Tunicamycin Depresses P-Glycoprotein Glycosylation Without an Effect on Its Membrane Localization and Drug Efflux Activity in L1210 Cells
Abstract
:1. Introduction
2. Results and Discussion
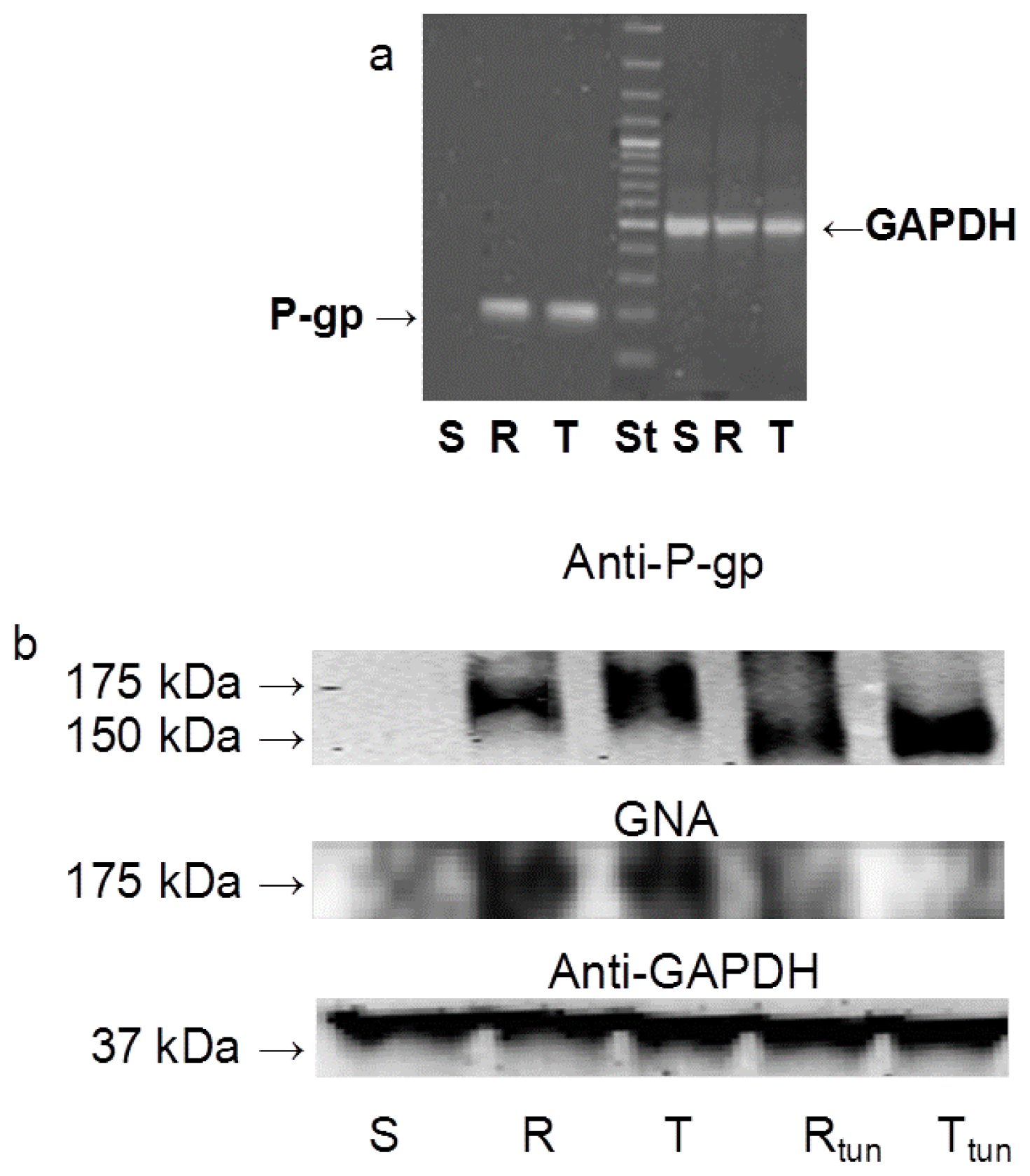
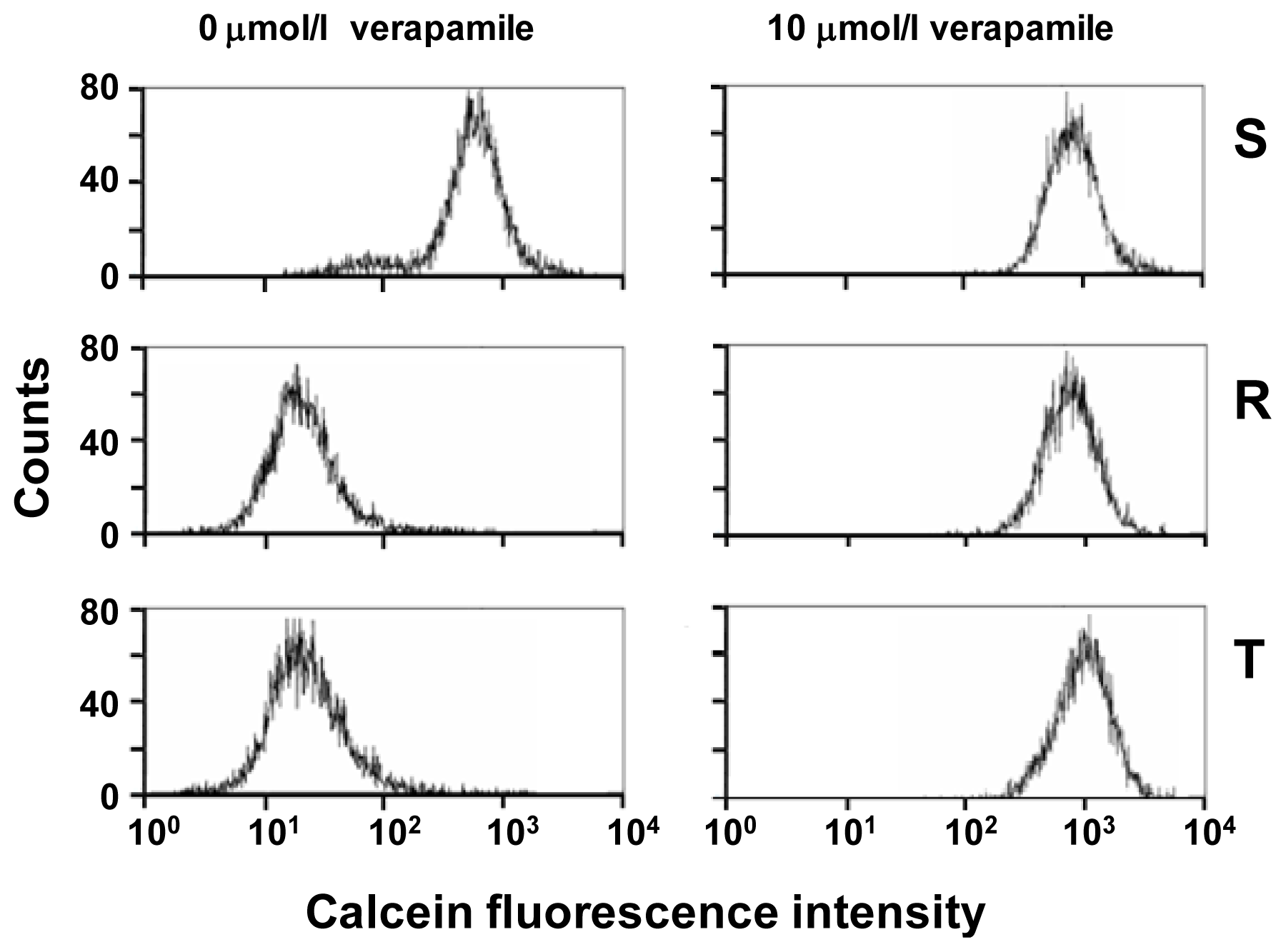
2.1. P-gp Expression and Transport Activity in P-gp-Positive L1210 Cell Variants
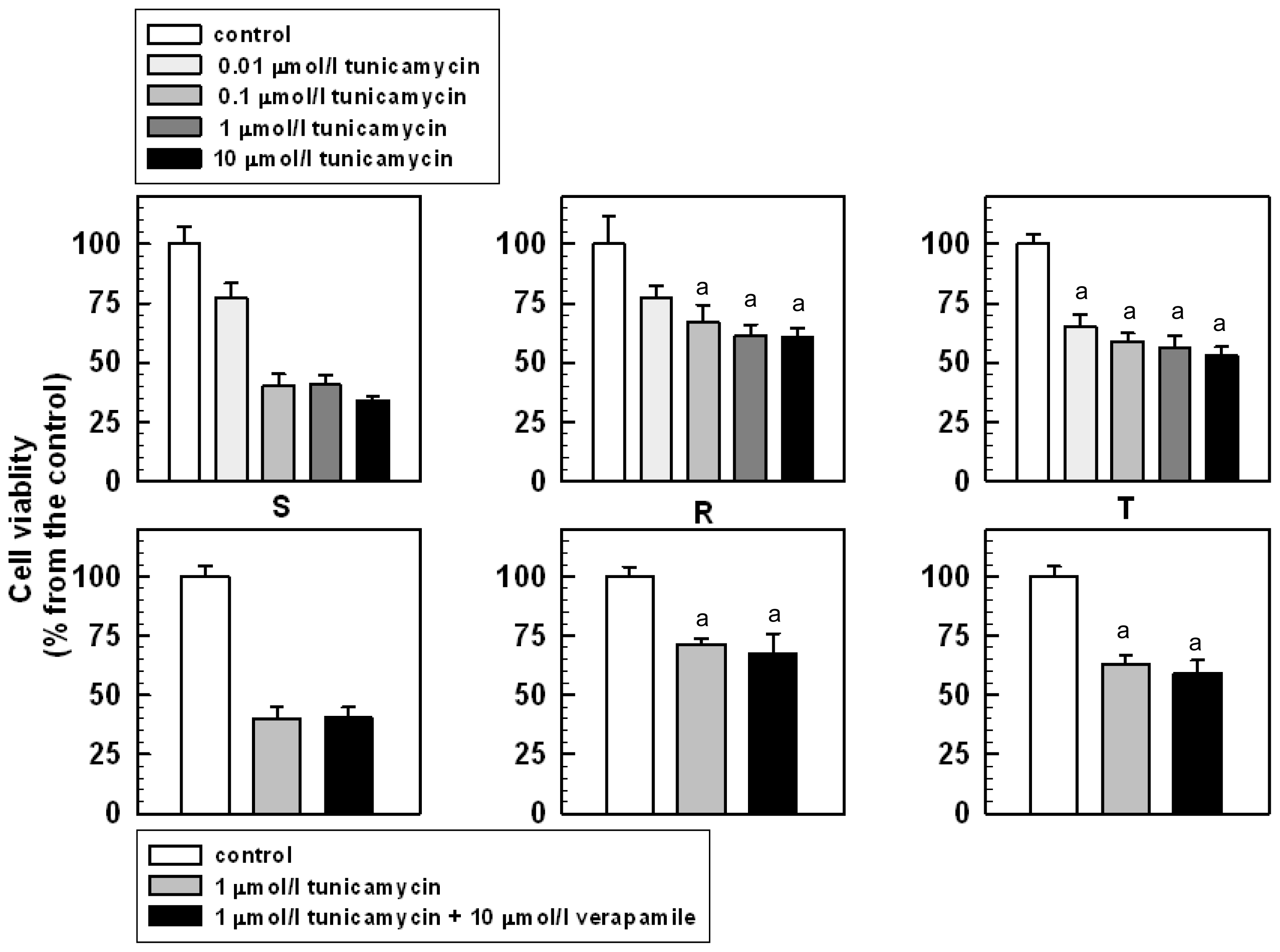
2.2. The Effect of Tunicamycin on the Viability and Proliferation of S, R and T Cells
2.3 The Effect of Tunicamycin on Glycosylation and Membrane Localization of P-gp
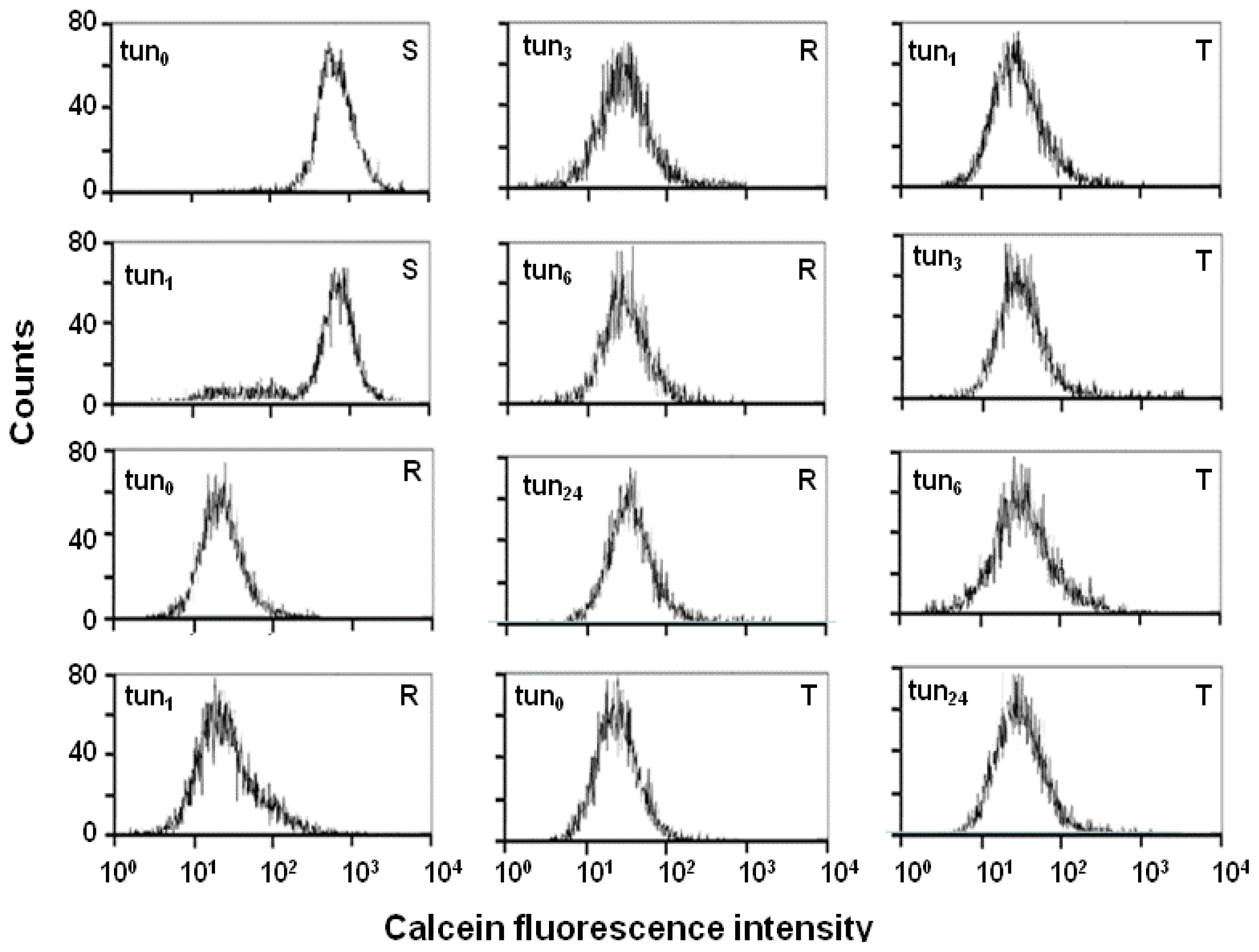
2.4. Effect of Tunicamycin on P-gp Transport Activity
3. Experimental Section
3.1. Cell Culture Conditions
3.2. Detection of the P-gp mRNA
3.3. Examination of P-gp Function Using Calcein/AM Assay
3.4. The Effect of Tunicamycin on S, R and T Cell Proliferation
3.5. Examination of the Effect of Tunicamycin on P-gp Levels in S, R and T Cells Using Western Blot and GNA Blot Analysis
3.6. Visualization of the P-gp in S, R and T Cells Using Immunofluorescence Confocal Microscopy
4. Conclusions
Supplementary Material
ijms-12-07772-s001.pdfAcknowledgments
References
- Breier, A.; Barancik, M.; Sulova, Z.; Uhrik, B. P-glycoprotein—implications of metabolism of neoplastic cells and cancer therapy. Curr. Cancer Drug Targets 2005, 5, 457–468. [Google Scholar]
- Baguley, B.C. Multidrug resistance in cancer. Methods Mol. Biol 2010, 596, 1–14. [Google Scholar]
