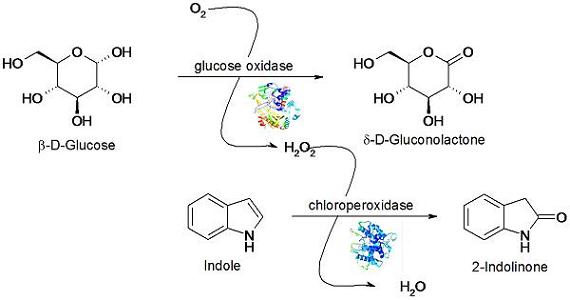
Covalent Anchoring of Chloroperoxidase and Glucose Oxidase on the Mesoporous Molecular Sieve SBA-15
Abstract
:1. Introduction
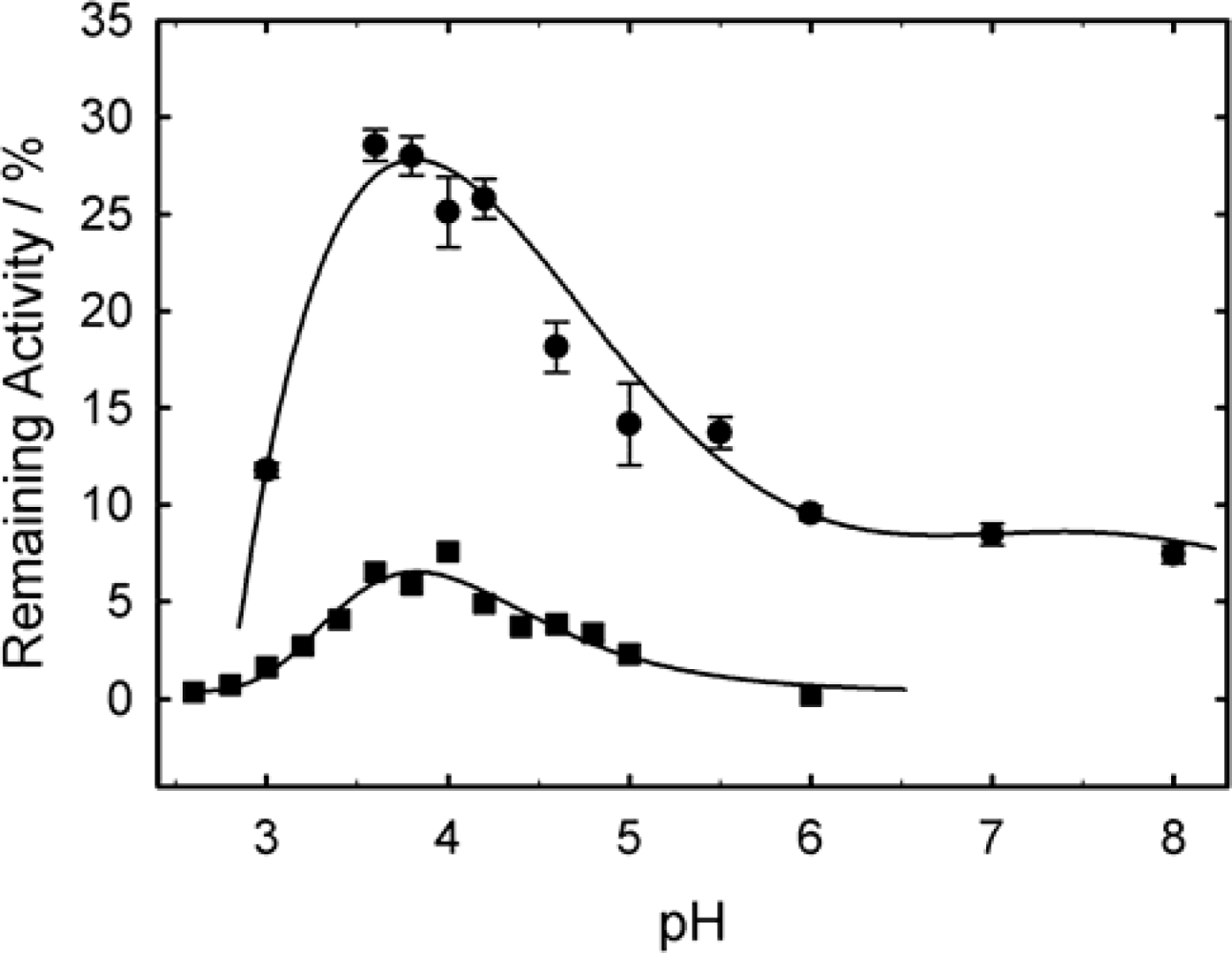
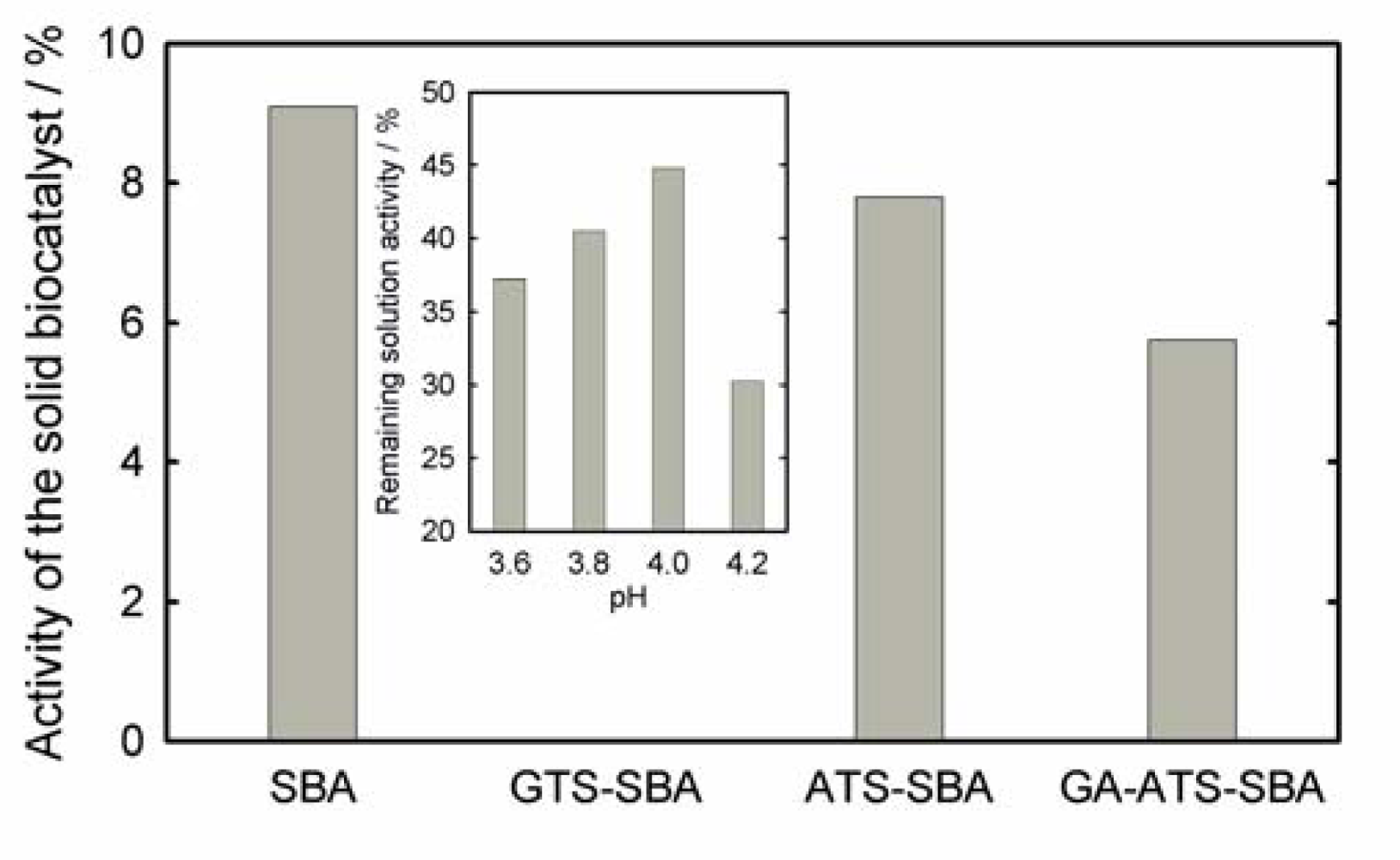
2. Results and Discussion
3. Experimental Section
3.1. Synthesis of SBA-15 Grafted with ATS, GTS and GA
3.2. Immobilization of CPO and GOx
3.3. Characterization of the Mesoporous Materials
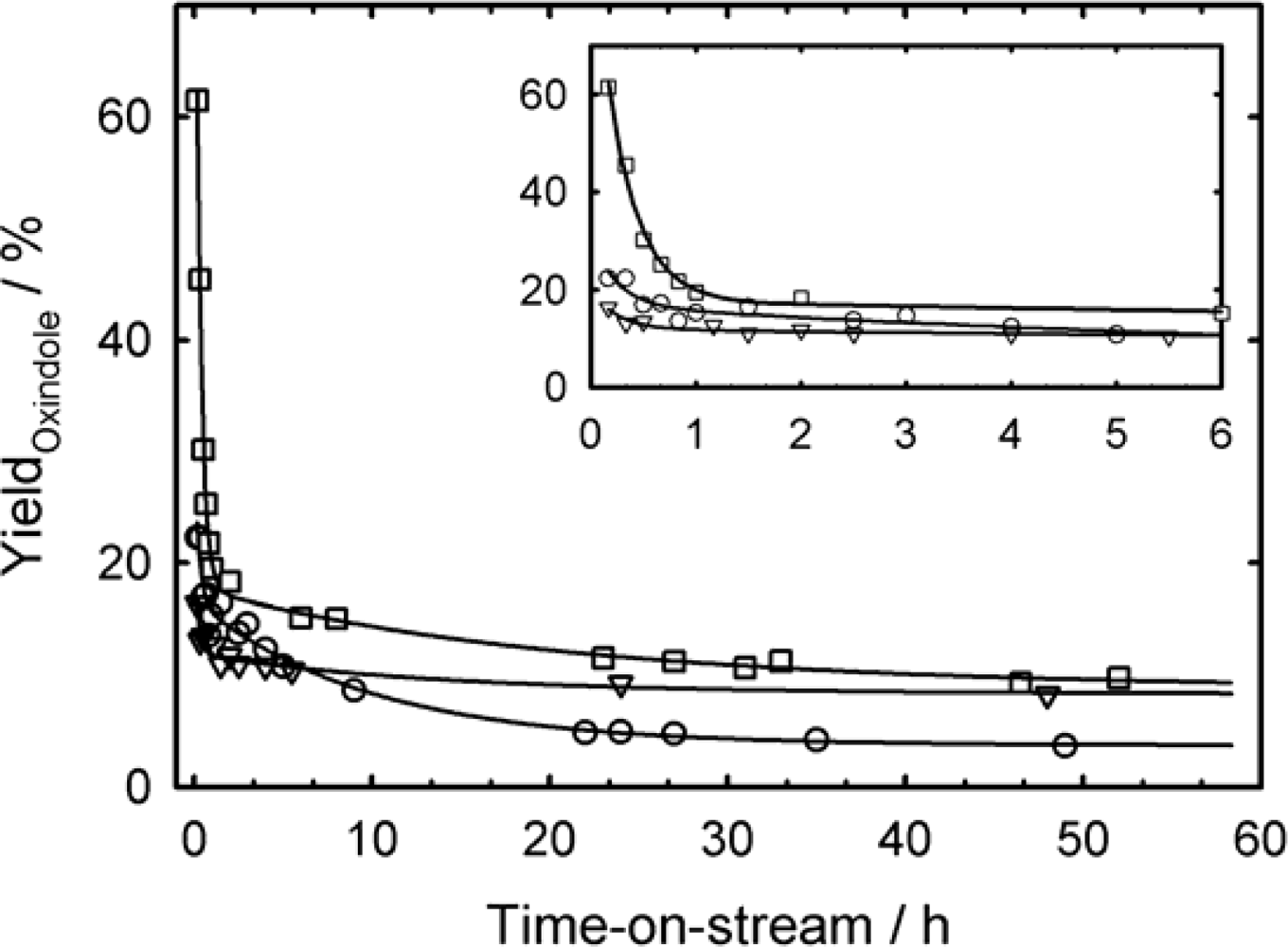
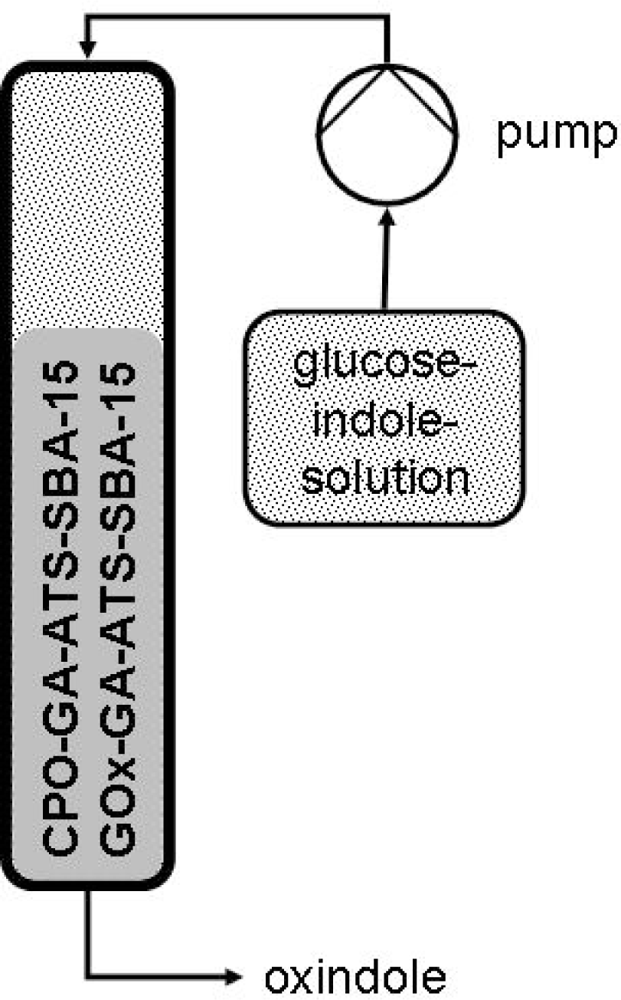
3.4. Indole Oxidation
4. Conclusions
Acknowledgments
References and Notes
- Hartmann, M; Jung, D. Biocatalysis with enzymes immobilized on mesoporous hosts: the status quo and future trends. J. Mater. Chem 2010, 20, 844–857. [Google Scholar]
- Ariga, K; Hill, JP; Lee, MV; Vinu, A; Charvet, R; Acharya, S. Challenges and breakthroughs in recent research on self-assembly. Sci Technol Adv Mater 2008, 9, 014109:1–014109:96. [Google Scholar]
- Cabral, JMS; Kennedy, JF. Thermostability of Enzymes; Gupta, MN, Ed.; Springer: Berlin, Germany, 1993; pp. 163–179. [Google Scholar]
- Ren, L; He, J; Zhang, S; Evans, DG; Duan, X. Immobilization of penicillin G acylase in layered double hydroxides pillared by glutamate ions. J. Mol. Catal. B: Enzym 2002, 18, 3–11. [Google Scholar]
- Pan, J-L; Syu, M-J. A thermal study on the use of immobilized penicillin G acylase in the formation of 7-amino-3-deacetoxy cephalosporanic acid from cephalosporin G. J. Chem. Technol 2004, 79, 1050–1056. [Google Scholar]
