1. Introduction
Protein microarrays are widely used tools for diagnostic biomarker analysis, with proven utility in such varied fields of human medicine as for instance autoimmunity, allergy, infection and cancer [
1]. In particular, the ability of protein microarrays to simultaneously determine presence or levels of a large number of biomolecules is useful as a means to screen a clinical sample for a variety of different conditions such as cancers or autoimmune diseases that have not yet started to manifest as symptoms or other detectable signs of disease [
2], or it can serve to refine the resolution in the differentiation between for instance infections from closely related bacterial hosts [
3]. Further, protein microarrays may improve the diagnostic accuracy in cancer diagnosis [
4]. As all the individual microspot assays simultaneously taking place on a microarray are directed to the exact same sample, the potential variability arising from aliquot preparations for the same number of simplex-assays is also conveniently avoided.
The assay procedures for protein microarrays are low- to moderately labor-intensive, and may due to the ambient-analyte conditions governing the microspot assay dynamics offer excellent sensitivity on par with ELISAs [
1]. However, due to the many assay steps involved and the required convective mixing in the sample incubation step, microarray assays are typically carried out manually, requiring skilled personnel and well-equipped laboratories. Further, as fluorescent probes remain the preferred choice for detection due to excellent sensitivity and high dynamic range, expensive and bulky laser-equipped high-end scanners are usually needed.
Manual handling and advanced detection strategies result in assay times normally spanning several hours and consequently current microarray technologies are not well suited for mobile, on-site or point of care applications. While it is likely that a large proportion of microarray analyses could preferentially continue to be performed within advanced health care sites such as hospitals or centralized laboratories, portable microarray solutions may support more rapid clinical decision making in emergency situations in the field or in such areas where little healthcare infrastructure exists. Further, point of care microarray tests might serve to relieve health care resources when patients or care-givers are able to conduct regular testing themselves. Finally, such portable tests could facilitate those unable to go to a hospital or health care point for testing [
5].
While there is a wide variety of simple portable tests commercially available, including for instance rapid tests for sexually transmitted diseases, cardiovascular disease and allergies, such tests are typically simplex or low-plex, with limited quantitative ability and sensitivity, and may further suffer in diagnostic accuracy [
6]. Thus, considering the great advances made in diagnostic protein microarrays, we believe that there exists a major potential health care benefit if microarray technologies could be made amenable for portable, rapid point-of-care use.
A considerable hurdle for creating portable, easy to use planar microarray solutions appears to be the accommodation of many sequential assay steps on-chip, as well as the need for comprehensive sample mixing in order to avoid long assay times due to large diffusion coefficients of protein biomarkers. These challenges are largely avoided in immunochromatographic/lateral flow-based tests, and to this end we previously demonstrated the feasibility of combining a lateral flow/dipstick assay framework commonly used in simplex/low-plex rapid tests with protein microarray patterning for rapid and accurate determination of levels of bovine IgG raised towards mycoplasma infection [
7] using a cell phone digital camera or consumer table top scanner for array imaging.
In this paper, we expand the concept into a general high-plexed lateral flow protein microarray (LFM) strategy, with 384 protein fragments (approximately 80–100 aa) [
8] immobilized in a 24 × 16 microarray pattern on nitrocellulose. The protein fragments are a subset of antigens used for the large-scale generation of monospecific antibodies within the Human Protein Atlas (HPA) [
9] where the aim is to by 2015 generate a set of highly specific antibody binders for the complete human proteome. In the HPA, bioinformatically designed protein fragments are employed as immunogens, as well as ligands in the subsequent affinity purification and quality control/validation of the generated antibody reagents. Together with the obvious benefit of providing reagents for determining presence or levels of virtually any expressed human protein in tissues or body fluids, there is ongoing work exploring the potential of using the protein immunogens as affinity binders for detection of disease-specific antibody populations [
10]. Thus, investigating alternative microarray assay formats employing protein immunogens as affinity binders could result in improved strategies for quality control of raised antibodies as well as in novel methods for diagnostic antigen microarray tests.
In this work, by employing a prototype lateral flow protein microarray device, we were able to accurately determine levels of 26 individual HPA antibodies, as well as up to 8 concurrent specific IgG analytes in approximately 10 min total assay time. We argue that: (a) the demonstrated concordance with an established glass/fluorescence microarray assay; (b) the simplicity of the assay procedure drawing from the benefits of common lateral flow/dipstick tests; (c) the highly multiplexed capacity of the assay; (d) the short assay time; and (e) the convenient read-out options, make the presented assay framework an interesting tool for rapid quality control of raised antibody reagents, and may further find use in translating the capabilities of protein microarrays to point of care/low resource settings.
