Biological Activities of Phenolic Compounds Present in Virgin Olive Oil
Abstract
:1. Introduction
2. Bioavailability of Olive Oil Phenolic Compounds
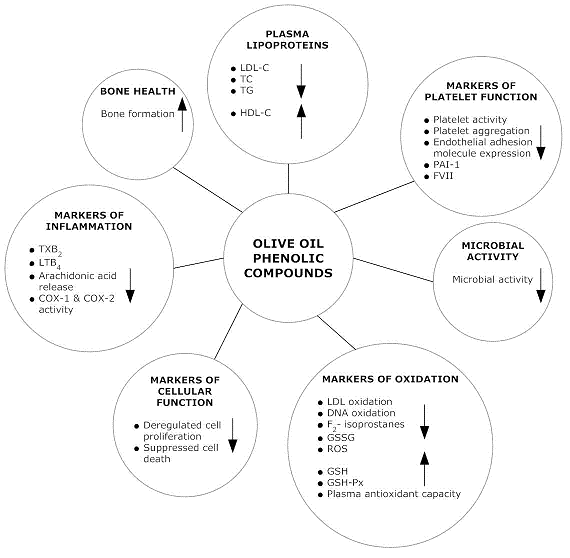
3. Olive Oil Phenolic Compounds and Health
4. Olive Oil Phenolic Compounds and Their Beneficial Effect on Plasma Lipoproteins
5. Olive Oil Phenolic Compounds and Their Beneficial Effect on Lipid Oxidation
6. Olive Oil Phenolic Compounds and Their Beneficial Effect on Oxidative DNA Damage
7. Olive Oil Phenolic Compounds and Their Beneficial Effect on Additional Markers of Oxidation
8. Olive Oil Phenolic Compounds and Their Beneficial Effect on Markers of Inflammation
9. Olive Oil Phenolic Compounds and Their Beneficial Effect on Platelet Function
10. Olive Oil Phenolic Compounds and Their Beneficial Effect on Cellular Function
11. Olive Oil Phenolic Compounds and Their Beneficial Effect on Microbial Activity
12. Olive Oil Phenolic Compounds and Their Beneficial Effect on Bone
13. Conclusions
Abbreviations:
| ADDL | beta-amyloid oligomers |
| CHD | coronary heart disease |
| COX-1 | cyclooxygenase-1 |
| COX-2 | cyclooxygenase-2 |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| DNA | deoxyribonucleic acid |
| FVII | factor VII |
| g | gram |
| GSSG | glutathionedissulfide |
| GSH | reduced glutathione |
| GSH-Px | glutathione peroxidise |
| HDL-C | high density lipoprotein cholesterol |
| IL-6 | interleukin-6 |
| LDL | low density lipoprotein |
| LDL-C | low density lipoprotein cholesterol |
| LPO | lipid peroxidation |
| LTB4 | leukotriene B4 |
| MUFA | monounsaturated fatty acid |
| μM | micromolar |
| μg | microgram |
| oxLDL | low density lipoprotein oxidation |
| 8-oxo-dg | 8-oxo-2’-deoxyguanosine |
| PAI-1 | plasminogen activator inhibitor-1 |
| ROS | reactive oxygen species |
| sICAM-1 | soluble intercellular molecules |
| sVCAM-1 | soluble vascular adhesion molecules |
| Tau | microtubule-associated protein |
| TC | total cholesterol |
| TG | triglyceride |
| TXB2 | thromboxane B2 |
Acknowledgments
References
- De Lorgeril, M; Salen, P; Martin, JL; Monjaud, I; Delaye, J; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar]
- Singh, RB; Dubnov, G; Niaz, MA; Ghosh, S; Singh, R; Rastogi, SS; Manor, O; Pella, D; Berry, EM. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): A randomised single-blind trial. Lancet 2002, 360, 1455–1461. [Google Scholar]
- Stark, AH; Madar, Z. Olive oil as a functional food: epidemiology and nutritional approaches. Nutr. Rev 2002, 60, 170–176. [Google Scholar]
- Trichopoulou, A; Costacou, T; Bamia, C; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med 2003, 348, 2599–2608. [Google Scholar]
- Visioli, F; Galli, C. Natural antioxidants and prevention of coronary heart disease: the potential role of olive oil and its minor constituents. Nutr. Metab. Cardio. Dis 1995, 5, 306–314. [Google Scholar]
- Fortes, C; Forastiere, F; Farchi, S; Mallone, S; Trequattrinni, T; Anatra, F; Schmid, G; Perucci, CA. The protective effect of the Mediterranean diet on lung cancer. Nutr Cancer 2003, 46, 30–37. [Google Scholar]
- Grasso, S; Siracusa, L; Spatafora, C; Renis, M; Tringali, C. Hydroxytyrosol lipophilic analogues: Enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection. Bioorg. Chem 2006, 35, 137–152. [Google Scholar]
- Herrera, MD; Perez-Guerrero, C; Marhuenda, E; Ruiz-Gutierrez, V. Effects of dietary oleic-rich oils (virgin olive and high-oleic-acid sunflower) on vascular reactivity in Wistar-Kyoto and spontaneously hypertensive rats. Br. J. Nutr 2001, 86, 349–357. [Google Scholar] [Green Version]
- Matalas, A-L; Zampelas, A; Stavrinos, V; Wolinsky, I. The Mediterranean Diet: Constituents and Health Promotion; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Galeone, C; Talamini, R; Levi, F; Pelucchi, C; Negri, E; Giacosa, A; Montella, M; Franceschi, S; La Vecchia, C. Fried foods, olive oil and colorectal cancer. Ann. Oncol 2006, 18, 36–39. [Google Scholar]
- Filik, L; Ozyilkan, O. Olive-oil consumption and cancer risk. Eur. J. Clin. Nutr 2003, 57, 191. [Google Scholar]
- Hu, FB. The Mediterranean diet and mortality–olive oil and beyond. N. Engl. J. Med 2003, 348, 2595–2596. [Google Scholar]
- Kok, FJ; Kromhout, D. Atherosclerosis–epidemiological studies on the health effects of a Mediterranean diet. Eur. J. Nutr 2004, 43, 1S–5S. [Google Scholar]
- Kris-Etherton, P; Eckel, RH; Howard, BV; St. Jeor, S; Bazzarre, TL. AHA Science advisory: Lyon diet heart study. benefits of a mediterranean-style, national cholesterol education program/american heart association step i dietary pattern on cardiovascular disease. Circulation 2001, 103, 1823–1825. [Google Scholar]
- Kushi, LH; Lenart, EB; Willett, WC. Health implications of Mediterranean diets in light of contemporary knowledge. 2. Meat, wine, fats, and oils. Am. J. Clin. Nutr 1995, 61, 1416S–1427S. [Google Scholar]
- Willett, WC; Sacks, F; Trichopoulou, A; Drescher, G; Ferro-Luzzi, A; Helsing, E; Trichopoulos, D. Mediterranean diet pyramid: a cultural model for healthy eating. Am. J. Clin. Nutr 1995, 61, 1402S–1406S. [Google Scholar]
- Fung, TT; Rexrode, KM; Mantzoros, CS; Manson, JE; Willett, WC; Hu, FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar]
