Extraction of Biomolecules Using Phosphonium-Based Ionic Liquids + K3PO4 Aqueous Biphasic Systems
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Phase Diagrams
2.3. Determination of Tie-Lines
2.4. Density and Viscosity Measurements
2.5. Partitioning of Biomolecules
3. Results and Discussion
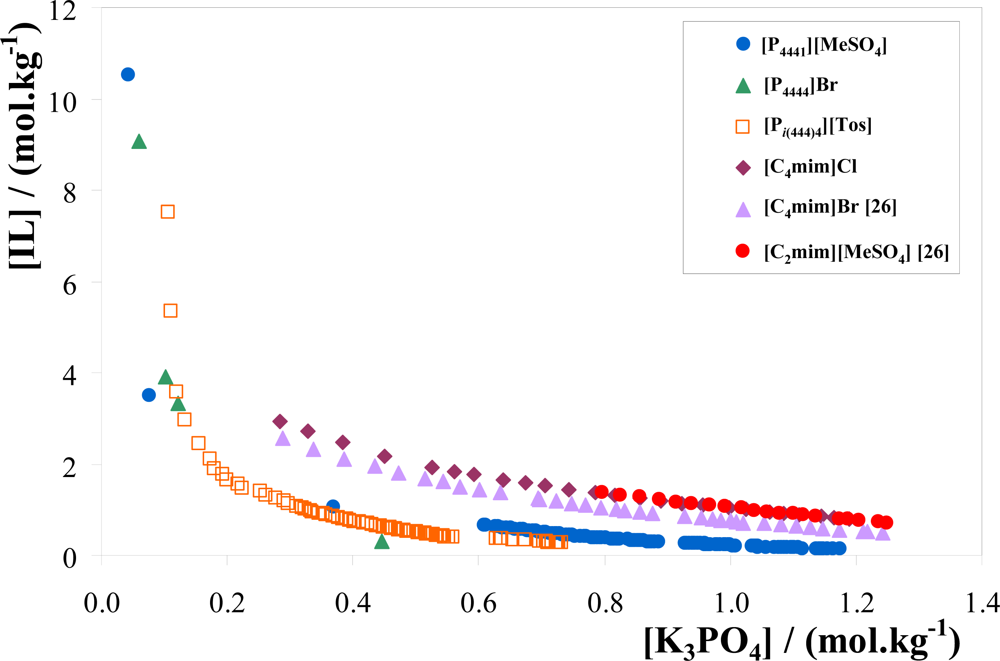
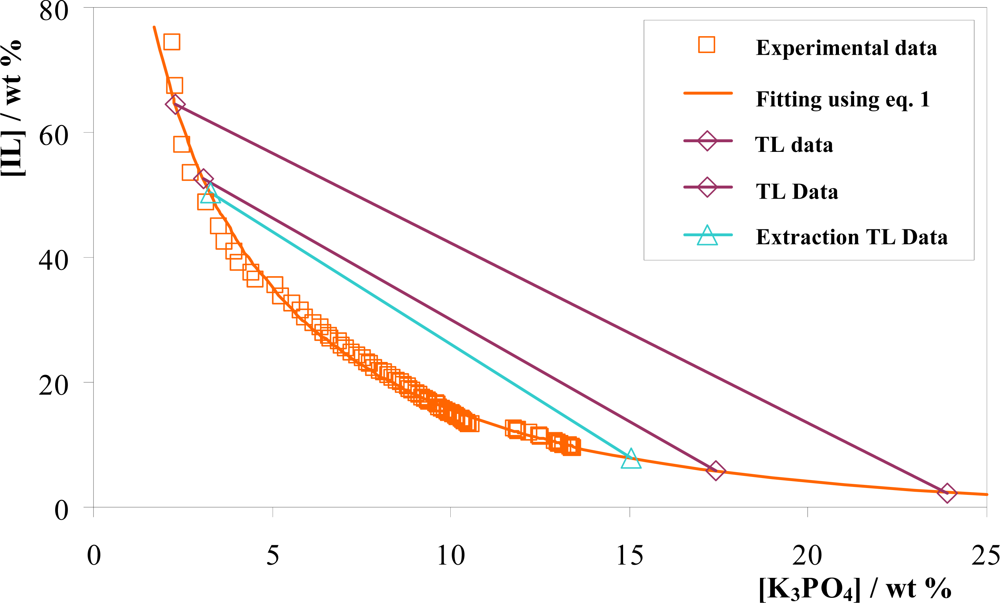
3.1. Phase Diagrams and Tie-Lines
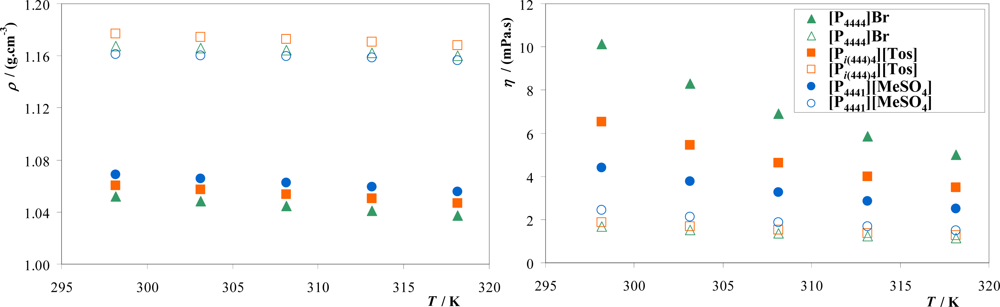
3.2. Density and Viscosity
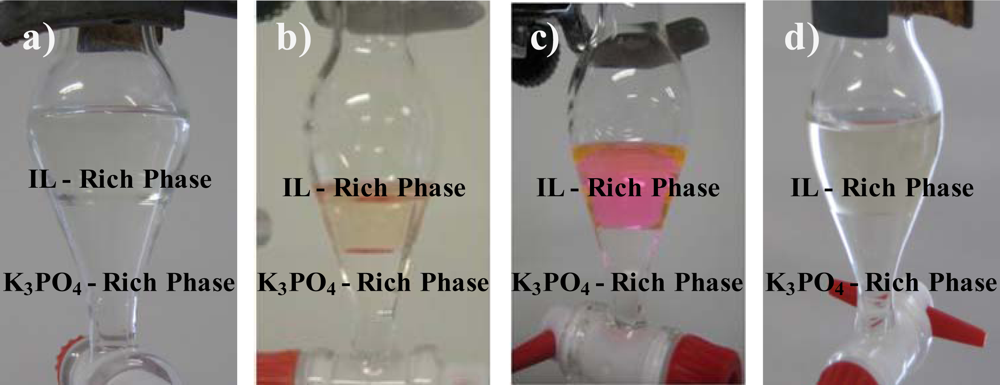
3.3. Partitioning of Biomolecules
4. Conclusions
Acknowledgments
References
- Albertsson, P-Å. Partitioning of Proteins in Liquid Polymer-Polymer Two-Phase Systems. Nature 1958, 182, 709–711. [Google Scholar]
- Hatti-Kaul, R. Aqueous Two-Phase Systems: A General Overview. Mol. Biotechnol 2001, 19, 269–277. [Google Scholar]
- Gutowski, KE; Broker, GA; Willauer, HD; Huddleston, JG; Swatloski, RP; Holbrey, JD; Rogers, RD. Controlling the Aqueous Miscibility of Ionic Liquids: Aqueous Biphasic Systems of Water-Miscible Ionic Liquids and Water-Structuring Salts for Recycle, Metathesis, and Separations. J. Am. Chem. Soc 2003, 125, 6632–6633. [Google Scholar]
- Abraham, MH; Zissimos, AM; Huddleston, JG; Swatloski, RP; Holbrey, JD; Rogers, RD; Acree, WE, Jr. Some Novel Liquid Partitioning Systems: Water-Ionic Liquids and Aqueous Biphasic Systems. Ind. Eng. Chem. Res 2003, 42, 413–418. [Google Scholar]
- Rogers, RD; Seddon, KR. Ionic Liquids - Solvents of the Future? Science 2003, 302, 792–293. [Google Scholar]
- Seddon, KR. Ionic Liquids for Clean Technology. J. Chem. Technol. Biotechnol 1997, 68, 351–356. [Google Scholar]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev 1999, 99, 2071–2083. [Google Scholar]
- Earle, MJ; Esperança, JMSS; Gilea, MA; Canongia Lopes, JN; Rebelo, LPN; Magee, JW; Seddon, KR; Widegren, JA. The Distillation and Volatility of Ionic Liquids. Nature 2006, 439, 831–834. [Google Scholar]
- Santos, LMNBF; Canongia Lopes, JN; Coutinho, JAP; Esperança, JMSS; Gomes, LR; Marrucho, IM; Rebelo, LPN. Ionic Liquids: First Direct Determination of their Cohesive Energy. J. Am. Chem. Soc 2007, 129, 284–285. [Google Scholar]