- Lü, Y; Lu, G; Wang, Y; Guo, Y; Guo, Y; Zhang, Z; Wang, Y; Liu, X. Functionalization of cubic Ia3d mesoporous silica for immobilization of penicillin G acylase. Adv. Funct. Mater 2007, 17, 2160–2166. [Google Scholar]
- Shah, P; Sridevi, N; Prabhune, A; Ramaswamy, V. Structural features of Penicillin acylase adsorption on APTES functionalized SBA-15. Microporous Mesoporous Mater 2008, 116, 157–165. [Google Scholar]
- Soares, CMF; Santana, MHA; Zanin, GM; de Castro, HF. Covalent coupling method for lipase immobilization on controlled pore silica in the presence of nonenzymatic proteins. Biotechnol. Prog 2003, 19, 803–807. [Google Scholar]
- Godjevargova, T; Nenkova, R; Dimora, N. Covalent Immobilization of Glucose Oxidase onto New Modified Acrylonitrile Copolymer/Silica Gel Hybrid Supports. Macromol. Biosci 2005, 5, 760–766. [Google Scholar]
- Bai, Y-X; Li, Y-F; Yang, Y; Yi, L-X. Covalent immobilization of triacylglycerol lipase onto functionalized nanoscale SiO2 spheres. Proc. Biochem 2006, 41, 770–777. [Google Scholar]
- Kima, MI; Hama, HO; Ohb, S-D; Park, HG; Changa, HN; Choi, S-H. Immobilization of Mucor javanicus lipase on effectively functionalized silica nanoparticles. J. Mol. Catal. B: Enzym 2006, 39, 62–68. [Google Scholar]
- Szymanska, K; Bryjak, J; Mrowiec-Bialon, J; Jarzebski, AB. Application and properties of siliceous mesostructured cellular foams as enzymes carriers to obtain efficient biocatalysts. Microporous Mesoporous Mater 2007, 99, 167–175. [Google Scholar]
