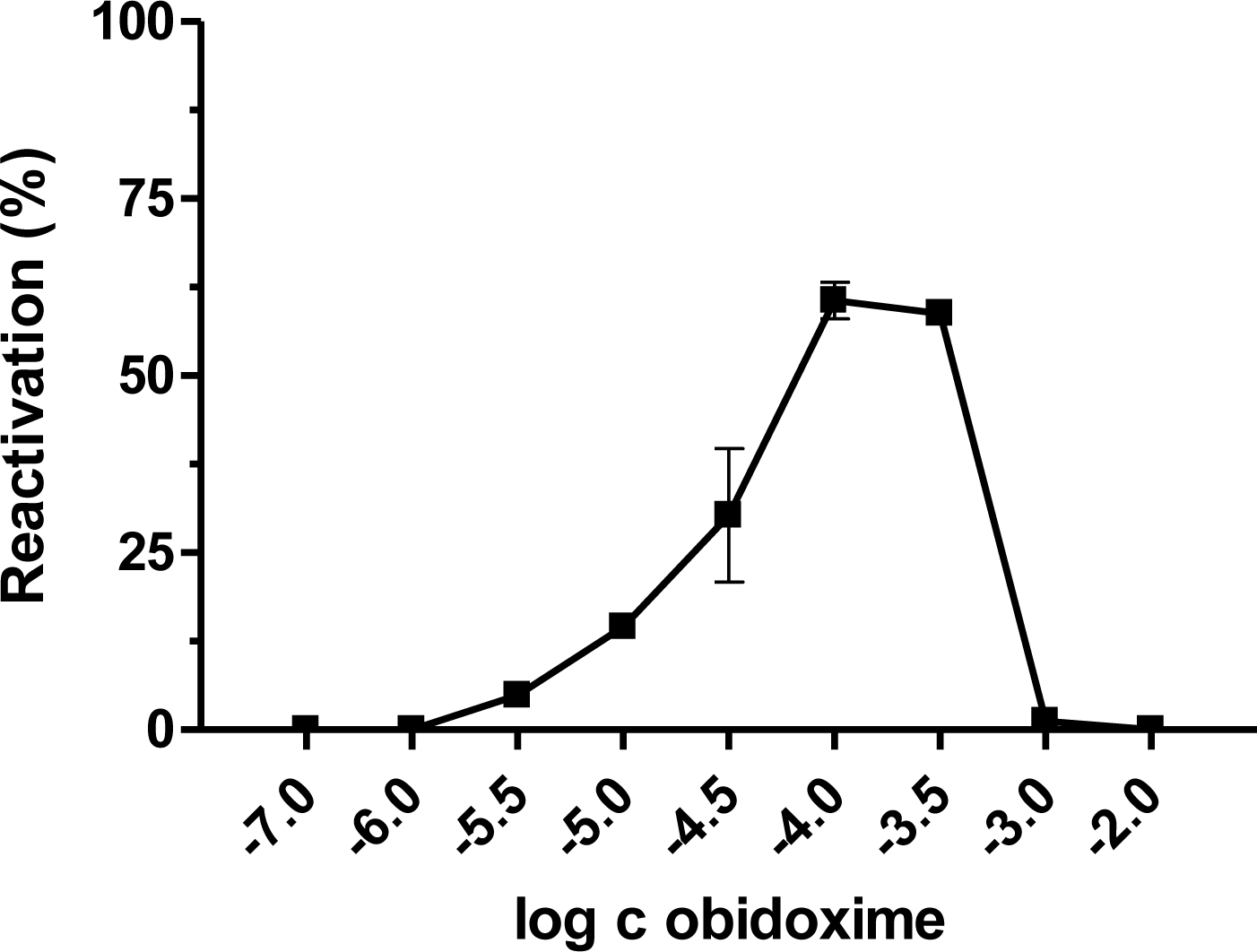


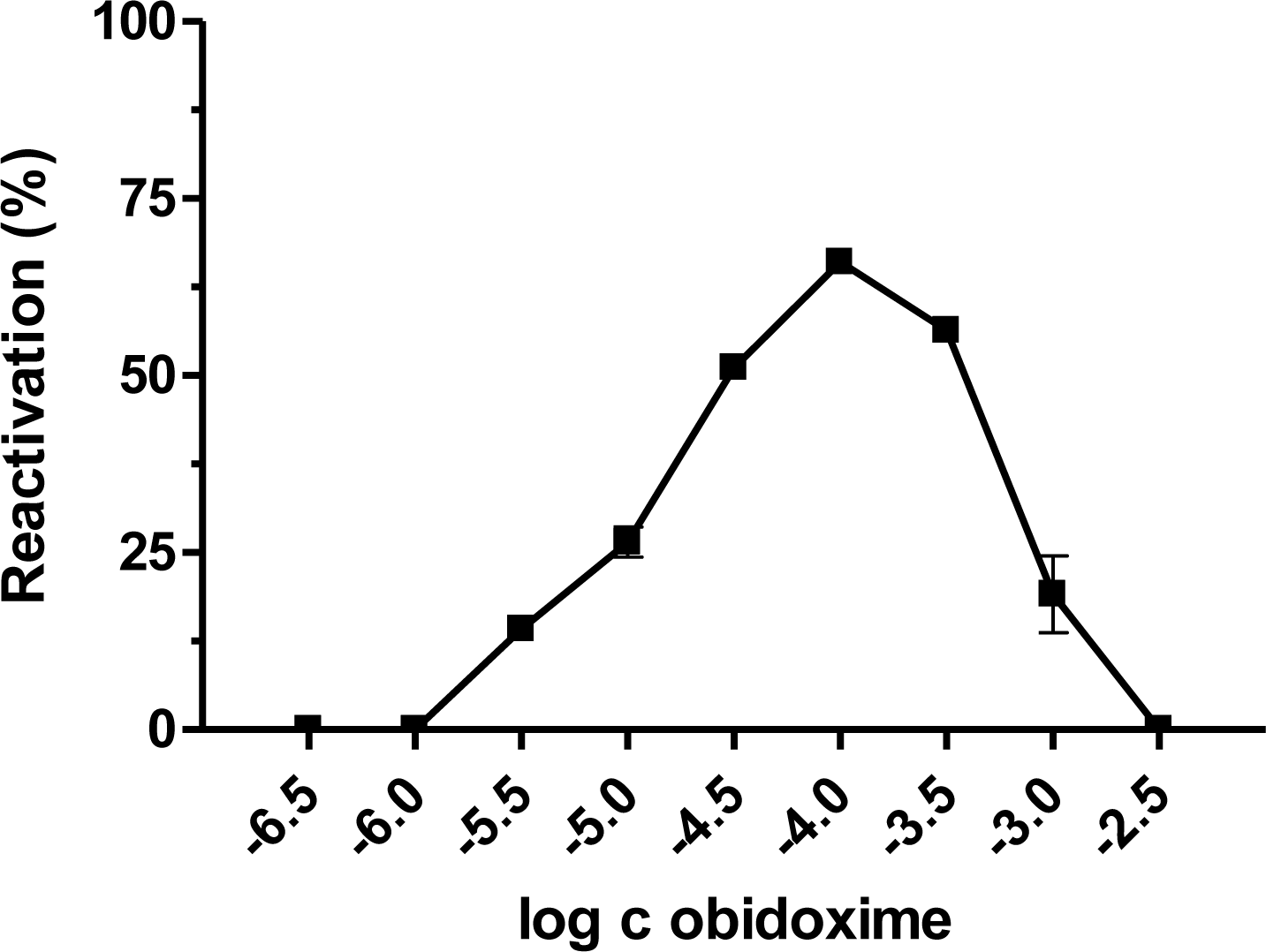
| AChE | Paraoxon [17] | Dichlorvos | DFP [18] | Leptophos-oxon [19] | Methamidophos | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 μM | 10 μM | 100 μM | 10 μM | 100 μM | 10 μM | 100 μM | 10 μM | 100 μM | 10 μM | |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Methoxime | 23.4 | 4.5 | 4.3 | 1.5 | 0 | 0 | 0.2 | 0.6 | 6.4 | 0.5 | 1.5 | 0.5 | 52.6 | 0.5 | 11.9 | 0.9 | 61.7 | 2.4 | 68.1 | 11.4 |
| Pralidoxime | 18.1 | 0.9 | 1.3 | 0.9 | 2.6 | 0.6 | 0.2 | 0.6 | 2.3 | 0.2 | 0 | 0 | 13.2 | 0.9 | 4.1 | 1.3 | 53.4 | 3.1 | 53.8 | 22.6 |
| Obidoxime | 96.8 | 0.9 | 59.4 | 0.9 | 0 | 0 | 0.6 | 0.1 | 17.1 | 0.1 | 7.4 | 0.5 | 50.3 | 0.9 | 31.4 | 0.2 | 5.8 | 4.8 | 57.0 | 18.7 |
| Trimedoxime | 86.0 | 1.6 | 45.3 | 0.8 | 0 | 0 | 0 | 0 | 23.8 | 0.2 | 6.4 | 0.2 | 51.3 | 0.5 | 26.4 | 2.7 | 9.4 | 7.5 | 53.1 | 10.9 |
| HI-6 | 16.1 | 0.2 | 3.9 | 0.9 | 0 | 0 | 0.6 | 1.1 | 0 | 0 | 0 | 0 | 32.8 | 8.0 | 11.6 | 0.4 | 37.4 | 12.3 | 75.2 | 14.6 |