- Freire, MG; Neves, MSS; Marrucho, IM; Coutinho, JAP; Fernandes, AM. Hydrolysis of Tetrafluoroborate and Hexafluorophosphate Counter Ions in Imidazolium-Based Ionic Liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar]
- Holbrey, JD; Seddon, KR. Ionic Liquids. Clean. Prod. Process 1999, 1, 223–236. [Google Scholar]
- Pei, Y; Wang, J; Liu, L; Wu, K; Zhao, Y. Liquid−Liquid Equilibria of Aqueous Biphasic Systems Containing Selected Imidazolium Ionic Liquids and Salts. J. Chem. Eng. Data 2007, 52, 2026–2031. [Google Scholar]
- Zafarani-Moattar, MT; Hamzehzadeh, S. Liquid−Liquid Equilibria of Aqueous Two-Phase Systems Containing 1-Butyl-3-methylimidazolium Bromide and Potassium Phosphate or Dipotassium Hydrogen Phosphate at 298.15 K. J. Chem. Eng. Data 2007, 52, 1686–1692. [Google Scholar]
- Bridges, NJ; Gutowski, KE; Rogers, RD. Investigation of Aqueous Biphasic Systems Formed from Solutions of Cchaotropic Salts with Kosmotropic Salts (Salt–Salt ABS). Green Chem 2007, 9, 177–183. [Google Scholar]
- Visak, ZP; Canongia Lopes, JN; Rebelo, LPN. Ionic Liquids in Polyethylene Glycol Aqueous Solutions: Salting-in and Salting-out Effects. Monatsh. Chem 2007, 138, 1153–1157. [Google Scholar]
- Najdanovic-Visak, V; Canongia Lopes, JN; Visak, ZP; Trindade, J; Rebelo, LPN. Salting-out in Aqueous Solutions of Ionic Liquids and K3PO4: Aqueous Biphasic Systems and Salt Precipitation. Int. J. Mol. Sci 2007, 8, 736–748. [Google Scholar]
- Wu, B; Zhang, Y; Wang, H. Phase Behavior for Ternary Systems Composed of Ionic Liquid + Saccharides + Water. J. Phys. Chem. B 2008, 112, 6426–6429. [Google Scholar]
- Zhang, Y; Zhang, S; Chen, Y; Zhang, J. Aqueous Biphasic Systems Composed of Ionic Liquid and Fructose. Fluid Phase Equilib 2007, 257, 173–176. [Google Scholar]
- Wu, B; Zhang, Y; Wang, H; Yang, L. Temperature Dependence of Phase Behavior for Ternary Systems Composed of Ionic Liquid + Sucrose + Water. J. Phys. Chem. B 2008, 112, 13163–13165. [Google Scholar]