2. Results and Discussion
In this study, a novel protein antigen lateral flow microarray containing 384 different capture microspots patterned onto a nitrocellulose substrate and with a gold nanobead detection strategy (
Figure 1) was evaluated in terms of absolute sensitivity and accuracy in comparison with a conventional glass/fluorescence-based microarray analysis of 26 IgG antibody species. We also investigated the method’s inherent variability as well as its ability to detect mixtures of up to eight different specific rabbit IgGs.
2.1. Analysis of 26 Specific Rabbit IgG and Assay Accuracy
A selection of 26 purified rabbit IgG isolates, diluted to 0.17–6.91 μg/mL was analyzed in triplicates on the lateral flow microarray as well as on the reference glass slide microarray (
Table 1). It was found that both platforms could correctly identify all 26 antibodies individually, with signal to noise values of 12–181 (mean 100) for the LFM and 23–347 (mean 192) for the glass array. For five samples, an additional strong common signal was found by both platforms. Further, on five occasions the glass array detected an additional strong signal which was not found by the LFM, whereas the LFM only once picked up an additional signal which was not found by the glass array. This may suggest that the glass array is more sensitive to weaker interactions, which may or may not be beneficial depending on the application. While higher signal to noise values were recorded from the glass array, the assay procedure was easier to perform for the lateral flow array, and the difference in assay time was substantial, around 10 min for the lateral flow array compared to more than two hours for the glass array assay. Further, the distinct red color arising on the lateral flow array allowed convenient options of employing a table top scanner or cell-phone camera for the array imaging. We propose that the excellent concordance suggests that the sensitivity, accuracy and multiplexing ability of glass-based protein microarrays can be retained in the translation into immunochromatographic/lateral flow assay frameworks.
A good agreement between the lateral flow array and the glass array results was found (
Figure 2) and the Pearson correlation coefficient was estimated to 0.74. Further, a receiver-operator-characteristics analysis was performed to reveal the concordance in terms of sensitivity and specificity for the comparison between the methods. While both the lateral flow and the glass array assays required thresholds of around 22 to accurately separate positive signals from negative, it was found that the area under the curve (AUC) could be estimated to 98%, indicating that the antigen microarray could be transferred from the conventional glass assay into the immunochromatographic/lateral flow platform with retained accuracy.