- Zhang, X; Guan, RF; Wu, DQ; Chan, KY. Enzyme immobilization on amino-functionalized mesostructured cellular foam surfaces, characterization and catalytic properties. J. Mol. Catal. B: Enzym 2005, 33, 43–50. [Google Scholar]
- Tortajada, M; Ramon, D; Beltran, D; Amoros, P. Hierarchical bimodal porous silicas and organosilicas for enzyme immobilization. J. Mater. Chem 2005, 15, 3859–3868. [Google Scholar]
- Pandya, PH; Jasra, RV; Newwalkar, BL; Bhatt, PN. Studies on the activity and stability of immobilized α-amylase in ordered mesoporous silicas. Microporous Mesoporous Mater 2005, 77, 67–77. [Google Scholar]
- Salis, A; Meloni, D; Ligas, S; Monduzzi, M; Solinas, V; Dumitriu, E. Physical and chemical adsorption of mucor javanicus lipase on SBA-15 mesoporous silica. Synthesis, structural characterization, and activity performance. Langmuir 2005, 21, 5511–5516. [Google Scholar]
- Lei, C; Shin, Y; Liu, J; Ackerman, EJ. Synergetic effects of nanoporous support and urea on enzyme activity. Nano Lett 2007, 7, 1050–1053. [Google Scholar]
- Yiu, HHP; Wright, PA; Botting, NP. Enzyme immobilisation using SBA-15 mesoporous molecular sieves with functionalised surfaces. J. Mol. Catal. B: Enzym 2001, 15, 81–92. [Google Scholar]
- Lei, C; Shin, Y; Liu, J; Ackerman, EJ. Entrapping Enzyme in a Functionalized Nanoporous Support. J. Am. Chem. Soc 2002, 124, 11242–11243. [Google Scholar]