- Wu, B; Zhang, YM; Wang, HP. Aqueous Biphasic Systems of Hydrophilic Ionic Liquids + Sucrose for Separation. J. Chem. Eng. Data 2008, 53, 983–985. [Google Scholar]
- Pei, Y; Wang, J; Wu, K; Xuan, X; Lu, X. Ionic Liquid-Based Aqueous Two-Phase Extraction of Selected Proteins. Sep. Purif. Technol 2009, 64, 288–295. [Google Scholar]
- Du, Z; Yu, Y-L; Wang, J-H. Extraction of Proteins from Biological Fluids by Use of an Ionic Liquid/Aqueous Two-Phase System. Chem. Eur. J 2007, 13, 2130–2137. [Google Scholar]
- Li, S; He, C; Liu, H; Li, K; Liu, F. Ionic Liquid-Based Aqueous Two-Phase System, a Sample Pretreatment Procedure Prior to High-Performance Liquid Chromatography of Opium Alkaloids. J. Chromatogr. B 2005, 826, 58–62. [Google Scholar]
- He, C; Li, S; Liu, H; Li, K; Liu, F. Extraction of Testosterone and Epitestosterone in Human Urine using Aqueous Two-Phase Systems of Ionic Liquid and Salt. J. Chromatogr. A 2005, 1082, 143–149. [Google Scholar]
- Neves, CMSS; Ventura, SPM; Freire, MG; Marrucho, IM; Coutinho, JAP. Evaluation of Cation Influence on the Formation and Extraction Capability of Ionic-Liquid-Based Aqueous Biphasic Systems. J. Phys. Chem. B 2009, 113, 5194–5199. [Google Scholar]
- Ventura, SPM; Neves, CMSS; Freire, MG; Marrucho, IM; Oliveira, J; Coutinho, JAP. Evaluation of Anion Influence on the Formation and Extraction Capacity of Ionic-Liquid-Based Aqueous Biphasic Systems. J. Phys. Chem. B 2009, 113, 9304–9310. [Google Scholar]
- Freire, MG; Neves, CMSS; Marrucho, IM; Canongia Lopes, JN; Rebelo, LPN; Coutinho, JAP. High-Performance Extraction of Alkaloids Using Aqueous Biphasic Systems with Ionic Liquids. 2010; , in preparation. [Google Scholar]
- Pereira, JFB; Lima, AS; Freire, MG; Coutinho, JAP. Ionic Liquids as Adjuvants for the Tailored Extraction of Biomolecules in Aqueous Biphasic Systems. Green Chem 2010. [Google Scholar]
- Domínguez-Pérez, M; Tomé, LIN; Freire, MG; Marrucho, IM; Cabeza, O; Coutinho, JAP. (Extraction of Biomolecules using) Aqueous Biphasic Systems formed by Ionic Liquids and Aminoacids. Sep. Purif. Technol 2010, 72, 85–91. [Google Scholar]
