Inhibition of Extracellular Signal-Regulated Kinases Ameliorates Hypertension-Induced Renal Vascular Remodeling in Rat Models
Abstract
:1. Introduction
2. Results and Discussion
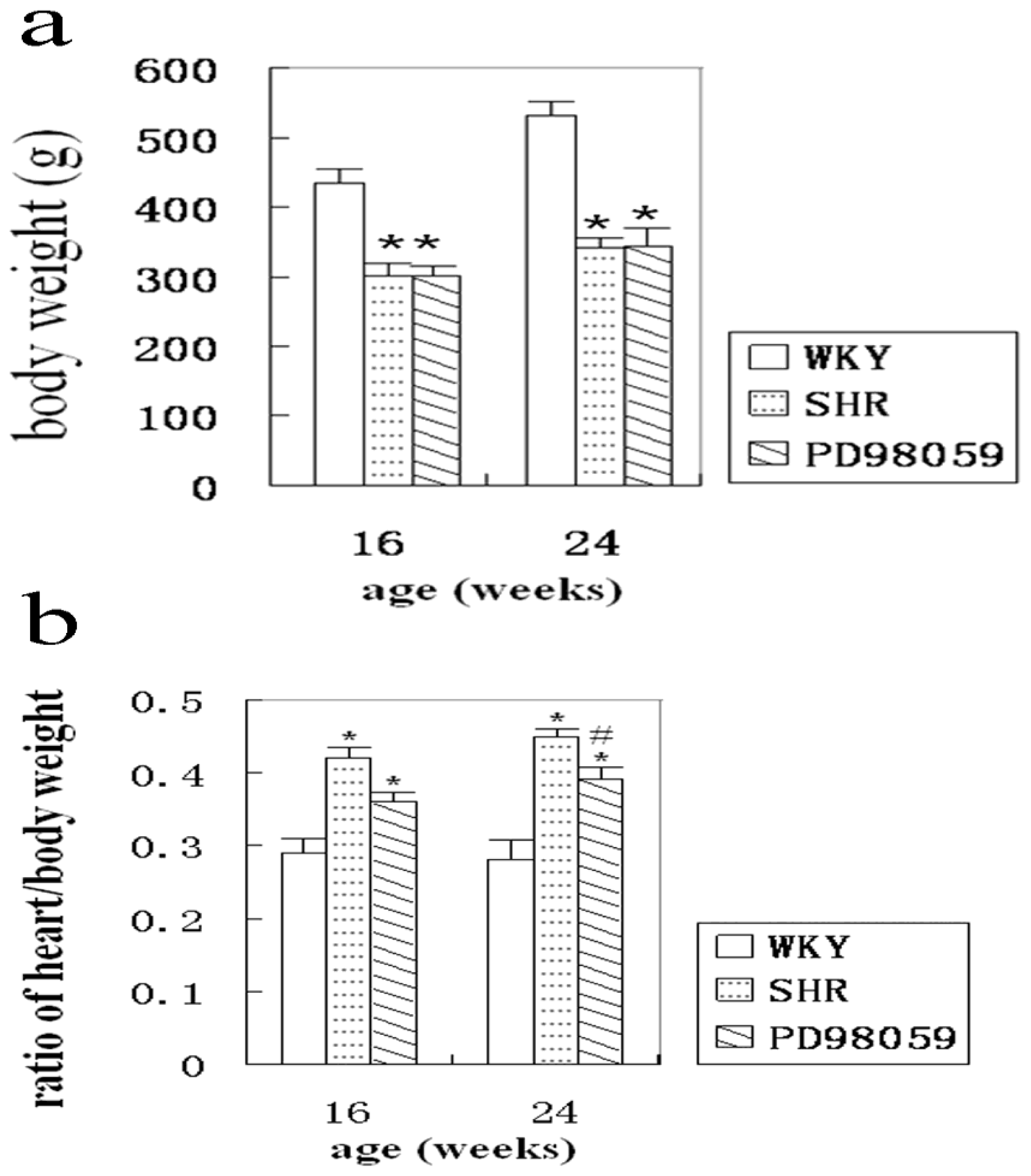
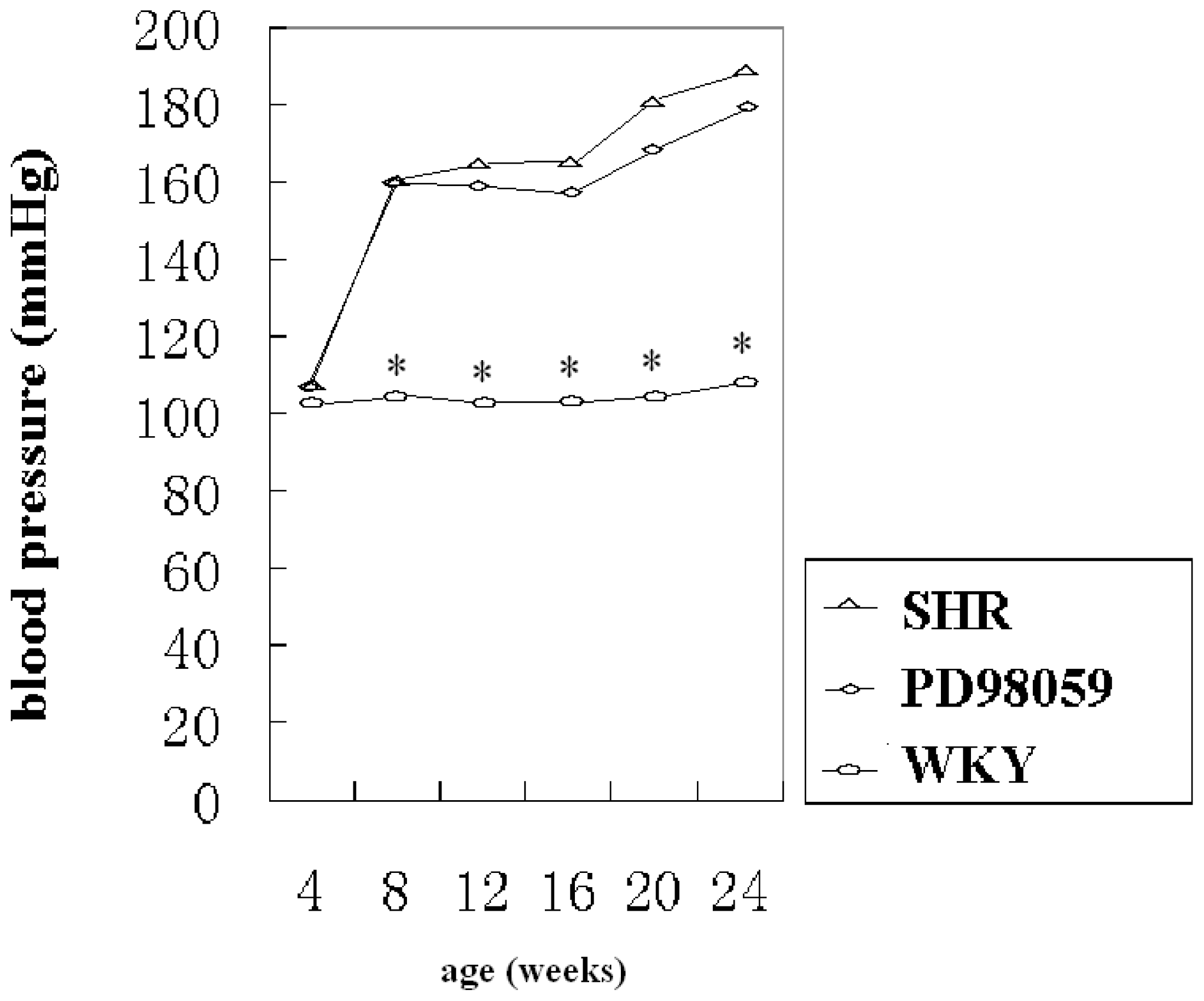
2.1. Physiological Variable
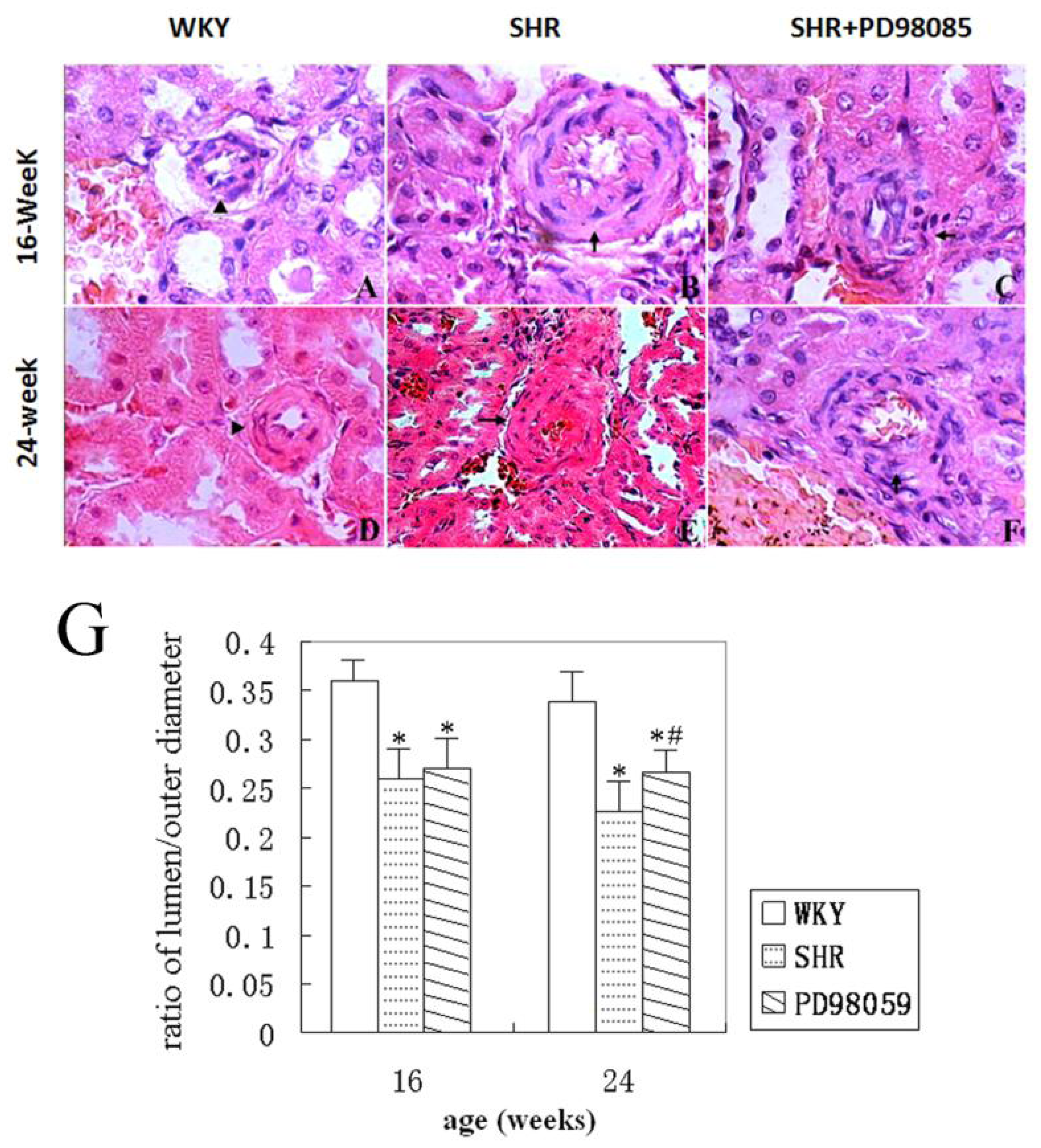
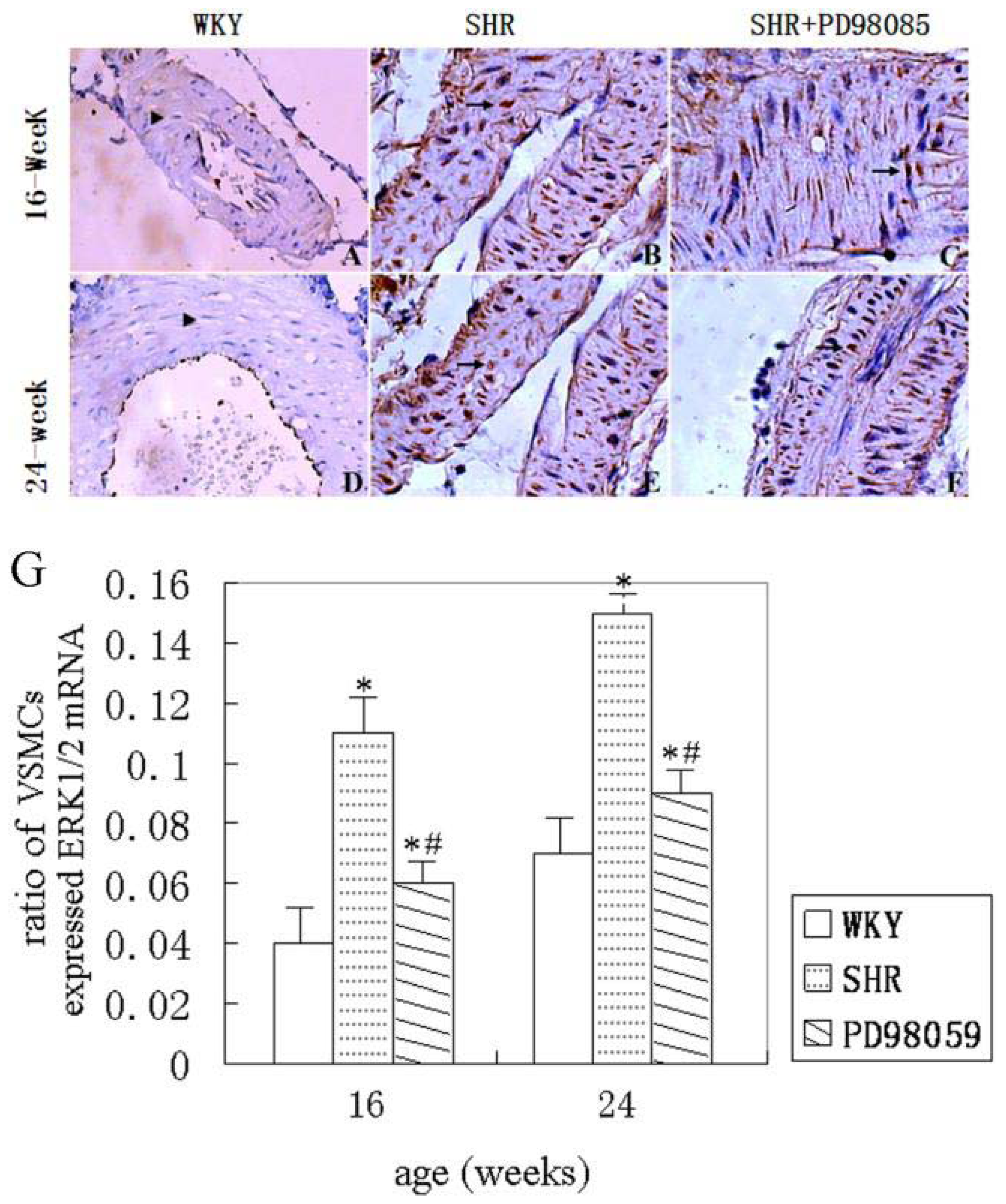
2.2. Vascular Wall Morphology
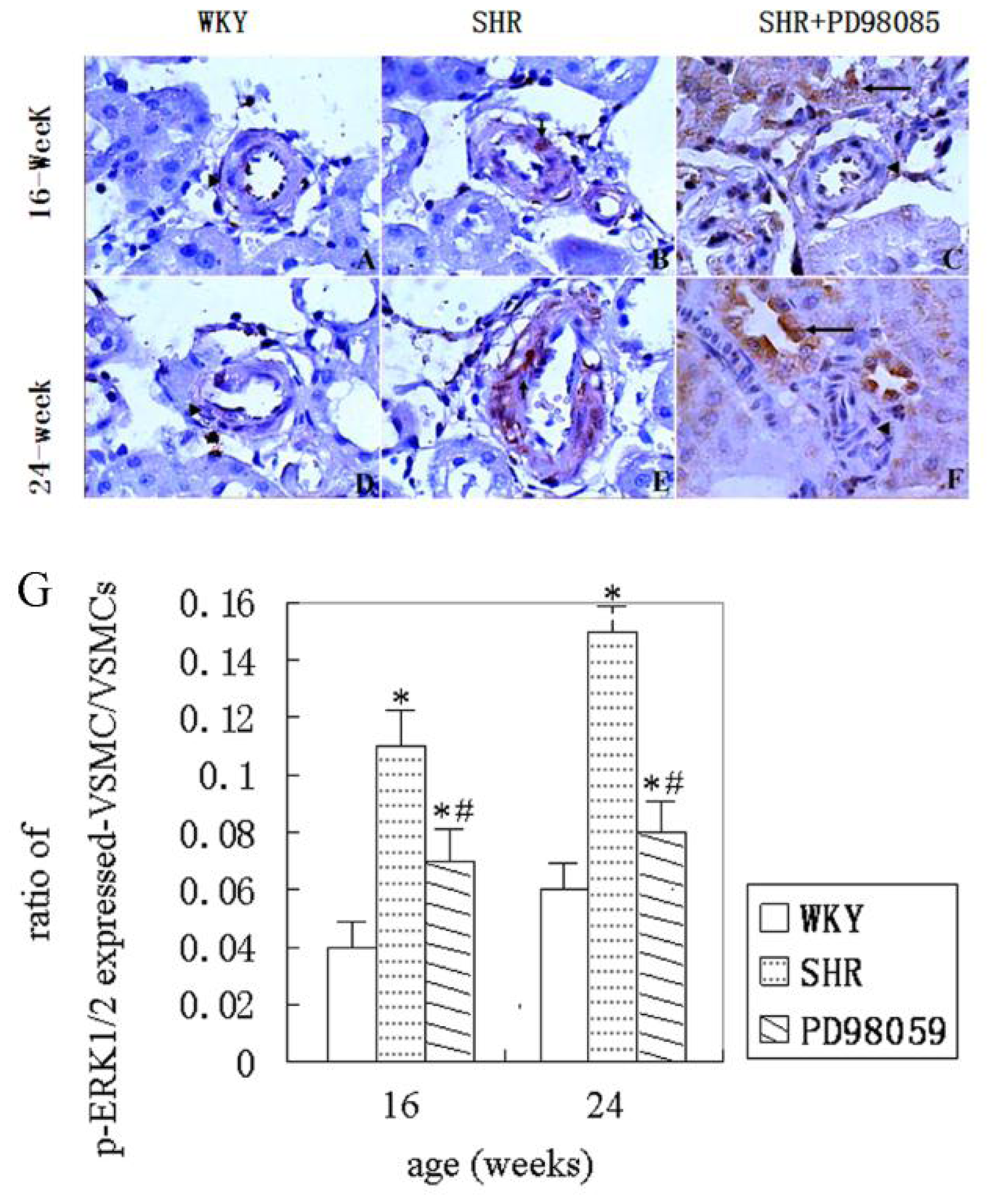
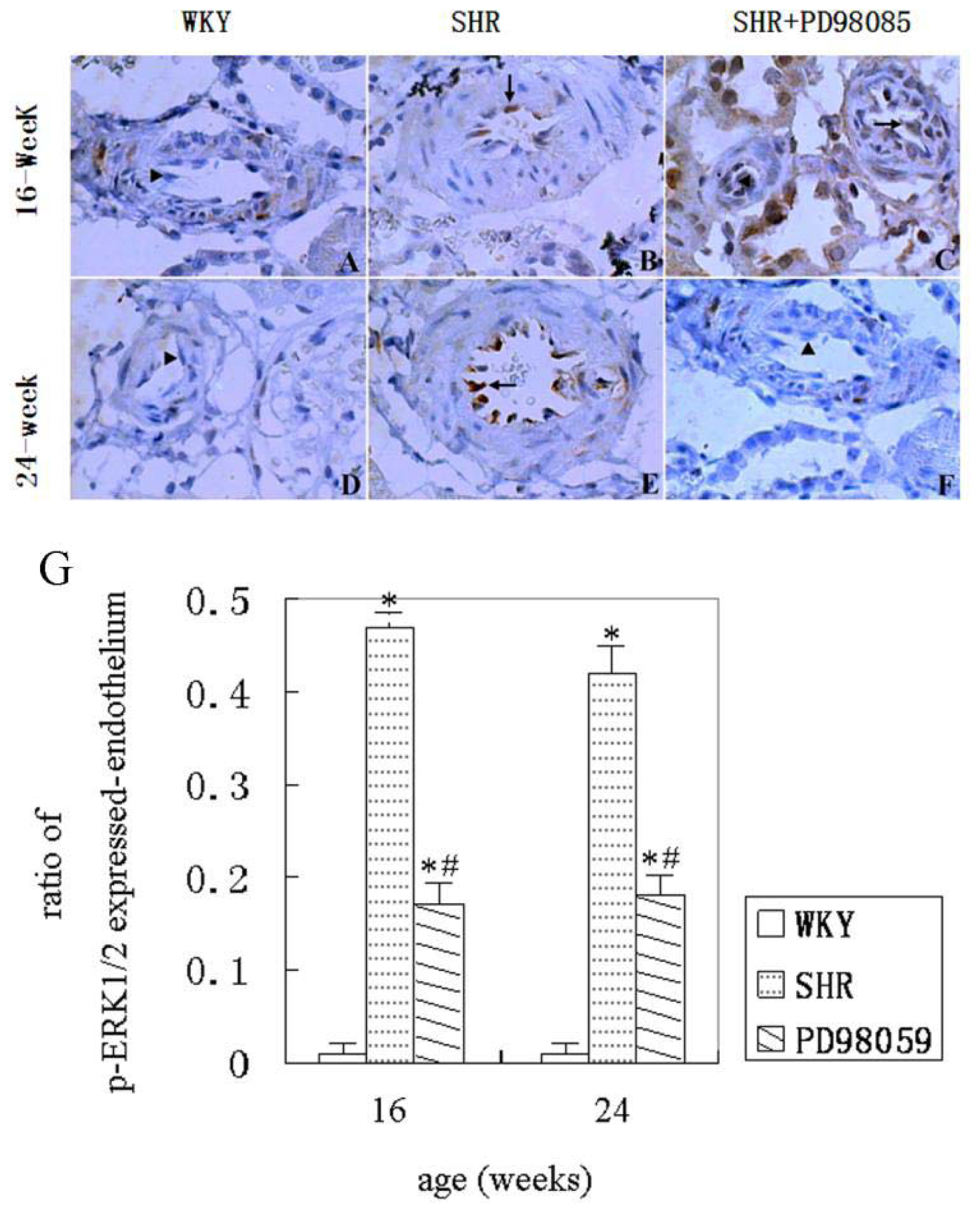
2.3. ERK Protein and mRNA Expression
2.4. Discussion
3. Materials and Methods
3.1. Animals and Reagents
3.2. Animal Treatments
3.3. Immunohistochemistry
3.4. In Situ Hybridization
3.5. Statistical Analysis
4. Conclusion
Acknowledgments
References
- Edgley, A.J.; Kett, M.M.; Anderson, W.P. Evidence for renal vascular remodeling in angiotensin II-induced hypertension. J. Hypertens 2003, 21, 1401–1406. [Google Scholar]
- Touyz, R.M. Vascular remodeling, retinal arteries, and hypertension. Hypertension 2007, 50, 603–604. [Google Scholar]
