Fe-Chlorophyllin Promotes the Growth of Wheat Roots Associated with Nitric Oxide Generation
Abstract
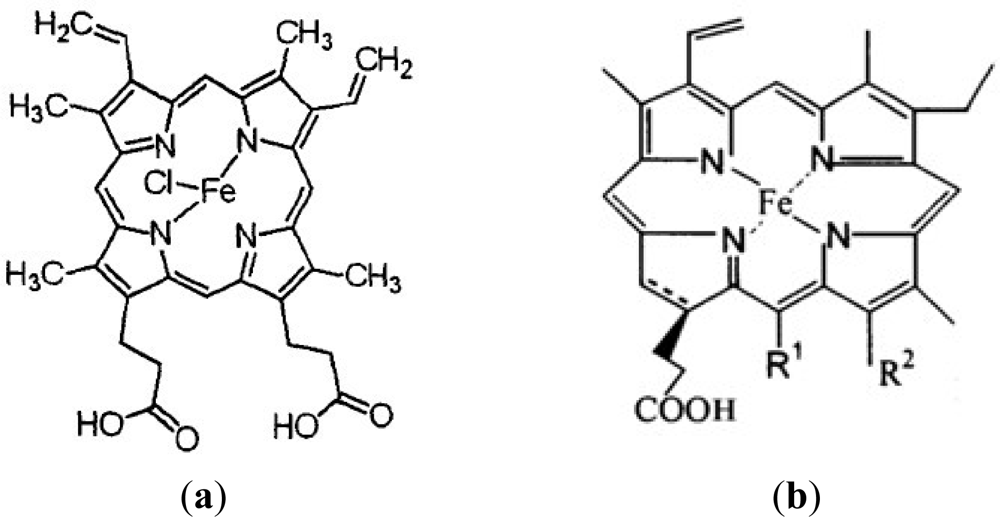
:1. Introduction
2. Results and Discussion
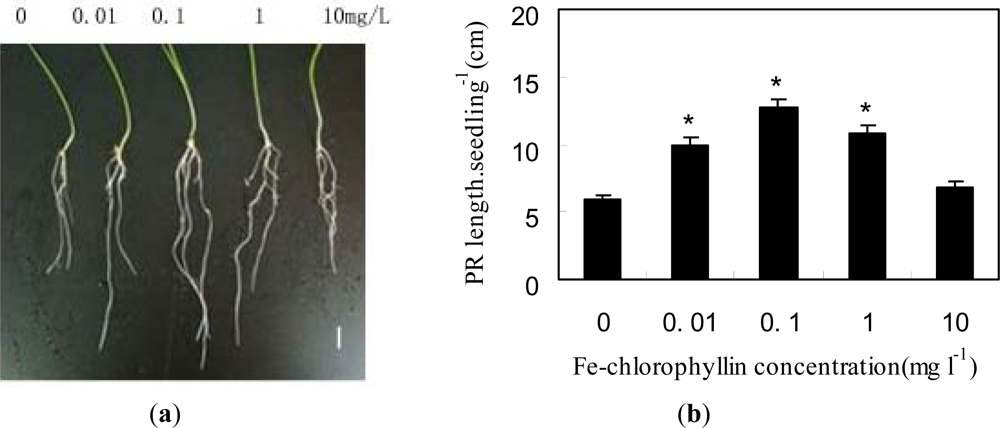
2.1. Fe-Chlorophyllin Promotes the Growth of Wheat Roots
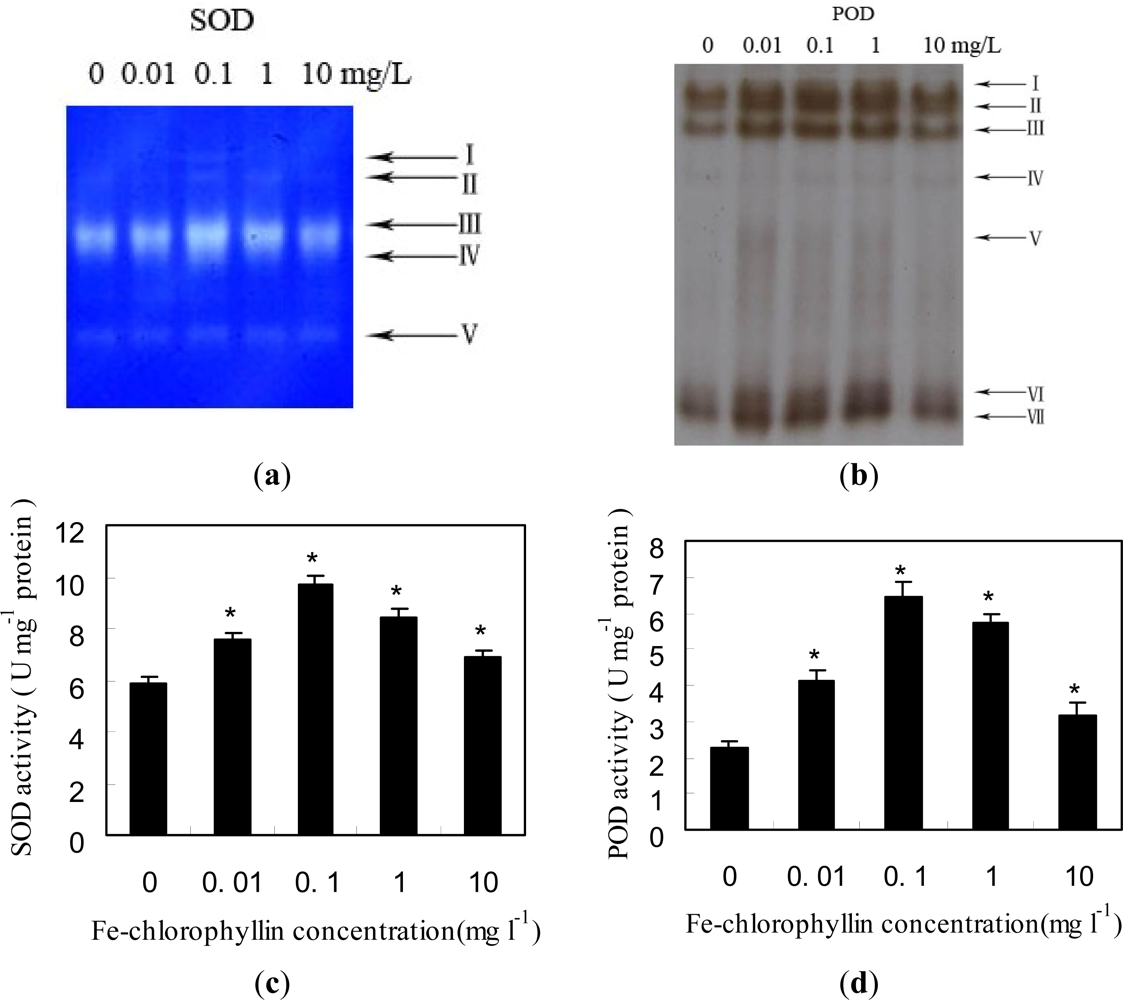
2.2. Changes of Anti-Oxidant Enzymes in Response to Fe-Chlorophyllin
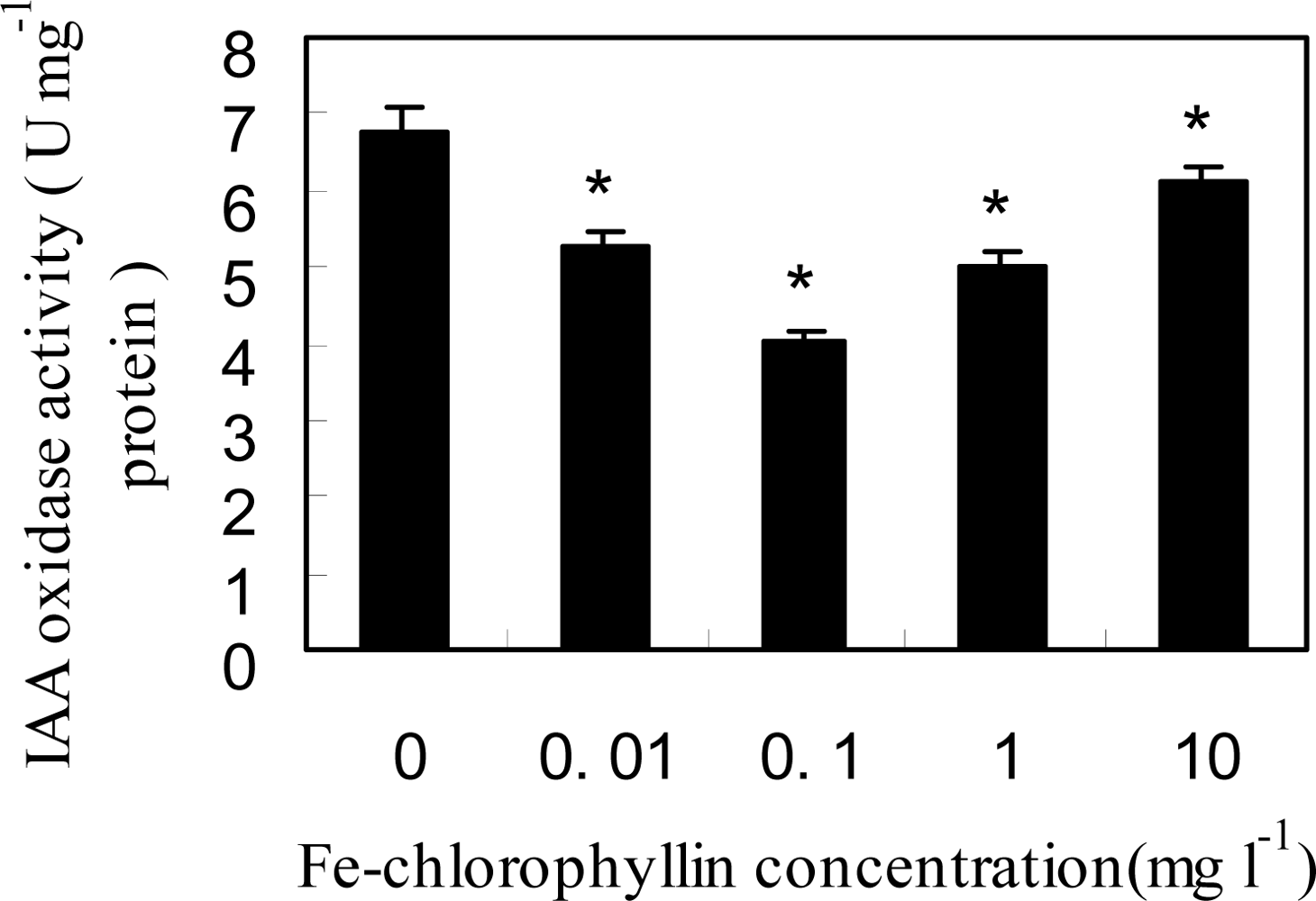
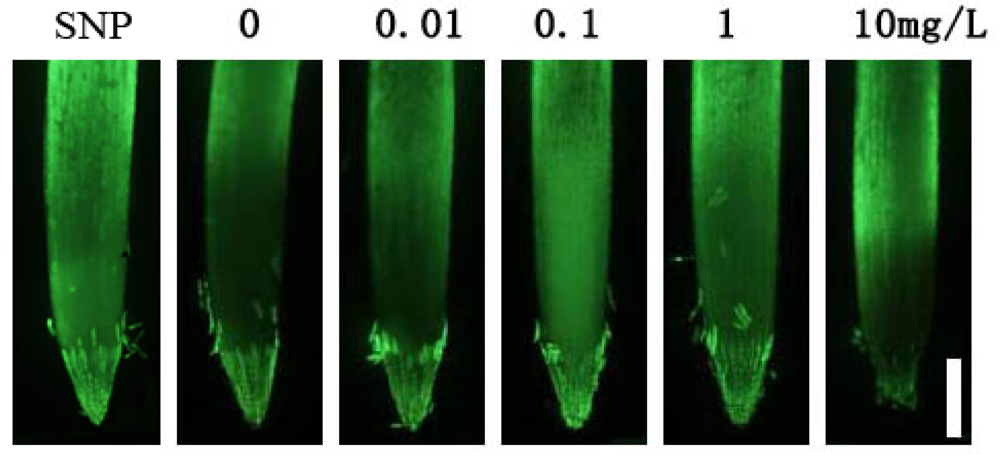
2.3. Fe-Chlorophyllin Promotes the Growth of Wheat Roots Involved in Auxin and NO Action
3. Experimental Section
3.1. Materials and Growth Conditions
3.2. Enzyme Activity Assays