- Breier, A.; Drobna, Z.; Docolomansky, P.; Barancik, M. Cytotoxic activity of several unrelated drugs on L1210 mouse leukemic cell sublines with P-glycoprotein (PGP) mediated multidrug resistance (MDR) phenotype. A QSAR study. Neoplasma 2000, 47, 100–106. [Google Scholar]
- Greer, D.A.; Ivey, S. Distinct N-glycan glycosylation of P-glycoprotein isolated from the human uterine sarcoma cell line MES-SA/Dx5. Biochim. Biophys. Acta 2007, 1770, 1275–1282. [Google Scholar]
- Loo, T.W.; Bartlett, M.C.; Clarke, D.M. Thapsigargin or curcumin does not promote maturation of processing mutants of the ABC transporters, CFTR, and P-glycoprotein. Biochem. Biophys. Res. Commun 2004, 325, 580–585. [Google Scholar]
- Schinkel, A.H.; Kemp, S.; Dolle, M.; Rudenko, G.; Wagenaar, E. N-glycosylation and deletion mutants of the human MDR1 P-glycoprotein. J. Biol. Chem 1993, 268, 7474–7481. [Google Scholar]
- Loo, T.W.; Clarke, D.M. Quality control by proteases in the endoplasmic reticulum. Removal of a protease-sensitive site enhances expression of human P-glycoprotein. J. Biol. Chem 1998, 273, 32373–32376. [Google Scholar]
- Bretthauer, R.K. Structure, expression, and regulation of UDP-GlcNAc: dolichol phosphate GlcNAc-1-phosphate transferase (DPAGT1). Curr. Drug Targets 2009, 10, 477–482. [Google Scholar]
- Ledoux, S.; Yang, R.; Friedlander, G.; Laouari, D. Glucose depletion enhances P-glycoprotein expression in hepatoma cells: role of endoplasmic reticulum stress response. Cancer Res 2003, 63, 7284–7290. [Google Scholar]