- Freire, MG; Neves, CMSS; Carvalho, PJ; Gardas, RL; Fernandes, AM; Marrucho, IM; Santos, LMNBF; Coutinho, JAP. Mutual Solubilities of Water and Hydrophobic Ionic Liquids. J. Phys. Chem. B 2007, 111, 13082–13089. [Google Scholar]
- Freire, MG; Santos, LMNBF; Fernandes, AM; Coutinho, JAP; Marrucho, IM. An Overview of the Mutual Solubilities of Water-Imidazolium-Based Ionic Liquids Systems. Fluid Phase Equilib 2007, 261, 449–454. [Google Scholar]
- Freire, MG; Carvalho, PJ; Gardas, RL; Marrucho, IM; Santos, LMNBF; Coutinho, JAP. Mutual Solubilities of Water and the [Cnmim][Tf2N] Hydrophobic Ionic Liquids. J. Phys. Chem. B 2008, 112, 1604–1610. [Google Scholar]
- Freire, MG; Carvalho, PJ; Gardas, RL; Santos, LMNBF; Marrucho, IM; Coutinho, JAP. Solubility of Water in Tetradecyltrihexylphosphonium-Based Ionic Liquids. J. Chem. Eng. Data 2008, 53, 2378–2382. [Google Scholar]
- Freire, MG; Neves, CMSS; Ventura, SPM; Pratas, MJ; Marrucho, IM; Oliveira, J; Coutinho, JAP; Fernandes, AM. Solubility of Non Aromatic Ionic Liquids in Water and Correlation Using a QSPR Approach. Fluid Phase Equilib 2010. [Google Scholar]
- Trindade, JR; Visak, ZP; Blesic, M; Marrucho, IM; Coutinho, JAP; Canongia Lopes, JN; Rebelo, LPN. Salting-Out Effects in Aqueous Ionic Liquid Solutions: Cloud-Point Temperature Shifts. J. Phys. Chem. B 2007, 111, 4737–4741. [Google Scholar]
- Freire, MG; Carvalho, PJ; Silva, AMS; Santos, LMNBF; Rebelo, LPN; Marrucho, IM; Coutinho, JAP. Ion Specific Effects on the Mutual Solubilities of Water and Hydrophobic Ionic Liquids. J. Phys. Chem. B 2009, 113, 202–211. [Google Scholar]
- Tomé, LIN; Varanda, FR; Freire, MG; Marrucho, IM; Coutinho, JAP. Towards an Understanding of the Mutual Solubilities of Water and Hydrophobic Ionic Liquids in the Presence of Salts: The Anion Effect. J. Phys. Chem. B 2009, 113, 2815–2825. [Google Scholar]
- Tomé, LIN; Domínguez-Perez, M; Cláudio, AFM; Freire, MG; Marrucho, IM; Cabeza, O; Coutinho, JAP. On the Interactions between Amino Acids and Ionic Liquids in Aqueous Media. J. Phys. Chem. B 2009, 113, 13971–13979. [Google Scholar]