- Corona, G; Spencer, JPE; Dessi, MA. Extra virgin olive oil phenolics: absorption, metabolism, and biological activities in the GI tract. Toxical. Ind. Health 2009, 25, 285–293. [Google Scholar]
- Tripoli, E; Giammanco, M; Tabacchi, G; Di Majo, D; Giammanco, S; La Guardia, M. The phenolic compounds of olive oil: structure, biological activity and beneficial effects on human health. Nutr. Res. Rev 2005, 18, 98–112. [Google Scholar]
- Harper, CR; Edwards, MC; Jacobson, TA. Flaxseed oil supplementation does not affect plasma lipoprotein concentration or particle size in human subjects. J. Nutr 2006, 136, 2844–2848. [Google Scholar]
- Aguilera, CM; Mesa, MD; Ramirez-Tortosa, MC; Nestares, MT; Ros, E; Gil, A. Sunflower oil does not protect against LDL oxidation as virgin olive oil does in patients with peripheral vascular disease. Clin. Nutr 2004, 23, 673–681. [Google Scholar]
- Visioli, F; Galli, C. Biological properties of olive oil phytochemicals. Crit. Rev. Food Sci. Nutr 2002, 42, 209–221. [Google Scholar]
- Psaltopoulou, T; Naska, A; Orfanos, P; Trichopoulos, D; Mountokalakis, T; Trichopoulou, A. Olive oil, the Mediterranean diet, and arterial blood pressure: the Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am. J. Clin. Nutr 2004, 80, 1012–1018. [Google Scholar]
- Martin-Moreno, JM; Willett, WC; Gorgojo, L; Banegas, JR; Rodriguez-Artalejo, F; Fernandez-Rodriguez, JC; Maisonneuve, P; Boyle, P. Dietary fat, olive oil intake and breast cancer risk. Int. J. Cancer 1994, 58, 774–780. [Google Scholar]
- Covas, MI; Nyyssonen, K; Poulsen, HE; Kaikkonen, J; Zunft, HJ; Kiesewetter, H; Gaddi, A; de la Torre, R; Mursu, J; Baumler, H; Nascetti, S; Salonen, JT; Fito, M; Virtanen, J; Marrugat, J; Group, ES. The effect of polyphenols in olive oil on heart disease risk factors: a randomized trial. Ann. Int. Med 2006, 145, 333–341. [Google Scholar]
- Nicolaiew, N; Lemort, N; Adorni, L; Berra, B; Montorfano, G; Rapelli, S; Cortesi, N; Jacotot, B. Comparison between extra virgin olive oil and oleic acid rich sunflower oil: effects on postprandial lipemia and LDL susceptibility to oxidation. Ann. Nutr. Metab 1998, 42, 251–260. [Google Scholar]
- Carluccio, MA; Siculella, L; Ancora, MA; Massaro, M; Scoditti, E; Storelli, C; Visioli, F; Distante, A; De Caterina, R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterio. Throm. Vasc. Bio 2003, 23, 622–629. [Google Scholar]
- Tuck, KL; Hayball, PJ. Major phenolic compounds in olive oil: metabolism and health effects. J. Nutr. Biochem 2002, 13, 636–644. [Google Scholar]
- Visioli, F; Caruso, D; Galli, C; Viappiani, S; Galli, G; Sala, A. Olive oils rich in natural catecholic phenols decrease isoprostane excretion in humans. Biochem. Biophys. Res. Comm 2000, 278, 797–799. [Google Scholar]
- Visioli, F; Galli, C; Bornet, F; Mattei, A; Patelli, R; Galli, G; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett 2000, 468, 159–160. [Google Scholar]
- Bai, C; Yan, X; Takenaka, M; Sekiya, K; Nagata, T. Determination of synthetic hydroxytyrosol in rat plasma by GC-MS. J. Agric. Food Chem 1998, 46, 3998–4001. [Google Scholar]
- Vissers, MN; Zock, PL; Roodenburg, AJ; Leenen, R; Katan, MB. Olive oil phenols are absorbed in humans. J. Nutr 2002, 132, 409–417. [Google Scholar]
- Miro Casas, E; Albadalejo, MF; Covas Planells, MI; Colomer, FM; Lamuela Raventos, RM; de la Torre Fornell, R. Tyrosol bioavailability in humans after ingestion of virgin olive oil. Clin. Chem 2001, 47, 341–343. [Google Scholar]
- Bruni, N; Cortesi, N; Fiorino, P. Influence of agricultural techniques, cultivar and area of origin on characteristics of virgin olive oil and levels of some of its “minor” components. Olivae 1994, 1, 28–34. [Google Scholar]
- Esti, M; Cinquanta, L; La Notte, E. Phenolic compounds in different olive varieties. J. Agric. Food Chem 1998, 46, 32–35. [Google Scholar]
- Gomez-Alonzo, S; Salvador, MD; Fregapane, G. Phenolic compounds profile of Cornicarba virgin olive oil. J. Agric. Food Chem 2002, 50, 6812–6817. [Google Scholar]
- Vinha, AF; Ferreres, F; Silva, BM; Valentao, P; Goncalves, A; Pereira, JA; Oliveira, MB; Seabra, RM; Andrade, PB. Phenolic profiles of Portuguese olive fruits (Olea europaea L.): Influences of cultivar and geographical origin. Food Chem 2005, 89, 561–568. [Google Scholar]
- Cerretani, L; Bendini, A; Rotondi, A; Lercker, G; Toschi, TG. Analytical comparison of monovarietal virgin olive oils obtained by both a continuous industrial plant and low-scale mill. Eur. J. Lipid Sci. Technol 2005, 107, 93–100. [Google Scholar]
- Sivakumar, G; Bati, CB; Uccella, N. HPLC-MS screening of the antioxidant profile of Italian olive cultivars. Chem. Nat. Comp 2005, 41, 588–591. [Google Scholar]
- Franconi, F; Coinu, R; Carta, S; Urgeghe, PP; Ieri, F; Mulinacci, N; Romani, A. Antioxidant effect of two virgin olive oils depends on the concentration and composition of minor polar compounds. J. Agric. Food Chem 2006, 54, 3121–3125. [Google Scholar]
- Carrasco Pancorbo, A; Cruces-Blanco, C; Segura Carretero, A; Fernandez Gutierrez, A. Sensitive determination of phenolic acids in extra-virgin olive oil by capillary zone electrophoresis. J. Agric. Food Chem 2004, 52, 6687–6693. [Google Scholar]