- Shenoy, V.; Ferreira, A.J.; Qi, Y.; Fraga-Silva, R.A.; Díez-Freire, C.; Dooies, A.; Jun, J.Y.; Sriramula, S.; Mariappan, N.; Pourang, D.; et al. The angiotensin-converting enzyme 2/angiogenesis-(1–7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am. J. Respir. Crit. Care Med 2010, 182, 1065–1072. [Google Scholar]
- Poli, G.; Sottero, B.; Gargiulo, S.; Leonarduzzi, G. Cholesterol oxidation products in the vascular remodeling due to atherosclerosis. Mol. Aspects Med 2009, 30, 180–189. [Google Scholar]
- Laplante, M.A.; Wu, R.; El Midaoui, A.; de Champlain, J. NAD(P)H oxidase activation by angiotensin II is dependent on p42/44 ERK-MAPK pathway activation in rat’s vascular smooth muscle cells. J. Hypertens 2003, 21, 927–936. [Google Scholar]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1284. [Google Scholar]
- Jing, L.; Zhang, J.Z.; Zhao, L.; Wang, Y.L.; Guo, F.Y. High-expression of transforming growth factor β1 and phosphorylation of extracellular signal-regulated protein kinase in vascular smooth muscle cells from aorta and renal arterioles of spontaneous hypertension rats. Clin. Exp. Hypertens 2007, 29, 107–117. [Google Scholar]
- Hayashi, K.; Naiki, T. Adaption and remodeling of vascular wall; biomechanical response to hypertension. J. Mech. Behav. Biomed. Mater 2009, 2, 3–19. [Google Scholar]
- Belmadani, S.; Zerfaoui, M.; Boulares, H.A.; Palen, D.I.; Matrougui, K. Micorvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, αvβ3-integrin, and TGF-β1 in response to ANG II and high glucose. Am. J. Physiol. Heart Circ. Physiol 2008, 295, H69–H76. [Google Scholar]
- Luo, D.X.; Cheng, J.; Xiong, Y.; Li, J.; Xia, C.; Wang, C.; Zhu, B.; Hu, Z.; Liao, D.F. Static pressure drives proliferation of vascular smooth muscle cells via caveolin-1/ERK1/2 pathway. Biochem. Biophys. Res. Commun 2010, 391, 1693–1697. [Google Scholar]