- Freire, MG; Neves, CMSS; Silva, AMS; Santos, LMNBF; Marrucho, IM; Rebelo, LPN; Shah, JK; Maginn, EJ; Coutinho, JAP. 1H NMR and Molecular Dynamics Evidence for an Unexpected Interaction on the Origin of Salting-In/Salting-Out Phenomena. J. Phys. Chem. B 2010, 114, 2004–2014. [Google Scholar]
- Marták, J; Schlosser, S. Extraction of Lactic Acid by Phosphonium Ionic Liquids. Sep. Purif. Technol 2007, 57, 483–494. [Google Scholar]
- Carvalho, PJ; Regueira, T; Santos, LMNBF; Fernandez, J; Coutinho, JAP. Effect of Water on the Viscosities and Densities of 1-Butyl-3-methylimidazolium Dicyanamide and 1-Butyl-3-methylimidazolium Tricyanomethane at Atmospheric Pressure. J. Chem. Eng. Data 2010, 55, 645–652. [Google Scholar]
- Atefi, F; Garcia, MT; Singer, RD; Scammels, PJ. Phosphonium Ionic Liquids: Design, Synthesis and Evaluation of Biodegrability. Green Chem 2009, 11, 1595–1604. [Google Scholar]
- Merchuk, JC; Andrews, BA; Asenjo, JA. Aqueous Two-Phase Systems for Protein Separation: Studies on Phase Inversion. J. Chromatogr. B 1998, 711, 285–293. [Google Scholar]
- Mei, L-H; Lin, D-Q; Zhu, Z-Q; Han, Z-X. Densities and Viscosities of Polyethylene Glycol + Salt + Water Systems at 20 °C. J. Chem. Eng. Data 1995, 40, 1168–1171. [Google Scholar]
- Treszczanowicz, T; Kasprzycka-Guttman, T; Cieślak, D; Treszczanowicz, AJ. Distribution Coefficient of β-Carotene between an Organic Solvent and Water. J. Chem. Eng. Data 1998, 43, 632–634. [Google Scholar]
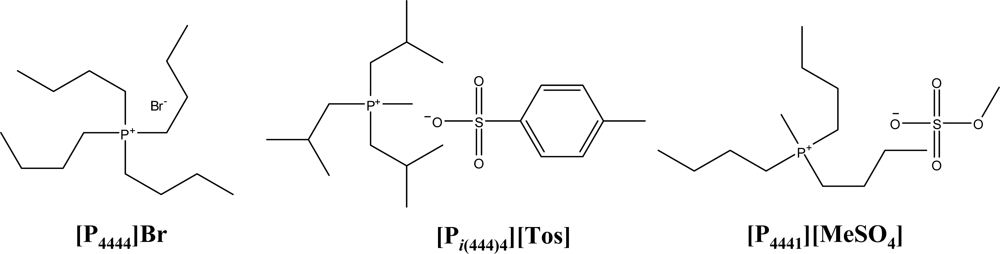
| IL + K3PO4 + Water system | A | B | C | R2 |
|---|---|---|---|---|
| [P4444]Br | 176.57 | −0.7562 | 1.100 × 10−3 | 0.9987 |
| [Pi(444)4][Tos] | 229.48 | −0.8378 | 3.614 × 10−5 | 0.9863 |
| [P4441][MeSO4] | 116.85 | −0.5131 | 1.217 × 10−4 | 0.9864 |
| IL + K3PO4 + Water system | Weight fraction composition/wt % | TL equation[a] | TLL | ||
|---|---|---|---|---|---|
| IL | K3PO4 | a | b | ||
| [P4444]Br | 40.98 | 5.930 | 71.70 | −5.181 | 62.38 |