- Caravaca, AMG; Pancorbo, AC; Diaz, BC; Carretero, AS; Gutierrez, AF. Electrophoretic identification and quantification of compounds in the polyphenolic fraction of extra-virgin olive oil. Electrophoresis 2005, 26, 3538–3551. [Google Scholar]
- Romero, MP; Tovar, MJ; Girona, J; Motilva, MJ. Changes in the HPLC phenolic profile of virgin olive oil from young trees (Olea europaea L. Cv. Arbequina) grown under different deficit irrigation strategies. J. Agric. Food Chem 2002, 50, 5349–5354. [Google Scholar]
- Gomez-Rico, A; Salvador, MD; La Greca, M; Fregapane, G. Phenolic and volatile compounds of extra virgin olive oil (Olea europaea L. Cv. Cornicabra) with regard to fruit ripening and irrigation management. J. Agric. Food Chem 2006, 54, 7130–7136. [Google Scholar]
- Gimeno, E; Castellote, AI; Lamuela-Raventos, RM; De la Torre, MC; Lopez-Sabater, MC. The effects of harvest and extraction methods on the antioxidant content (phenolics, alpha-tocopherol, and beta-carotene) in virgin olive oil. Food Chem 2002, 78, 207–211. [Google Scholar]
- Brenes, M; Garcia, A; Garcia, P; Rios, JJ; Garrido, A. Phenolic compounds in Spanish olive oils. J. Agric. Food Chem 1999, 47, 3535–3540. [Google Scholar]
- Kalua, CM; Allen, MS; Bedgood, DR, Jr; Bishop, AG; Prenzler, PD. Discrimination of olive oils and fruits into cultivars and maturity stages based on phenolic and volatile compounds. J. Agric. Food Chem 2005, 53, 8054–8062. [Google Scholar]
- Tovar, MJ; Motilva, MJ; Romero, MP. Changes in the phenolic composition of virgin olive oil from young trees (Olea europaea L. cv. Arbequina) grown under linear irrigation strategies. J. Agric. Food Chem 2001, 49, 5502–5508. [Google Scholar]
- Ranalli, A; Ferrante, ML; De Mattia, G; Costantini, N. Analytical evaluation of virgin olive oil of first and second extraction. J. Agric. Food Chem 1999, 47, 417–424. [Google Scholar]
- Kalua, CM; Bedgood, DR, Jr; Bishop, AG; Prenzler, PD. Changes in volatile and phenolic compounds with malaxation time and temperature during virgin olive oil production. J. Agric. Food Chem 2006, 54, 7641–7651. [Google Scholar]
- Mailer, RJ; Ayton, J. Comparison of olive oil (Olea europaea) quality extracted by stonemill and hammermill. NZ. J. Crop Hort. Sci 2004, 32, 325–330. [Google Scholar]
- Fregapane, G; Lavelli, V; Leon, S; Kapuralin, J; Desamparados Salvador, M. Effect of filtration on virgin olive oil stability during storage. Eur. J. Lip. Sci. Technol 2006, 108, 134–142. [Google Scholar]
- Di Giovacchino, L; Sestili, S; Di Vincenzo, D. Influence of olive processing on virgin olive oil quality. Eur. J. Lip. Sci. Technol 2002, 104, 587–601. [Google Scholar]
- Brenes, M; Garcia, A; Garcia, P; Garrido, A. Acid hydrolysis of secoiridoid aglycons during storage of virgin olive oil. J. Agric. Food Chem 2001, 49, 5609–5614. [Google Scholar]
- Gutierrez, F; Fernandez, JL. Determinant parameters and components in the storage of virgin olive oil. Prediction of storage time beyond which the oil is no longer of “extra” quality. J. Agric. Food Chem 2002, 50, 571–577. [Google Scholar]
- Okogeri, O; Tasioula-Margari, M. Changes occurring in phenolic compounds and alpha-tocopherol of virgin olive oil during storage. J. Agric. Food Chem 2002, 50, 1077–1080. [Google Scholar]
- Rastrelli, L; Passi, S; Ippolito, F; Vacca, G; De Simone, F. Rate of degradation of alpha-tocopherol, squalene, phenolics, and polyunsaturated fatty acids in olive oil during different storage conditions. J. Agric. Food Chem 2002, 50, 5566–5570. [Google Scholar]
- Brenes, M; Garcia, A; Dobarganes, MC; Velasco, J; Romero, C. Influence of thermal treatments simulating cooking processes on the polyphenol content in virgin olive oil. J. Agric. Food Chem 2002, 50, 5962–5967. [Google Scholar]
- Gomez-Alonso, S; Fregapane, G; Salvador, MD; Gordon, MH. Changes in phenolic composition and antioxidant activity of virgin olive oil during frying. J. Agric. Food Chem 2003, 51, 667–672. [Google Scholar]
- Cicerale, S; Conlan, XA; Barnett, NW; Sinclair, AJ; Keast, RS. Influence of heat on biological activity and concentration of oleocanthal—A natural anti-inflammatory agent in virgin olive oil. J. Agric. Food Chem 2009, 57, 1326–1330. [Google Scholar]
- Carrasco-Pancorbo, A; Cerretani, L; Bendini, A; Segura-Carretero, A; Gallina-Toschi, T; Fernandez-Gutierez, A. Analytical determination of polyphenols in olive oils. J Sep Sci 2005, 28, 837–858. [Google Scholar]
- Cicerale, S; Conlan, XA; Sinclair, AJ; Keast, RSJ. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr 2009, 49, 218–236. [Google Scholar]
- Martini, FH. Fundamentals of Anatomy and Physiology, 7 ed; Pearson Education Inc: San Francisco, CA, USA, 2006. [Google Scholar]
- Visioli, F; Galli, C; Plasmati, E; Viappiani, S; Hernandez, A; Colombo, C; Sala, A. Olive phenol hydroxytyrosol prevents passive smoking-induced oxidative stress. Circulation 2000, 102, 2169–2171. [Google Scholar]
- Caruso, D; Visioli, F; Patelli, R; Galli, C; Galli, G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism 2001, 50, 1426–1428. [Google Scholar]
- Tuck, KL; Freeman, MP; Hayball, PJ; Stretch, GL; Stupans, I. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J. Nutr 2001, 131, 1993–1996. [Google Scholar]