A moderate correlation coefficient is to be expected due to differences in substrate coupling to the protein antigens, as well as substantial differences in assay dynamics. However, the sensitive and specific binding of the sample antibodies on the corresponding antigen microspots should be retained, and this is indeed what the ROC analysis demonstrates.
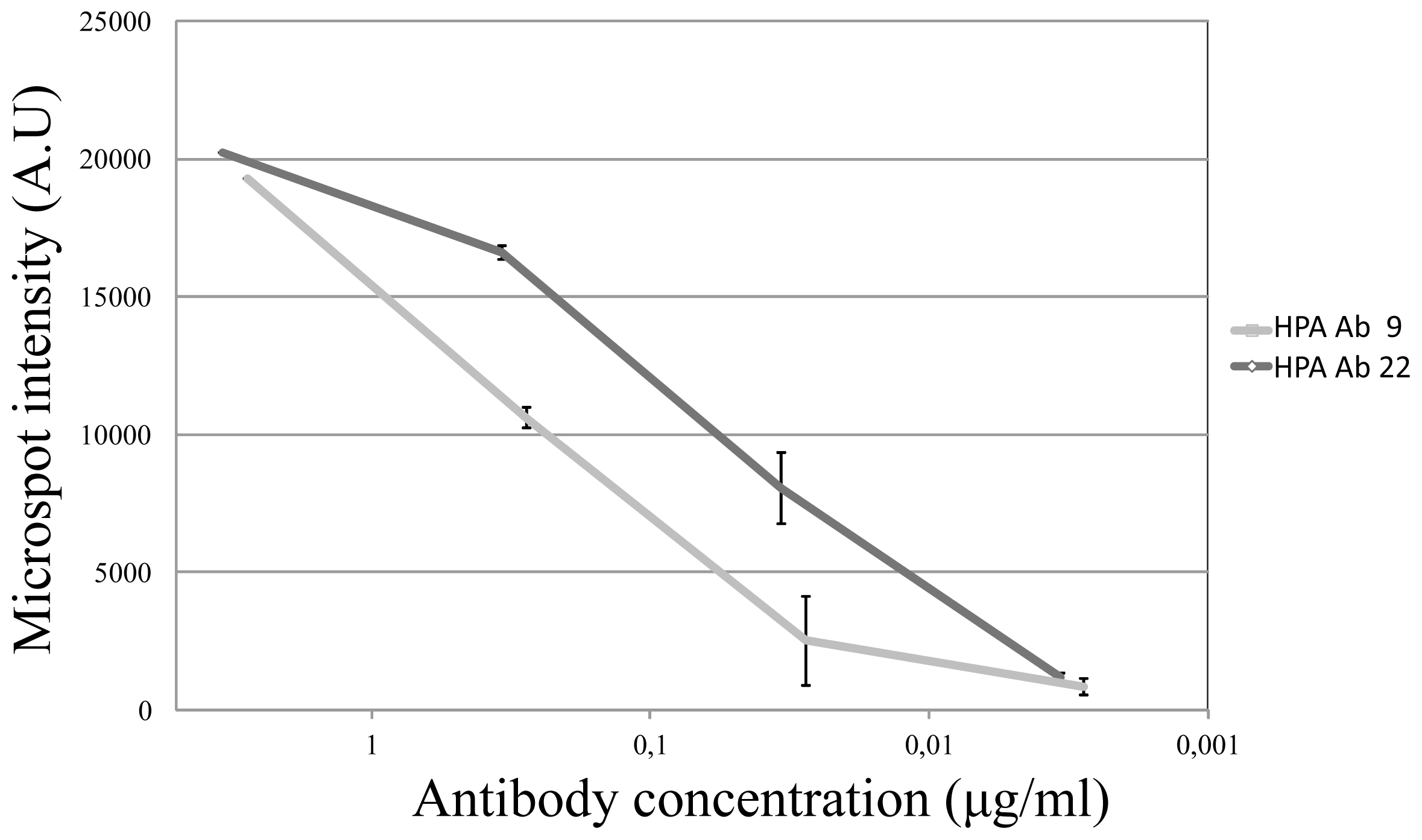
2.2. Assay Sensitivity
Two antibodies (HPA Ab 9 and HPA Ab 22, due to suitably high initial concentrations) were selected for a sensitivity analysis. Each antibody was diluted 1:100, 1:1000, 1:10.000, 1:100.000 in assay buffer, and three replicates per antibody were run for each dilution. As compared with the negative control, it was found that the sensitivity could be estimated to around 30 ng/mL or better and that the response tended to be log-linear (
Figure 3).
2.3. Assay Variability Investigation
From the triplicate runs of the total cohort of 26 specific IgG species included in this study (and under the assumption that the uncontrolled variation was Gaussian distributed), we calculated the standard deviation of each analyzed antibody. We found that the average coefficient of variation (%CV) was 13% [5%–21%]. The level of variability may impede highly resolved quantitative analysis, but could still be acceptable for semi-quantitative approaches.
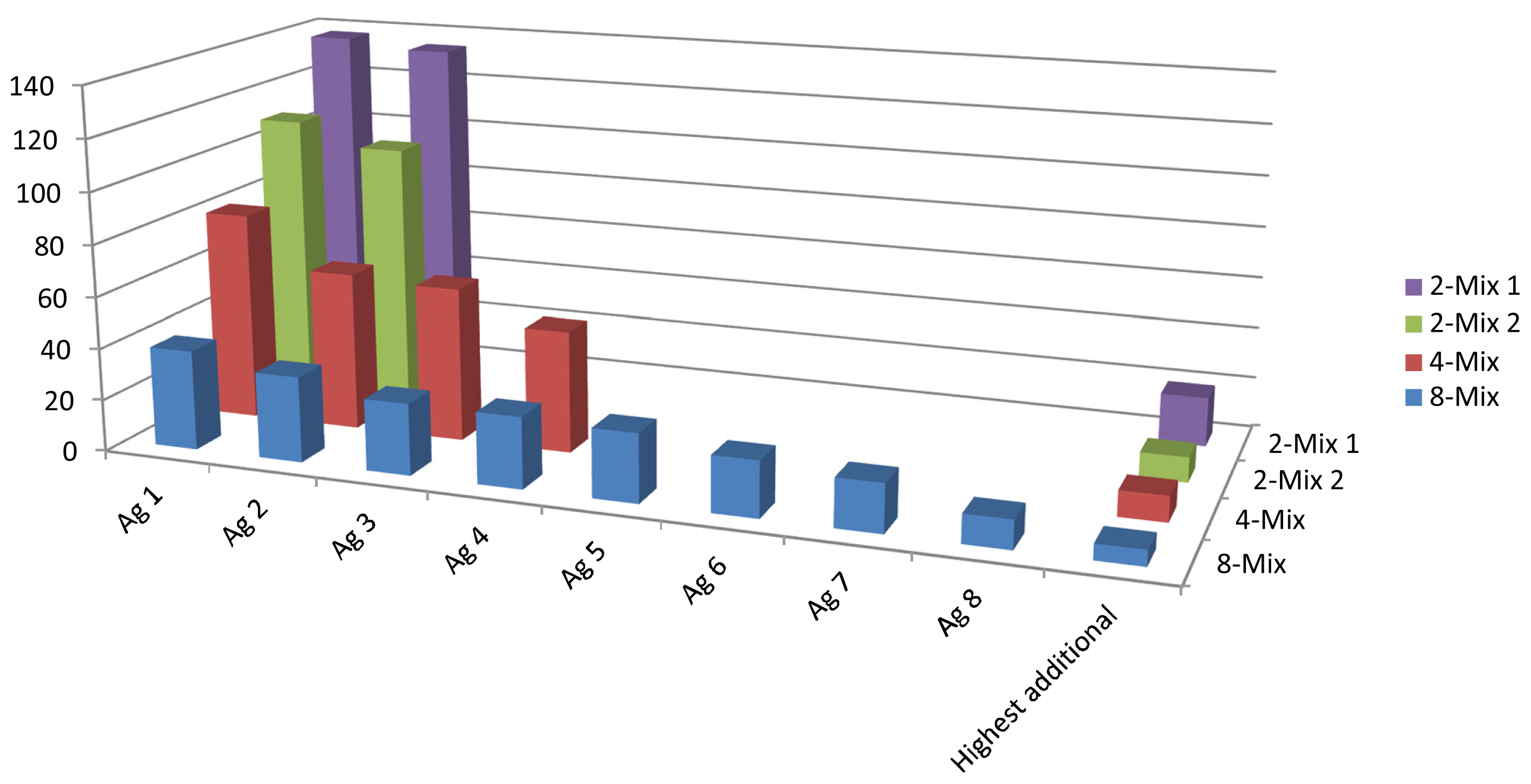
2.4. Analysis of IgG Mixed Samples
In order to demonstrate the array’s capability of detecting an increasing number of antibody analytes, we created mixtures of 2, 2, 4 and 8 antibodies to be analyzed on the lateral flow microarray. It was found that the array could accurately identify all the constituents of the mixes, although the positive signal to noise ratios gradually decreased (
Figure 4) due to increasing background/array mean intensities. The increased array mean was expected, and did conceivably arise due to the increased concentration of total IgG in the mixed samples, which amounted to around 15 μg/mL in total for the 8-mix, in comparison to between 0.17 and 6.9 for the individual antibodies (in the case of the antibody HPA Ab 26 which was analyzed at 6.9 μg/mL, the background was already substantial). Thus, it appears likely that at least 8 different antibody species may be detected simultaneously by the lateral flow microarray, given that the sample dilution keeps the total IgG concentration at or below a critical level (15 μg/mL in this study).