- Yiu, HHP; Botting, CH; Botting, NP; Wright, PA. Size selective protein adsorption on thiol-functionalised SBA-15 mesoporous molecular sieve. Phys. Chem.: Chem. Phys 2001, 3, 2983–2985. [Google Scholar]
- Wang, P; Dai, S; Waezsada, SD; Tsao, AY; Davison, BH. Enzyme stabilization by covalent binding in nanoporous sol-gel glass for nonaqueous biocatalysis. Biotech. Bioeng 2001, 74, 249–255. [Google Scholar]
- Schlossbauer, A; Schaffert, D; Kecht, J; Wagner, E; Bein, T. Click chemistry for high-density biofunctionalization of mesoporous silica. J. Am. Chem. Soc 2008, 130, 12558–12559. [Google Scholar]
- Oha, C; Lee, J-H; Lee, Y-G; Lee, Y-H; Kimb, J-W; Kangb, H-H; Oha, S-G. New approach to the immobilization of glucose oxidase on non-porous silica microspheres functionalized by (3-aminopropyl)trimethoxysilane (APTMS). Colloid. Surface. B: Biointerfaces 2006, 53, 225–232. [Google Scholar]
- Jung, D; Streb, C; Hartmann, M. Oxidation of indole using chloroperoxidase and glucose oxidase immobilized on SBA-15 as tandem biocatalyst. Microporous Mesoporous Mater 2008, 113, 523–529. [Google Scholar]
- Aburto, J; Ayala, M; Bustos-Jaimes, I; Montiel, C; Terres, E; Dominguez, JM; Torres, E. Stability and catalytic properties of chloroperoxidase immobilized on SBA-16 mesoporous materials. Microporous Mesoporous Mater 2005, 83, 193–200. [Google Scholar]
- Montiel, C; Terres, E; Dominguez, J-M; Aburto, J. Immobilization of chloroperoxidase on silica-based materials for 4,6-dimethyl dibenzothiophene oxidation. J. Mol. Catal. B: Enzym 2007, 48, 90–98. [Google Scholar]