- Gaddameedhi, S.; Chatterjee, S. Association between the unfolded protein response, induced by 2-deoxyglucose, and hypersensitivity to cisplatin: a mechanistic study employing molecular genomics. J. Cancer Res. Ther 2009, 5, S61–S66. [Google Scholar]
- Werno, C.; Zhou, J.; Brune, B. A23187, ionomycin and thapsigargin upregulate mRNA of HIF-1alpha via endoplasmic reticulum stress rather than a rise in intracellular calcium. J. Cell Physiol 2008, 215, 708–714. [Google Scholar]
- Hiss, D.C.; Gabriels, G.A.; Folb, P.I. Combination of tunicamycin with anticancer drugs synergistically enhances their toxicity in multidrug-resistant human ovarian cystadenocarcinoma cells. Cancer Cell Int 2007, 7, 5. [Google Scholar] [Green Version]
- Zhang, Z.; Wu, J.Y.; Hait, W.N.; Yang, J.M. Regulation of the stability of P-glycoprotein by ubiquitination. Mol. Pharmacol 2004, 66, 395–403. [Google Scholar]
- Polekova, L.; Barancik, M.; Mrazova, T.; Pirker, R.; Wallner, J.; Sulova, Z.; Breier, A. Adaptation of mouse leukemia cells L1210 to vincristine. Evidence for expression of P-glycoprotein. Neoplasma 1992, 39, 73–77. [Google Scholar]
- Sulova, Z.; Ditte, P.; Kurucova, T.; Polakova, E.; Rogozanova, K.; Gibalova, L.; Seres, M.; Skvarkova, L.; Sedlak, J.; Pastorek, J.; et al. The presence of P-glycoprotein in L1210 cells directly induces down-regulation of cell surface saccharide targets of concanavalin A. Anticancer Res 2010, 30, 3661–3668. [Google Scholar]