| BChE | Paraoxon [17] | Dichlorvos | DFP [18] | Leptophos-oxon [19] | Methamidophos | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 μM | 10 μM | 100 μM | 10 μM | 100 μM | 10 μM | 100 μM | 10 μM | 100 μM | 10 μM | |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Methoxime | 6.1 | 0.6 | 0.9 | 0.8 | 0.2 | 0.1 | 0.2 | 0.1 | 8.2 | 1.3 | 0.8 | 0.5 | 6.4 | 0.4 | 1.9 | 1.8 | 4.8 | 0.2 | 1.0 | 0.2 |
| Pralidoxime | 5.5 | 0.1 | 1.0 | 0.3 | 1.0 | 0.1 | 0.4 | 0.2 | 6.4 | 0.8 | 0.7 | 0.1 | 2.3 | 1.8 | 0 | 0 | 3.5 | 0.3 | 0 | 0 |
| Obidoxime | 9.9 | 0.4 | 2.2 | 0.4 | 3.1 | 0.2 | 1.6 | 0.4 | 9.5 | 1.0 | 1.5 | 0.6 | 14.3 | 0.6 | 6.5 | 4.2 | 4.2 | 0.3 | 1.0 | 0.2 |
| Trimedoxime | 12.1 | 1.7 | 1.3 | 0.3 | 1.2 | 0.1 | 0.4 | 0.2 | 7.3 | 0.5 | 0.8 | 0.5 | 8.5 | 2.4 | 2.1 | 0.4 | 5.2 | 0.7 | 0.6 | 0.8 |
| HI-6 | 2.3 | 0.3 | 0.8 | 0.5 | 0.6 | 0.1 | 0.4 | 0.1 | 3.8 | 0.1 | 0.7 | 0.2 | 5.6 | 4.9 | 0 | 0 | 4.8 | 0.2 | 0.1 | 0.2 |
| Inhibitor | AChE | BChE | ||
|---|---|---|---|---|
| IC95 (M) | 7 × T1/2 (min) | IC95 (M) | 7 × T1/2 (min) | |
| Paraoxon | 3.38 × 10−6 | 2.17 | 1.41 × 10−7 | 1.82 |
| Dichlorvos | 3.30 × 10−4 | 0.32 | 2.08 × 10−6 | 2.20 |
| DFP | 5.00 × 10−6 | 0.93 | 8.30 × 10−8 | 1.75 |