- Hudson, S; Cooney, J; Hodnett, BK; Magner, E. Chloroperoxidase on periodic mesoporous organosilanes: Immobilization and reuse. Chem. Mater 2007, 19, 2049–2055. [Google Scholar]
- Subramanian, A; Kennel, SJ; Oden, PI; Jacobson, KB; Woodward, J; Doktycz, MJ. Comparison of techniques for enzyme immobilization on silicon supports. Enzyme Microb. Technol 1999, 24, 26–34. [Google Scholar]
- Impens, NREN; van der Voort, P; Vansant, EF. Silylation of micro-, meso- and non-porous oxides: A review. Microporous Mesoporous Mater 1999, 28, 217–232. [Google Scholar]
- Chong, ASM; Zhao, XS. Functionalization of SBA-15 with APTES and Characterization of Functionalized Materials. J. Phys. Chem. B 2003, 107, 12650–12657. [Google Scholar]
- Yiu, HHP; Wright, PA. Enzymes supported on ordered mesoporous solids: a special case of an inorganic–organic hybrid. J Mater Chem 2005, 15, 3690–3699. [Google Scholar]
- Burkett, SL; Sims, SD; Mann, S. Synthesis of hybrid inorganic–organic mesoporous silica by co-condensation of siloxane and organosiloxane precursors. Chem Commun 1996, 1367–1368. [Google Scholar]
- Macquarrie, JD. Direct preparation of organically modified MCM-type materials. Preparation and characterisation of aminopropyl–MCM and 2-cyanoethyl–MCM. Chem. Commun 1996, 16, 1961–1962. [Google Scholar]