- Fiala, R.; Sulova, Z.; El-Saggan, A.H.; Uhrik, B.; Liptaj, T.; Dovinova, I.; Hanusovska, E.; Drobna, Z.; Barancik, M.; Breier, A. P-glycoprotein-mediated multidrug resistance phenotype of L1210/VCR cells is associated with decreases of oligo- and/or polysaccharide contents. Biochim. Biophys. Acta 2003, 1639, 213–224. [Google Scholar]
- Sulova, Z.; Mislovicova, D.; Gibalova, L.; Vajcnerova, Z.; Polakova, E.; Uhrik, B.; Tylkova, L.; Kovarova, A.; Sedlak, J.; Breier, A. Vincristine-induced overexpression of P-glycoprotein in L1210 cells is associated with remodeling of cell surface saccharides. J. Proteome Res 2009, 8, 513–520. [Google Scholar]
- Parfett, C.L.; Jamieson, J.C.; Wright, J.A. Changes in cell surface glycoproteins on non-differentiating L6 rat myoblasts selected for resistance to concanavalin A. Exp. Cell Res 1983, 144, 405–415. [Google Scholar]
- Eneroth, A.; Astrom, E.; Hoogstraate, J.; Schrenk, D.; Conrad, S.; Kauffmann, H.M.; Gjellan, K. Evaluation of a vincristine resistant Caco-2 cell line for use in a calcein AM extrusion screening assay for P-glycoprotein interaction. Eur. J. Pharm. Sci 2001, 12, 205–214. [Google Scholar]
- Karaszi, E.; Jakab, K.; Homolya, L.; Szakacs, G.; Hollo, Z.; Telek, B.; Kiss, A.; Rejto, L.; Nahajevszky, S.; Sarkadi, B.; et al. Calcein assay for multidrug resistance reliably predicts therapy response and survival rate in acute myeloid leukaemia. Br. J. Haematol 2001, 112, 308–314. [Google Scholar]