- Jing, L.; Zhang, J.Z.; Guo, F.Y. Over-expression of extracellular signal-regulated kinase in vascular smooth muscle cell of hypertensive rats. Chin. Med. Sci. J 2006, 21, 36–40. [Google Scholar]
- St Lezin, E.; Zhang, L.; Yang, Y.; Wang, J.M.; Wang, N.; Qi, N.; Steadman, J.S.; Liu, W.; Kren, V.; Zidek, V.; et al. Effect of chromosome 19 transfer on blood pressure in the spontaneously hypertensive rat. Hypertension 1999, 33, 256–260. [Google Scholar]
- van den Akker, J.; Schoorl, M.J.; Bakker, E.N.; Vanbavel, E. Small Artery Remodeling: Current Concepts and Questions. J. Vasc. Res 2009, 47, 183–202. [Google Scholar]
- Ibrahim, J.; Berk, B.C. Flow-mediated vascular remodeling in hypertension: relation to hemodyamics. Stroke 2009, 40, 582–590. [Google Scholar]
- Resta, T.C.; Broughton, B.R.; Jernigan, N.L. Reactive oxygen species and RhoA signaling in vascular smooth muscle: role in chronic hypoxia-induced pulmonary hypertension. Adv. Exp. Med. Biol 2010, 661, 355–373. [Google Scholar]