4. Conclusions
Efforts to discover novel clinically useful biomarkers is continuously generating better molecular tools that through earlier and more accurate diagnosis and better disease monitoring may eventually greatly reduce the global burden of disease [
14]. A key challenge lies in translating the wealth of improved diagnostic potential from research into clinical practice. While current microarray technologies are well suited to handle a high number of novel affinity binders, point of care tests are lagging behind in terms of multiplexing ability, sensitivity and diagnostic accuracy. Here, we present a novel device which draws its speed, portability and ease of use from the widely used lateral flow rapid test and the sensitivity, accuracy and multiplexing ability from the planar microarray. The sensitivity was found to be at or better than around 30 ng/mL, a level which should be acceptable in many IgG-based assays where disease-specific IgG are typically in the order of μg/mL or higher. The average variability was estimated to 13% CV which could be suitable at least for semi-quantitative analyses. Further, in comparison with a conventional glass/fluorescence microarray assay, we show that the concordance between the methods for a set of 26 rabbit IgG antibodies was excellent, with an AUC of 98%, suggesting that the lateral flow microarray could prove useful as an alternative in quality control for high-throughput antibody generation as well as for future potential rapid and portable diagnostic protein antigen microarrays. A brief qualitative comparison between the lateral flow array and glass array assay systems with regards to some relevant performance parameters can be found in
table 2. We believe an important novel finding is that the nitrocellulose substrate, which has proven immensely useful for rapid and simple immunochromatography tests, appears amenable for high-density protein microarray analysis with retained diagnostic accuracy. Coupled to a detection strategy involving gold nanobeads, the LFM allowed for a flexible and convenient detection and read-out strategy along with the powerful analytic ability of the microarray. In future work we will select a similarly sized set of protein antigens which have demonstrated a clinically relevant diagnostic utility and evaluate the lateral flow microarray’s ability on clinical samples. We will also attempt to develop the device further for less manual operation and towards integration of filtration steps for whole-blood analysis and automated image analysis.