- Ross, D.D. Modulation of drug resistance transporters as a strategy for treating myelodysplastic syndrome. Best Pract. Res. Clin. Haematol 2004, 17, 641–651. [Google Scholar]
- Morin, M.J.; Porter, C.W.; McKernan, P.; Bernacki, R.J. The biochemical and ultrastructural effects of tunicamycin and d-glucosamine in L1210 leukemic cells. J. Cell Physiol 1983, 114, 162–172. [Google Scholar]
- Seres, M.; Ditte, P.; Breier, A.; Sulova, Z. Effect of thapsigargin on P-glycoprotein-negative and P-glycoprotein-positive L1210 mouse leukaemia cells. Gen. Physiol. Biophys 2010, 29, 396–401. [Google Scholar]
- Kramer, R.; Weber, T.K.; Arceci, R.; Ramchurren, N.; Kastrinakis, W.V.; Steele, G., Jr; Summerhayes, I.C. Inhibition of N-linked glycosylation of P-glycoprotein by tunicamycin results in a reduced multidrug resistance phenotype. Br. J. Cancer 1995, 71, 670–675. [Google Scholar]
- Bentley, J.; Quinn, D.M.; Pitman, R.S.; Warr, J.R.; Kellett, G.L. The human KB multidrug-resistant cell line KB-C1 is hypersensitive to inhibitors of glycosylation. Cancer Lett 1997, 115, 221–227. [Google Scholar]
- Gervasoni, J.E., Jr; Taub, R.N.; Rosado, M.; Krishna, S.; Stewart, V.J.; Knowles, D.M.; Bhalla, K.; Ross, D.D.; Baker, M.A.; Lutzky, J.; et al. Membrane glycoprotein changes associated with anthracycline resistance in HL-60 cells. Cancer Chemother. Pharmacol. 1991, 28, 93–101. [Google Scholar]