| Leptophos−oxon | 4.16 × 10−7 | 2.45 | 7.06 × 10−6 | 0.75 |
| Methamidophos | 4.26 × 10−5 | 2.22 | 2.08 × 10−4 | 2.83 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jun, D.; Musilova, L.; Musilek, K.; Kuca, K. In Vitro Ability of Currently Available Oximes to Reactivate Organophosphate Pesticide-Inhibited Human Acetylcholinesterase and Butyrylcholinesterase. Int. J. Mol. Sci. 2011, 12, 2077-2087. https://doi.org/10.3390/ijms12032077
Jun D, Musilova L, Musilek K, Kuca K. In Vitro Ability of Currently Available Oximes to Reactivate Organophosphate Pesticide-Inhibited Human Acetylcholinesterase and Butyrylcholinesterase. International Journal of Molecular Sciences. 2011; 12(3):2077-2087. https://doi.org/10.3390/ijms12032077
Chicago/Turabian StyleJun, Daniel, Lucie Musilova, Kamil Musilek, and Kamil Kuca. 2011. "In Vitro Ability of Currently Available Oximes to Reactivate Organophosphate Pesticide-Inhibited Human Acetylcholinesterase and Butyrylcholinesterase" International Journal of Molecular Sciences 12, no. 3: 2077-2087. https://doi.org/10.3390/ijms12032077