- Castro, M.M.; Rizzi, E.; Figueiredo-Lopes, L.; Fernandes, K.; Bendhack, L.M.; Pitol, D.L.; Gerlach, R.F.; Tanus-Santos, J.E. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis 2008, 198, 320–331. [Google Scholar]
- Bonnet, S.; Paulin, R.; Sutendra, G.; Dromparis, P.; Roy, M.; Watson, K.O.; Nagendran, J.; Haromy, A.; Dyck, J.R.; Michelakis, E.D. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-β/NFAT axis. Circulation 2009, 120, 1231–1240. [Google Scholar]
- Lu, X.; Murphy, T.C.; Nanes, M.S.; Hart, C.M. PPARγ regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-κB. Am. J. Physiol. Lung Cell Mol. Physiol 2010, 299, 559–566. [Google Scholar]
- Hänze, J.; Weissmann, N.; Grimminger, F.; Seeger, W.; Rose, F. Cellular and molecular mechanisms of hypoxia-inducible factor driven vascular remodeling. Thromb. Haemost 2007, 97, 774–787. [Google Scholar]
- Miyata, K.; Hitomi, H.; Guo, P.; Zhang, G.X.; Kimura, S.; Kiyomoto, H.; Hosomi, N.; Kagami, S.; Kohno, M.; Nishiyama, A. Possible involvement of Rho-kinase in aldosterone-induced vascular smooth muscle cell remodeling. Hypertens. Res 2008, 31, 1407–1413. [Google Scholar]
- Meredith, D.; Panchatcharam, M.; Miriyala, S.; Tsai, Y.S.; Morris, A.J.; Maeda, N.; Stouffer, G.A.; Smyth, S.S. Dominant-negative loss of PPAR gamma function enhances smooth muscle cell proliferation, migration, and vascular remodeling. Arterioscler. Thromb. Vasc. Biol 2009, 29, 465–471. [Google Scholar]
- Wong, K.K. Recent developments in anti-cancer agents targeting the Ras/Raf/MEK/ERK pathway. Recent Pat. Anticancer Drug Discov 2009, 4, 28–35. [Google Scholar]
- Schnidar, H.; Eberl, M.; Klingler, S.; Mangelberger, D.; Kasper, M.; Hauser-Kronberger, C.; Regl, G.; Kroismayr, R.; Moriggl, R.; Sibilia, M.; Aberger, F. Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res 2009, 69, 1284–1292. [Google Scholar]