- Chong, MAS; Zhao, XS; Kustedjo, AT; Qiao, SZ. Functionalization of large-pore mesoporous silicas with organosilanes by direct synthesis. Microporous Mesoporous Mater 2004, 72, 33–42. [Google Scholar]
- Faber, K. Biotransformations in Organic Chemistry, 5th ed; Springer Verlag: Berlin-Heidelberg, Germany, 2004. [Google Scholar]
- Felix, G; Descorps, V. Stereochemical resolution of racemates, in HPLC, using a chiral stationary phase based upon immobilized α-chymotrypsin. I. Structural chiral separations. Chromatographia 1999, 49, 595–605. [Google Scholar]
- Ispas, C; Sokolov, I; Andreescu, S. Enzyme-functionalized mesoporous silica for bioanalytical applications. Anal. Bioanal. Chem 2009, 393, 543–554. [Google Scholar]
- Mason, RD; Detar, CC; Weetall, HH. Protease covalently coupled to porous glass: Preparation and characterization. Biotechnol. Bioeng 1975, 17, 1019–1027. [Google Scholar]
- Hartmann, M; Streb, C. Selective oxidation of indole by chloroperoxidase immobilized on the mesoporous molecular sieve SBA-15. J. Porous Mater 2006, 13, 347–352. [Google Scholar]
- Morris, DR; Hager, LP. Chloroperoxidase: I. Isolation and properties of the crystalline glycoprotein. J. Biol. Chem 1966, 241, 1763–1768. [Google Scholar]
- Shaw, PD; Hager, LP. Biological Chlorination: VI. Chloroperoxidase: A component of the β-ketoadipate chlorinase system. J. Biol. Chem 1961, 236, 1626–1630. [Google Scholar]
- Barret, EP; Joyner, LG; Halenda, PP. The Determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc 1951, 73, 373–380. [Google Scholar]