- Edgecombe, S; Stretch, GL; Hayball, PJ. Oleuropein, an antioxidant polyphenol from olive oil, is poorly absorbed from isolated perfused rat intestine. J. Nutr 2000, 130, 2996–3002. [Google Scholar]
- Manna, C; Galletti, P; Maisto, G; Cucciolla, V; D’Angelo, S; Zappia, V. Transport mechanism and metabolsi of olive oil hydroxytyrosol in Caco-2 cells. FEBS Letters 2000, 470, 341–344. [Google Scholar]
- Visioli, F; Caruso, D; Plasmati, E; Patelli, R; Mulinacci, N; Romani, A; Galli, G; Galli, C. Hydroxytyrosol, as a component of olive mill waste water, is dose- dependently absorbed and increases the antioxidant capacity of rat plasma. Free Rad. Res 2001, 34, 301–305. [Google Scholar]
- Fito, M; Cladellas, M; de la Torre, R; Marti, J; Munoz, D; Schroder, H; Alcantara, M; Pujadas-Bastardes, M; Marrugat, J; Lopez-Sabater, MC; Bruguera, J; Covas, MI. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: a randomized, crossover, controlled trial. Eur. J. Clin. Nutr 2008, 62, 570–574. [Google Scholar]
- Ruano, J; Lopez-Miranda, J; de la Torre, R; Delgado-Lista, J; Fernandez, J; Caballero, J; Covas, MI; Jimenez, Y; Perez-Martinez, P; Marin, C; Fuentes, F; Perez-Jimenez, F. Intake of phenol-rich virgin olive oil improves the postprandial prothrombotic profile in hypercholesterolemic patients. Am. J. Clin. Nutr 2007, 86, 341–346. [Google Scholar]
- Gimeno, E; de la Torre-Carbot, K; Lamuela-Raventos, RM; Castellote, AI; Fito, M; de la Torre, R; Covas, MI; Carmen Lopez-Sabater, M. Changes in the phenolic content of low density lipoprotein after olive oil consumption in men. A randomized crossover controlled trial. Br J Nutr 2007, 1–8. [Google Scholar]
- Bogani, P; Galli, C; Villa, M; Visioli, F. Postprandial anti-inflammatory and antioxidant effects of extra virgin olive oil. Atherosclerosis 2007, 190, 181–186. [Google Scholar]
- Salvini, S; Sera, F; Caruso, D; Giovannelli, L; Visioli, F; Saieva, C; Masala, G; Ceroti, M; Giovacchini, V; Pitozzi, V; Galli, C; Romani, A; Mulinacci, N; Bortolomeazzi, R; Dolara, P; Palli, D. Daily consumption of a high-phenol extra-virgin olive oil reduces oxidative DNA damage in postmenopausal women. Br. J. Nutr 2006, 95, 742–751. [Google Scholar]
- Covas, MI; de la Torre, K; Farre-Albaladejo, M; Kaikkonen, J; Fito, M; Lopez-Sabater, C; Pujadas-Bastardes, MA; Joglar, J; Weinbrenner, T; Lamuela-Raventos, RM; de la Torre, R. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Rad. Biol. Med 2006, 40, 608–616. [Google Scholar]
- Ruano, J; Lopez-Miranda, J; Fuentes, F; Moreno, JA; Bellido, C; Perez-Martinez, P; Lozano, A; Gomez, P; Jimenez, Y; Perez Jimenez, F. Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J. Am. Coll. Cardiol 2005, 46, 1864–1868. [Google Scholar]
- Visioli, F; Caruso, D; Grande, S; Bosisio, R; Villa, M; Galli, G; Sirtori, C; Galli, C. Virgin Olive Oil Study (VOLOS): Vasoprotective potential of extra virgin olive oil in mildly dyslipidemic patients. Eur. J. Nutr 2005, 44, 121–127. [Google Scholar]
- Fito, M; Cladellas, M; de la Torre, R; Marti, J; Alcantara, M; Pujadas-Bastardes, M; Marrugat, J; Bruguera, J; Lopez-Sabater, MC; Vila, J; Covas, MI. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: a randomized, crossover, controlled, clinical trial. Atherosclerosis 2005, 181, 149–158. [Google Scholar]
- Marrugat, J; Covas, MI; Fito, M; Schroder, H; Miro-Casas, E; Gimeno, E; Lopez-Sabater, MC; de la Torre, R; Farre, M. Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation–a randomized controlled trial. Eur. J. Nutr 2004, 43, 140–147. [Google Scholar]
- Weinbrenner, T; Fito, M; de la Torre, R; Saez, GT; Rijken, P; Tormos, C; Coolen, S; Albaladejo, MF; Abanades, S; Schroder, H; Marrugat, J; Covas, MI. Olive oils high in phenolic compounds modulate oxidative/antioxidative status in men. J. Nutr 2004, 134, 2314–2321. [Google Scholar]
- Moschandreas, J; Vissers, MN; Wiseman, S; van Putte, KP; Kafatos, A. Extra virgin olive oil phenols and markers of oxidation in Greek smokers: a randomized cross-over study. Eur. J. Clin. Nutr 2002, 56, 1024–1029. [Google Scholar]
- Vissers, MN; Zock, PL; Wiseman, SA; Meyboom, S; Katan, MB. Effect of phenol-rich extra virgin olive oil on markers of oxidation in healthy volunteers. Eur. J. Clin. Nutr 2001, 55, 334–341. [Google Scholar]
- Bonanome, A; Pagnan, A; Caruso, D; Toia, A; Xamin, A; Fedeli, E; Berra, B; Zamburlini, A; Ursini, F; Galli, G. Evidence of postprandial absorption of olive oil phenols in humans. Nutr. Metab. Cardiovas. Dis 2000, 10, 111–120. [Google Scholar]
- Ramirez-Tortosa, MC; Urbano, G; Lopez-Jurado, M; Nestares, T; Gomez, MC; Mir, A; Ros, E; Mataix, J; Gil, A. Extra-virgin olive oil increases the resistance of LDL to oxidation more than refined olive oil in free-living men with peripheral vascular disease. J. Nutr 1999, 129, 2177–2183. [Google Scholar]
- Gordon, T; Kannel, WB; Castelli, WP; Dawber, TR. Lipoproteins, cardiovascular disease, and death. The Framingham study. Arch. Intern. Med 1981, 141, 1128–1131. [Google Scholar]
- Chrysohoou, C; Pitsavos, C; Skoumas, J; Masoura, C; Katinioti, A; Panagiotakos, D; Stefanadis, C. The emerging anti-inflammatory role of HDL-cholesterol, illustrated in cardiovascular disease free population; the ATTICA study. Int. J. Cardio 2006, 122, 29–33. [Google Scholar]