- Thaler, S.; Hähnel, P.S.; Schadn, A.; Dammann, R.; Schuler, M. RASSF1A mediates p21Cip1/Waf1-dependent cell cycle arrest and senescence through modulation of the Raf-MEK-ERK pathway and inhibition of Akt. Cancer Res 2009, 69, 1748–1757. [Google Scholar]
- Nakano, S.; Kobayashi, N.; Yoshida, K.; Ohno, T.; Matsuoka, H. Cardioprotective mechanisms of spironolactone associated with the angiotensin-converting enzyme/epidermal growth factor receptor/extracellular signal-regulated kinases, NAD(P)H oxidase/lectin-like oxidized low-density lipoprotein receptor-1, and Rho-kinase pathways in aldosterone/salt-induced hypertensive rats. Hypertens. Res 2005, 28, 925–936. [Google Scholar]
- Wojcicka, G.; Jamroz-Wisniewska, A.; Windomska, S.; Ksiazek, M.; Beltowski, J. Role of extracellular signal-regulated kinases (ERK) in leptin-induced hypertension. Life Sci 2008, 82, 402–412. [Google Scholar]
- Ljuca, F.; Drevenšek, G.; Zerem, E. Contribution of Ras farnesyl transferase, MAP kinase and cytochrome P-450 metabolites to endothelin-1 induced hypertension. Bosn. J. Basic Med. Sci 2011, 11, 84–86. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jing, L.; Zhang, J.; Sun, J.; Guo, F.; An, X.; Yang, K.; Li, P.A. Inhibition of Extracellular Signal-Regulated Kinases Ameliorates Hypertension-Induced Renal Vascular Remodeling in Rat Models. Int. J. Mol. Sci. 2011, 12, 8333-8346. https://doi.org/10.3390/ijms12128333
Jing L, Zhang J, Sun J, Guo F, An X, Yang K, Li PA. Inhibition of Extracellular Signal-Regulated Kinases Ameliorates Hypertension-Induced Renal Vascular Remodeling in Rat Models. International Journal of Molecular Sciences. 2011; 12(12):8333-8346. https://doi.org/10.3390/ijms12128333
Chicago/Turabian StyleJing, Li, Jianzhong Zhang, Jinping Sun, Fengying Guo, Xin An, Kan Yang, and Ping Andy Li. 2011. "Inhibition of Extracellular Signal-Regulated Kinases Ameliorates Hypertension-Induced Renal Vascular Remodeling in Rat Models" International Journal of Molecular Sciences 12, no. 12: 8333-8346. https://doi.org/10.3390/ijms12128333