- Zhao, DY; Huo, QS; Feng, JL; Chmelka, BF; Stucky, GD. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc 1998, 120, 6024–6036. [Google Scholar]
- Zhao, DY; Feng, JL; Huo, QS; Melosh, N; Fredrickson, GH; Chmelka, BF; Stucky, GD. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar]
- Sing, KSW; Everett, DH; Haul, RAW; Moscow, L; Pierotti, RA; Rouquérol, J; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem 1985, 57, 603–619. [Google Scholar]
- Saam, WF; Cole, MW. Excitations and thermodynamics for liquid-helium films. Phys. Rev. B 1975, 11, 1086–1105. [Google Scholar]
- Jaroniec, CP; Gilpin, RK; Jaroniec, M. Comparative studies of chromatographic properties of silica-based amide-bonded phases under hydro–organic conditions. J. Chromatogr. A 1998, 797, 103–110. [Google Scholar]
- Chiang, CH; Ishida, H; Koenig, JL. The structure of γ-aminopropyltriethoxysilane on glass surfaces. J. Colloid Interface Sci 1980, 74, 396–404. [Google Scholar]
- Huang, X; Wang, J; Liu, X; Cong, R. A new type of chemically bonded phase for reversedphase HPLC. Anal. Sci 2003, 19, 1391–1394. [Google Scholar]
- Hesse, M; Meier, H; Zeeh, B. Spektroskopische Methoden in der organischen Chemie, 5th ed; Georg Thieme Verlag: New York, NY, USA, 1995. [Google Scholar]
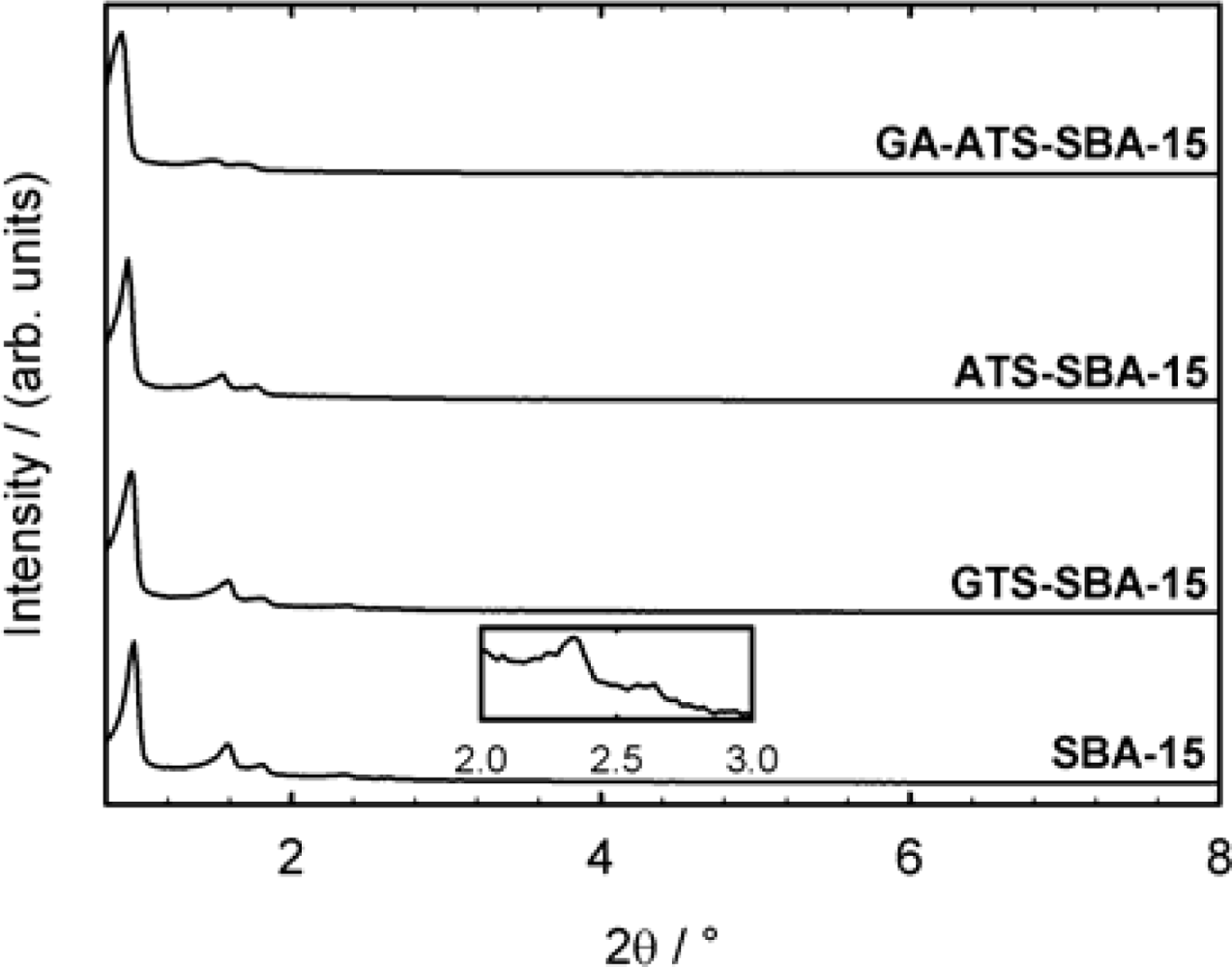
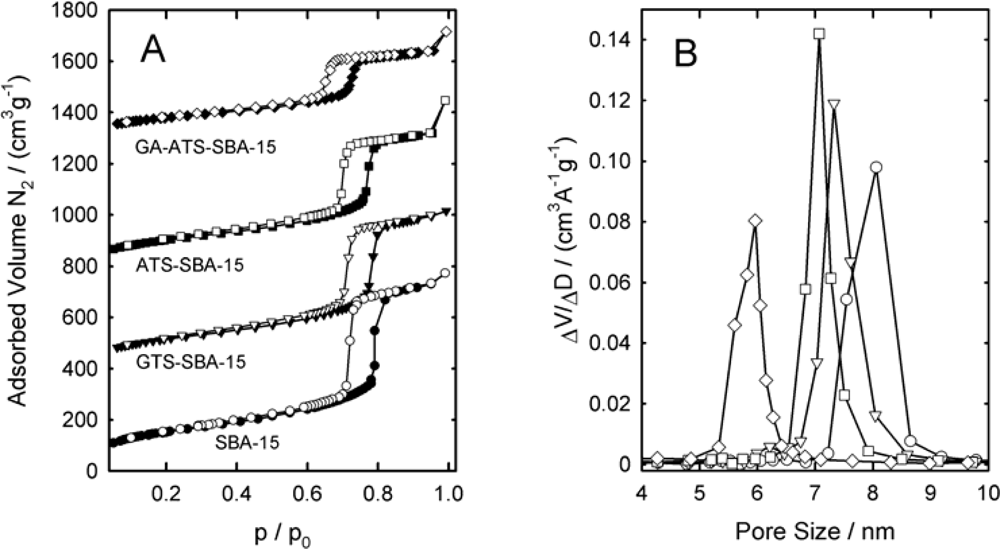

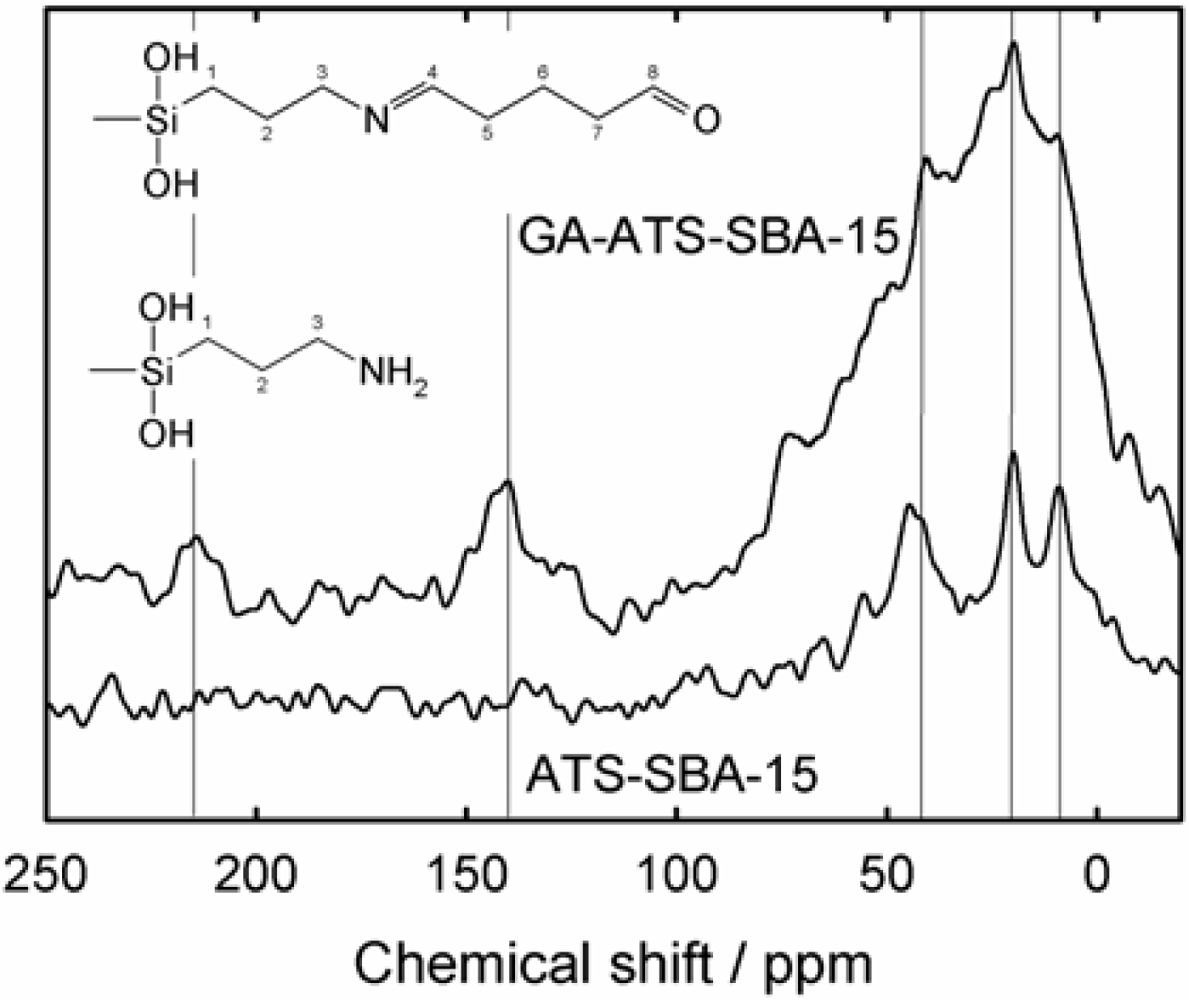



| Sample | dP / nm | ABET / (m2g−1) | VP / (cm3g−1) | a0 / nm |
|---|---|---|---|---|