- Gimeno, E; Fito, M; Lamuela-Raventos, RM; Castellote, AI; Covas, M; Farre, M; de La Torre-Boronat, MC; Lopez-Sabater, MC. Effect of ingestion of virgin olive oil on human low-density lipoprotein composition. Eur. J. Clin. Nutr 2002, 56, 114–120. [Google Scholar]
- Gorinstein, S; Leontowicz, H; Lojek, A; Leontowicz, M; Ciz, M; Krzeminski, R; Gralak, M; Czerwinski, J; Jastrzebski, Z; Trakhtenberg, S; Grigelmo-Miguel, N; Soliva-Fortuny, R; Martin-Belloso, O. Olive oils improve lipid metabolism and increase antioxidant potential in rats fed diets containing cholesterol. J. Agric. Food Chem 2002, 50, 6102–6108. [Google Scholar]
- Mangas-Cruz, MA; Fernandez-Moyano, A; Albi, T; Guinda, A; Relimpio, F; Lanzon, A; Pereira, JL; Serrera, JL; Montilla, C; Astorga, R; Garcia-Luna, PP. Effects of minor constituents (non-glyceride compounds) of virgin olive oil on plasma lipid concentrations in male Wistar rats. Clin. Nutr 2001, 20, 211–215. [Google Scholar]
- Witztum, JL. The oxidation hypothesis of atherosclerosis. Lancet 1994, 344, 793–795. [Google Scholar]
- Coni, E; Di Benedetto, R; Di Pasquale, M; Masella, R; Modesti, D; Mattei, R; Carlini, EA. Protective effect of oleuropein, an olive oil biophenol, on low density lipoprotein oxidizability in rabbits. Lipids 2000, 35, 45–54. [Google Scholar]
- Patrick, L; Uzick, M. Cardiovascular disease: C-reactive protein and the inflammatory disease paradigm: HMG-CoA reductase inhibitors, alpha-tocopherol, red yeast rice, and olive oil polyphenols. A review of the literature. Altern. Med. Rev 2001, 6, 248–271. [Google Scholar]
- Ramirez-Tortosa, C; Lopez-Pedrosa, JM; Suarez, A; Ros, E; Mataix, J; Gil, A. Olive oil- and fish oil-enriched diets modify plasma lipids and susceptibility of LDL to oxidative modification in free-living male patients with peripheral vascular disease: the Spanish Nutrition Study. Br. J. Nutr 1999, 82, 31–39. [Google Scholar]
- Ochoa, JJ; Quiles, JL; Ramirez-Tortosa, C; Mataix, J; Huertas, JR. Dietary oil high in oleic acid but with different unsaponifiable fraction contents have different effects in fatty acid composition and peroxidation in rabit LDL. Nutrition 2002, 18, 60–65. [Google Scholar]
- De la Torre-Carbot, K; Chavez-Servin, JL; Jauregui, O; Castellote, AI; Lamuela-Raventos, RM; Fito, M; Covas, MI; Munoz-Aguayo, D; Lopez-Sabater, MC. Presence of virgin olive oil phenolic metabolites in human low density lipoprotein fraction: determination by high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Analyt. Chim. Acta 2007, 583, 402–410. [Google Scholar]
- Berrougui, H; Cloutier, M; Isabelle, M; Khalil, A. Phenolic-extract from argan oil (Argania spinosa L.) inhibits human low-density lipoprotein (LDL) oxidation and enhances cholesterol efflux from human THP-1 macrophages. Atherosclerosis 2006, 184, 389–396. [Google Scholar]
- Masella, R; Vari, R; D’Archivio, M; Di Benedetto, R; Matarrese, P; Malorni, W; Scazzocchio, B; Giovannini, C. Extra virgin olive oil biophenols inhibit cell-mediated oxidation of LDL by increasing the mRNA transcription of glutathione-related enzymes. J. Nutr 2004, 134, 785–791. [Google Scholar]
- Visioli, F; Bellomo, G; Montedoro, G; Galli, C. Low density lipoprotein oxidation is inhibited in vitro by olive oil constituents. Atherosclerosis 1995, 117, 25–32. [Google Scholar]
- Cooke, MS; Evans, MD; Dizdaroglu, M; Lunec, J. Oxidative DNA damage: mechanisms, mutation, and disease. Fed. Am. Soc. Exper. Biol. J 2003, 17, 1195–1214. [Google Scholar]
- Machowetz, A; Poulsen, HE; Gruendel, S; Weimann, A; Fito, M; Marrugat, J; de la Torre, R; Salonen, JT; Nyyssonen, K; Mursu, J; Nascetti, S; Gaddi, A; Kiesewetter, H; Baumler, H; Selmi, H; Kaikkonen, J; Zunft, HJ; Covas, MI; Koebnick, C. Effect of olive oils on biomarkers of oxidative DNA stress in Northern and Southern Europeans. FASEB J 2007, 21, 45–52. [Google Scholar]
- Jacomelli, M; Pitozzi, V; Zaid, M; Larrosa, M; Tonini, G; Martini, A; Urbani, S; Taticchi, A; Servili, M; Dolara, P; Giovannelli, L. Dietary extra-virgin olive oil rich in phenolic antioxidants and the aging process: long-term effects in the rat. J Nutr Biochem 2009. [Google Scholar]
- Quiles, JL; Farquharson, AJ; Simpson, DK; Grant, I; Wahle, KW. Olive oil phenolics: effects on DNA oxidation and redox enzyme mRNA in prostate cells. Br. J. Nutr 2002, 88, 225–234. [Google Scholar]
- Fabiani, R; Rosignoli, P; De Bartolomeo, A; Fuccelli, R; Servili, M; Montedoro, GF; Morozzi, G. Oxidative DNA damage is prevented by extracts of olive oil, hydroxytyrosol, and other olive phenolic compounds in human blood mononuclear cells and HL60 cells. J. Nutr 2008, 138, 1411–1416. [Google Scholar]
- Reinisch, N; Kiechl, S; Mayr, C; Schratzberger, P; Dunzendorfer, S; Kahler, CM; Buratti, T; Willeit, J; Wiedermann, CJ. Association of high plasma antioxidant capacity with new lesion formation in carotid atherosclerosis: a prospective study. Eur. J. Clin. Invest 1998, 28, 787–792. [Google Scholar]
- Goya, L; Mateos, R; Bravo, L. Effect of the olive oil phenol hydroxytyrosol on human hepatoma HepG2 cells: Protection against oxidative stress induced by tert-butylhydroperoxide. Eur. J. Nutr 2007, 46, 70–78. [Google Scholar]