| 49.95 | 6.516 | 79.54 | −4.542 | 75.21 | |
| 40.12[b] | 6.010[b] | 70.96 | −5.132 | 61.47 | |
| [Pi(444)4][Tos] | 39.71 | 10.90 | 71.07 | −2.878 | 65.77 |
| 30.07 | 9.980 | 62.76 | −3.276 | 49.04 | |
| 39.93[b] | 6.062[b] | 62.04 | −3.648 | 43.38 | |
| [P4441][MeSO4] | 22.98 | 9.986 | 48.60 | −2.566 | 24.88 |
| 20.13 | 15.11 | 57.16 | −2.451 | 51.80 | |
| 22.38[b] | 10.61[b] | 48.46 | −2.458 | 28.75 | |
| IL + K3PO4 + Water system | Weight fraction composition/wt % | TL equation[a] | TLL | Ki | ||
|---|---|---|---|---|---|---|
| IL | K3PO4 | a | b | |||
| l-tryptophan | ||||||
| [P4441][MeSO4] | 22.95 | 10.66 | 50.39 | −2.574 | 31.86 | 9.0 ± 0.1 |
| β-carotene | ||||||
| [Pi(444)4][Tos] | 39.62 | 6.516 | 64.22 | −3.776 | 46.04 | 61 ± 5 |
| rhodamine 6G | ||||||
| [P4444]Br | 39.92 | 5.995 | 72.86 | −5.494 | 62.73 | 0.018 ± 0.007 |
| [Pi(444)4][Tos] | 40.11 | 6.118 | 62.23 | −3.615 | 44.14 | 3.6 ± 0.1 |
| [P4441][MeSO4] | 23.66 | 10.22 | 48.61 | −2.442 | 29.94 | 8 ± 1 |
| caffeine | ||||||
| [P4441][MeSO4] | 23.62 | 10.26 | 49.49 | −2.521 | 30.38 | 4.75 ± 0.01 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Louros, C.L.S.; Cláudio, A.F.M.; Neves, C.M.S.S.; Freire, M.G.; Marrucho, I.M.; Pauly, J.; Coutinho, J.A.P. Extraction of Biomolecules Using Phosphonium-Based Ionic Liquids + K3PO4 Aqueous Biphasic Systems. Int. J. Mol. Sci. 2010, 11, 1777-1791. https://doi.org/10.3390/ijms11041777
Louros CLS, Cláudio AFM, Neves CMSS, Freire MG, Marrucho IM, Pauly J, Coutinho JAP. Extraction of Biomolecules Using Phosphonium-Based Ionic Liquids + K3PO4 Aqueous Biphasic Systems. International Journal of Molecular Sciences. 2010; 11(4):1777-1791. https://doi.org/10.3390/ijms11041777
Chicago/Turabian StyleLouros, Cláudia L. S., Ana Filipa M. Cláudio, Catarina M. S. S. Neves, Mara G. Freire, Isabel M. Marrucho, Jérôme Pauly, and João A. P. Coutinho. 2010. "Extraction of Biomolecules Using Phosphonium-Based Ionic Liquids + K3PO4 Aqueous Biphasic Systems" International Journal of Molecular Sciences 11, no. 4: 1777-1791. https://doi.org/10.3390/ijms11041777