| SBA-15 | 8.0 | 530 | 1.05 | 12.1 |
| GTS-SBA-15 | 7.3 | 433 | 0.90 | 11.1 |
| ATS-SBA-15 | 7.0 | 392 | 0.79 | 11.3 |
| GA-ATS-SBA-15 | 6.0 | 294 | 0.53 | 11.3 |
| Moities (calculated) | Elemental content/wt.-% | Molecules/nm2 | ||
|---|---|---|---|---|
| C | H | N | ||
| ATS | 9.3 | 2.1 | 2.2 | 4.7 |
| GA-ATS | 19.6 | 3.4 | 2.1 | |
| GA-ATS/ATS = 2/1 | 16.9 | 3.0 | 2.1 | |
| Sample (observed) | ||||
| GA-ATS-SBA-15 | 16.57 | 2.79 | 2.09 | 5.9 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jung, D.; Streb, C.; Hartmann, M. Covalent Anchoring of Chloroperoxidase and Glucose Oxidase on the Mesoporous Molecular Sieve SBA-15. Int. J. Mol. Sci. 2010, 11, 762-778. https://doi.org/10.3390/ijms11020762
Jung D, Streb C, Hartmann M. Covalent Anchoring of Chloroperoxidase and Glucose Oxidase on the Mesoporous Molecular Sieve SBA-15. International Journal of Molecular Sciences. 2010; 11(2):762-778. https://doi.org/10.3390/ijms11020762
Chicago/Turabian StyleJung, Dirk, Carsten Streb, and Martin Hartmann. 2010. "Covalent Anchoring of Chloroperoxidase and Glucose Oxidase on the Mesoporous Molecular Sieve SBA-15" International Journal of Molecular Sciences 11, no. 2: 762-778. https://doi.org/10.3390/ijms11020762
APA StyleJung, D., Streb, C., & Hartmann, M. (2010). Covalent Anchoring of Chloroperoxidase and Glucose Oxidase on the Mesoporous Molecular Sieve SBA-15. International Journal of Molecular Sciences, 11(2), 762-778. https://doi.org/10.3390/ijms11020762