- De la Puerta, R; Martinez Dominguez, ME; Ruiz-Gutierrez, V; Flavill, JA; Hoult, JR. Effects of virgin olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci 2001, 69, 1213–1222. [Google Scholar]
- Owen, RW; Giacosa, A; Hull, WE; Haubner, R; Spiegelhalder, B; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar]
- Paiva-Martins, F; Fernandes, J; Rocha, S; Nascimento, H; Vitorino, R; Amado, F; Borges, F; Belo, L; Santos-Silva, A. Effects of olive oil polyphenols on erythrocyte oxidative damage. Mol. Nutr. Food Res 2009, 53, 609–616. [Google Scholar]
- Moreno, JJ. Effect of olive oil minor components on oxidative stress and arachidonic acid mobilization and metabolism by macrophages. Free Rad. Biol. Med 2003, 35, 1073–1081. [Google Scholar]
- Biswas, SK; Newby, DE; Rahman, I; Megson, IL. Depressed glutathione synthesis precedes oxidative stress and atherogenesis in Apo-E(–/–) mice. Biochem. Biophys. Res. Comm 2005, 338, 1368–1373. [Google Scholar]
- Visioli, F; Wolfram, R; Richard, D; Abdullah, MICB; Crea, R. Olive phenolics increase glutathione levelsin healthy volunteers. J. Agric. Food Chem 2009, 57, 1793–1796. [Google Scholar]
- Loru, D; Incani, A; Deiaa, M; Corona, G; Atzeri, A; Melis, MP; Rosa, A; Dessi, MA. Protective effect of hydroxytyrosol and tyrosol against oxidative stress in kidney cells. Toxicol. Ind. Health 2009, 25, 301–310. [Google Scholar]
- Packard, RRS; Libby, P. Inflammation in atherosclerosis: From vascular biology to biomarker discovery and risk prediction. Clin. Chem 2008, 54, 24–38. [Google Scholar]
- Groff, JL; Gropper, SS. Advanced Nutrition and Human Metabolism, 3rd ed; Wadsworth Thomson Learning: Belmont, CA, USA, 2000. [Google Scholar]
- Tiziani, A. Harvard’s Nursing Guide to Drugs; Elsevier Australia: Sydney, Australia, 2006. [Google Scholar]
- Leger, CL; Carbonneau, MA; Michel, F; Mas, E; Monnier, L; Cristol, JP; Descomps, B. A thromboxane effect of a hydroxytyrosol-rich olive oil wastewater extract in patients with uncomplicated type I diabetes. Eur. J. Clin. Nutr 2005, 59, 727–730. [Google Scholar]
- Beauchamp, GK; Keast, RS; Morel, D; Lin, J; Pika, J; Han, Q; Lee, CH; Smith, AB; Breslin, PA. Phytochemistry: ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar]
- Brody, T. Nutritional Biochemistry, 2nd ed; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- White, JG. Platelets and atherosclerosis. Eur J Clin Invest 1994, 24(Suppl 1), 25–29. [Google Scholar]
- De La Cruz, JP; Villalobos, MA; Carmona, JA; Martin-Romero, M; Smith-Agreda, JM; de la Cuesta, FS. Antithrombotic potential of olive oil administration in rabbits with elevated cholesterol. Thromb. Res 2000, 100, 305–315. [Google Scholar]
- Perona, JS; Cabello-Moruno, R; Ruiz-Gutierrez, V. The role of virgin olive oil components in the modulation of endothelial function. J. Nutr. Biochem 2006, 17, 429–445. [Google Scholar] [Green Version]
- Togna, GI; Togna, AR; Franconi, M; Marra, C; Guiso, M. Olive oil isochromans inhibit human platelet reactivity. J. Nutr 2003, 133, 2532–2536. [Google Scholar]
- Petroni, A; Blasevich, M; Salami, M; Papini, N; Montedoro, G; Galli, C. Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thromb Res 1995, 78, 151–160. [Google Scholar]
- Dell’ Agli, M; Maschi, O; Galli, GV; Fagnani, R; Dal Cero, E; Caruso, D; Bosisio, E. Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. Br. J. Nutr 2008, 99, 945–951. [Google Scholar]
- Manna, C; Napoli, D; Cacciapuoti, G; Porcelli, M; Zappia, V. Olive oil phenolic compounds inhibit homocysteine-induced endothelial cell adhesion regardless of their different antioxidant activity. J. Agric. Food Chem 2009, 57, 3478–3482. [Google Scholar]
- Evan, GI; Vousden, KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar]
- Fabiani, R; De Bartolomeo, A; Rosignoli, P; Servili, M; Selvaggini, R; Montedoro, GF; Di Saverio, C; Morozzi, G. Virgin olive oil phenols inhibit proliferation of human promyelocytic leukemia cells (HL60) by inducing apoptosis and differentiation. J. Nutr 2006, 136, 614–619. [Google Scholar]
- Gill, CI; Boyd, A; McDermott, E; McCann, M; Servili, M; Selvaggini, R; Taticchi, A; Esposto, S; Montedoro, G; McGlynn, H; Rowland, I. Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int. J. Cancer 2005, 117, 1–7. [Google Scholar]
- Fini, L; Hotchkiss, E; Fogliano, V; Graziani, G; Romano, M; De Vol, EB; Qin, H; Selgrad, M; Boland, CR; Ricciardiello, L. Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines. Carcinogenesis 2008, 29, 139–146. [Google Scholar]
- Hashim, YZ; Rowland, IR; McGlynn, H; Servili, M; Selvaggini, R; Taticchi, A; Esposto, S; Montedoro, G; Kaisalo, L; Wahala, K; Gill, CI. Inhibitory effects of olive oil phenolics on invasion in human colon adenocarcinoma cells in vitro. Int. J. Cancer 2008, 122, 495–500. [Google Scholar]
- Corona, G; Deiana, M; Incani, A; Vauzour, D; Dessi, MA; Spencer, JPE. Hydroxytyrosol inhibits the proliferation of human colon adeocarcinoma cells through ihibition of ERK1/2 ad cyclin D1. Mol. Nutr. Food Res 2009, 53, 897–903. [Google Scholar]
- Menendez, JA; Vazquez-Martin, A; Colomer, R; Brunet, J; Carrasco-Pancorbo, A; Garcia-Villalba, R; Fernandez-Gutierrez, A; Segura-Carretero, A. Olive oil’s bitter principle reverses acquired autoresistance to trastuzumab (Herceptin) in HER2-overexpressing breast cancer cells. BMC Cancer 2007, 7, 80–99. [Google Scholar]