- Pastan, I.; Gottesman, M.M.; Ueda, K.; Lovelace, E.; Rutherford, A.V.; Willingham, M.C. A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P-glycoprotein in MDCK cells. Proc. Natl. Acad. Sci. USA 1988, 85, 4486–4490. [Google Scholar]
- Gerlier, D.; Thomasset, N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 1986, 94, 57–63. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar]
- Krishan, A.; Dandekar, P.D. DAPI fluorescence in nuclei isolated from tumors. J. Histochem. Cytochem 2005, 53, 1033–1036. [Google Scholar]

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Šereš, M.; Cholujová, D.; Bubenčíkova, T.; Breier, A.; Sulová, Z. Tunicamycin Depresses P-Glycoprotein Glycosylation Without an Effect on Its Membrane Localization and Drug Efflux Activity in L1210 Cells. Int. J. Mol. Sci. 2011, 12, 7772-7784. https://doi.org/10.3390/ijms12117772
Šereš M, Cholujová D, Bubenčíkova T, Breier A, Sulová Z. Tunicamycin Depresses P-Glycoprotein Glycosylation Without an Effect on Its Membrane Localization and Drug Efflux Activity in L1210 Cells. International Journal of Molecular Sciences. 2011; 12(11):7772-7784. https://doi.org/10.3390/ijms12117772
Chicago/Turabian StyleŠereš, Mário, Dana Cholujová, Tatiana Bubenčíkova, Albert Breier, and Zdenka Sulová. 2011. "Tunicamycin Depresses P-Glycoprotein Glycosylation Without an Effect on Its Membrane Localization and Drug Efflux Activity in L1210 Cells" International Journal of Molecular Sciences 12, no. 11: 7772-7784. https://doi.org/10.3390/ijms12117772