- Menendez, JA; Vazquez-Martin, A; Oliveras-Ferraros, C; Garcia-Villalba, R; Carrasco Pancorbo, A; Fernandez-Gutierez, A; Segura-Carretero, A. Analysing effects of extra-virgin olive oil polyphenols on breast cancer-associated fatty acid synthase protein expression using reverse-phase protein microarrays. Int. J. Mol. Med 2008, 22, 433–439. [Google Scholar]
- Menendez, JA; Vazquez-Martin, A; Oliveras-Ferraros, C; Garcia-Villalba, R; Carrasco Pancorbo, A; Fernandez-Gutierrez, A; Segura-Carretero, A. Extra-virgin olive oil polyphenolics inhibit HER2 (erbB-2)-induced malignant transformation in human breast epithelial cells: Relationship between the chemical structures of extra-virgin olive oil secoiridoids and lignans and their inhibitory activities on the tyrosine kinase activity of HER2. Int. J. Oncol 2009, 34, 43–51. [Google Scholar]
- Han, J; Talorete, TPN; Yamada, P; Isoda, H. Anti-proliferative and apototic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology 2009, 59, 45–53. [Google Scholar]
- Manna, C; Galletti, P; Cucciolla, V; Moltedo, O; Leone, A; Zappia, V. The protective effect of the olive oil polyphenol (3,4-dihydroxyphenyl)-ethanol counteracts reactive oxygen metabolite-induced cytotoxicity in Caco-2 cells. J. Nutr 1997, 127, 286–292. [Google Scholar]
- Li, W; Sperry, JB; Crowe, A; Trojanowki, JQ; Smith, AB; Lee, VMY. Inhibition of tau fibrillization by oleocanthal via reaction with amino groups of tau. J. Neurochem 2009, 110, 1339–1351. [Google Scholar]
- Pitt, J; Roth, W; Lacor, P; Blankenship, M; Velasco, P; De Felice, F; Breslin, PA; Klein, WL. Alzheimer’s-associated A-beta oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol Appl Pharmacol 2009. [Google Scholar]
- Gonzalez-Correa, JA; Navas, MD; Lopez-Villodres, JA; Trujillo, M; Espartero, JL; De la Cruz, JP. Neuroprotective effect of hydroxytyrosol and hydroxytyrosol acetate in rat brain slices subjected to hypoxia-reoxygeation. Neurosci. Lett 2008, 446, 143–146. [Google Scholar]
- Medina, E; de Castro, A; Romero, C; Brenes, M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: correlation with antimicrobial activity. J. Agric. Food Chem 2006, 54, 4954–4961. [Google Scholar]
- Romero, C; Medina, E; Vargas, J; Brenes, M; De Castro, A. In vitro activity of olive oil polyphenols against Helicobacter pylori. J. Agric. Food Chem 2007, 55, 680–686. [Google Scholar]
- Bisignano, G; Tomaino, A; Lo Cascio, R; Crisafi, G; Uccella, N; Saija, A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharma. Pharmacol 1999, 51, 971–974. [Google Scholar]
- Puel, C; Mardon, J; Agalias, A; Davicco, MJ; Lebecque, P; Mazur, A; Horcajada, MN; Skaltsounis, AL; Coxam, V. Major phenolic compounds in olive oil modulate bone loss in an ovariectomy/inflammation experimental model. J. Agric. Food Chem 2008, 56, 9417–9422. [Google Scholar]
| Treatment | Subject number | Olive oil phenolic concentration | Study design | Investigated biomarker | Key findings | Ref. |
|---|---|---|---|---|---|---|
| High phenolic concentration vs. low phenolic concentration olive oil | 28 coronary heart disease subjects | 161 vs. 14.67 mg/kg | 3 week, crossover | IL-6, C-reactive protein, sICAM-1, sVCAM-1 and plasma lipids | Interleukin-6 and C-reactive protein decreased after phenol-rich olive oil consumption. However, no changes in soluble intercellular (sICAM-1) and vascular adhesion (sVCAM-1) molecules and lipid profile were observed. | [70] |
| High phenolic concentration vs. low phenolic concentration-enriched breakfast | 21 hypercholesterolemic subjects | 400 vs. 80 mg/kg | Acute dose, crossover | FVIIa and PAI-1 | Concentrations of FVIIa increased less and PAI-1 activity decreased more after the high phenolic breakfast than after the low phenolic breakfast. | [71] |
| High phenolic concentration vs. moderate phenolic concentration vs. poor phenolic concentration olive oil | 30 healthy subjects | 825 vs. 370 vs. 0 μmol CAE/kg | 3 week, crossover | Plasma lipids and oxLDL | An increase in phenolic content of LDL-C and decrease in oxLDL was noted after consumption of oil rich in phenolic compounds. | [72] |
| High phenolic concentration vs. low phenolic concentration olive oil vs. corn oil | 12 healthy subjects | 607 vs. 16 vs. 0 mg/kg | Acute dose, crossover | Plasma TXB2, plasma LTB4 and plasma antioxidant capacity | Decrease in TXB2 and LTB4 with increasing phenolic content of olive oil and concomitant increase in plasma antioxidant capacity with increased phenolic content of olive oil. | [73] |
| High phenolic concentration vs. low phenolic concentration olive oil | 10 healthy subjects | 592 vs. 147 mg/kg | 8 week, crossover | Oxidative DNA damage and plasma antioxidant capacity | A reduction in DNA damage with the consumption of a phenol-rich olive oil diet was demonstrated. No difference was seen in plasma antioxidant capacity. | [74] |
| High phenolic concentration vs. moderate phenolic concentration vs. low phenolic concentration olive oil | 200 healthy subjects | 366 vs. 164 vs. 2.7 mg/kg | 3 week, crossover | Plasma lipids, plasma oxLDL, plasma F2-isoprostanes, GSH and GSSG | A linear increase in HDL-C was observed for low-, medium-, and high phenolic olive oil. Furthermore, TC to HDL-C ratio decreased linearly with the increasing phenolic content of the olive oil. OxLDL decreased linearly with increasing phenolic content of the olive oil and TG levels decreased for all olive oils. Oxidative stress markers indicated by GSH and GSSG decreased linearly with increasing phenolic content. | [25] |
| High phenolic concentration vs. moderate phenolic concentration vs. low phenolic concentration olive oil | 12 healthy subjects | 366 vs. 164 vs. 2.7 mg/kg | Acute dose, crossover | Plasma F2-isoprostanes and plasma oxLDL | All olive oils promoted postprandial oxidative stress indicated by increased F2-isoprostanes, however, the degree of LDL oxidation decreased as the phenolic content in administered oil increased. | [75] |
| High phenolic concentration vs. low phenolic-enriched breakfast | 21 hypercholesterolemic subjects | 400 vs. 80 mg/kg | Acute dose, crossover | Plasma LPO and plasma F2-isoprostanes | Decrease in LPO and F2-isoprostanes with intake of the phenol-enriched breakfast compared to low phenol-enriched breakfast. | [76] |
| High phenolic concentration vs. low phenolic concentration olive oil | 22 mild dyslipidemic subjects | 166 vs. 2 mg/kg | 49 day, crossover | Plasma lipids, plasma TXB2, plasma antioxidant capacity and urinary F2-isoprostanes | Plasma TXB2 decreased with phenol-rich olive oil supplementation. Plasma antioxidant capacity increased after phenol-rich olive oil administration. No effect on urinary F2-isoprostanes and plasma lipids between phenol-rich and phenol-poor olive oil were observed. | [77] |
| High phenolic concentration vs. low phenolic concentration olive oil | 43 coronary heart disease subjects | 161 vs. 14.67 mg/kg | 3 week, crossover | Plasma oxLDL, plasma LPO and whole blood GSH-Px | Decrease in oxLDL and LPO and increase in GSH-Px upon phenol-rich olive oil consumption. | [78] |
| High phenolic concentration vs. moderate phenolic concentration vs. low phenolic concentration olive oil | 30 healthy subjects | 150 vs. 68 vs. 0 mg/kg | 3 week, crossover | Plasma lipids and oxLDL | Sustained consumption of phenol-rich olive oil was more effective in protecting LDL from oxidation and in raising HDL-C than olive oils with lesser quantities of phenolics. | [79] |
| High phenolic concentration vs. moderate phenolic concentration vs. low phenolic concentration olive oil | 12 healthy subjects | 486 vs. 133 vs. 10 mg/kg | 4 day, crossover | Plasma lipids, plasma oxLDL, plasma GSH-Px and urinary 8-oxo-dG | Short-term consumption of phenol-rich olive oil decreased plasma oxLDL, urinary 8-oxo-dG, and increased plasma HDL-C and GSH-Px, in a dose-dependent manner with the increasing phenolic content of the olive oil administered. | [80] |
| High phenolic concentration vs. low phenolic concentration olive oil | 25 healthy subjects | 21.6 vs. 3.0 mg/kg | 3 week, crossover | Plasma antioxidant capacity and oxLDL | Plasma antioxidant capacity and oxLDL did not differ significantly between the phenol-rich and phenol-poor olive oil. | [81] |
| High phenolic concentration vs. low phenolic concentration olive oil | 46 health subjects | 308 vs. 43 mg/kg | 3 week, crossover | Plasma lipids, plasma oxLDL and plasma LPO | No effect on plasma lipids, oxLDL, and LPO were noted between the phenol-rich and phenol-poor olive oils. | [82] |
| Olive oil with different phenolic concentrations | 6 healthy subjects | 1950 vs. 1462.5 vs. 975 vs. 487.5 mg/kg | Acute dose, cross over | Urinary F2-isoprostanes | A dose-dependent decrease in urinary excretion of F2-isoprostanes was noted upon administration of phenol-rich olive oil. | [29] |
| High phenolic concentration vs. low phenolic concentration olive oil | 14 healthy subjects | 303 vs. 0.3 mg/kg | 4 week, crossover | Plasma oxLDL and serum antioxidant capacity | Increase in plasma antioxidant capacity but no change in oxLDL. | [83] |
| High phenolic concentration vs. low phenolic concentration olive oil | 24 peripheral vascular disease subjects | 800 vs. 60 mg/kg | 12 week, crossover | Plasma lipids and plasma oxLDL | A lower oxLDL was noted in subjects after administration of phenol-rich olive oil. No difference in plasma lipids was observed. | [84] |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cicerale, S.; Lucas, L.; Keast, R. Biological Activities of Phenolic Compounds Present in Virgin Olive Oil. Int. J. Mol. Sci. 2010, 11, 458-479. https://doi.org/10.3390/ijms11020458
Cicerale S, Lucas L, Keast R. Biological Activities of Phenolic Compounds Present in Virgin Olive Oil. International Journal of Molecular Sciences. 2010; 11(2):458-479. https://doi.org/10.3390/ijms11020458
Chicago/Turabian StyleCicerale, Sara, Lisa Lucas, and Russell Keast. 2010. "Biological Activities of Phenolic Compounds Present in Virgin Olive Oil" International Journal of Molecular Sciences 11, no. 2: 458-479. https://doi.org/10.3390/ijms11020458
APA StyleCicerale, S., Lucas, L., & Keast, R. (2010). Biological Activities of Phenolic Compounds Present in Virgin Olive Oil. International Journal of Molecular Sciences, 11(2), 458-479. https://doi.org/10.3390/ijms11020458