3.3. Gel Electrophoresis
3.4. IAA Oxidase Assay
3.5. Detection of Intracellular Nitric Oxide
3.6. Statistical Analysis
4. Conclusion
Acknowledgments
References
- Gill, SS; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem 2010, 48, 909–930. [Google Scholar]
- Schoneich, C. Reactive oxygen species and biological aging: a mechanistic approach. Exp. Gerontol 1999, 34, 19–34. [Google Scholar]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 2006, 141, 312–322. [Google Scholar]
- Chernomorsky, S; Rancourt, R; Virdi, K; Segelman, A; Poretz, RD. Antimutagenicity, cytotoxicity and composition of chlorophyllin copper complex. Cancer Lett 1997, 120, 141–147. [Google Scholar]
- Chung, WY; Lee, JM; Park, MY; Yook, JI; Kim, J; Chung, AS; Surh, YJ; Park, KK. Inhibitory effects of chlorophyllin on 7,12-dimethylbenz anthracene-induced bacterial mutagenesis and mouse skin carcinogenesis. Cancer Lett 1999, 145, 57–64. [Google Scholar]
- Kamat, JP; Boloor, KK; Devasagayam, TPA. Chlorophyllin as an effective antioxidant against membrane damage in vitro and ex vivo. Biochim. Biophys. Acta 2000, 1487, 113–127. [Google Scholar]
- Sato, M; Fujimoto, I; Sakai, T; Aimoto, T; Kimura, R; Murata, T. Effect of Sodium Copper Chlorophyllin on Lipid Peroxidation. IV. The Antioxidative Action of Copper Chlorins. Chem. Pharm. Bull 1986, 34, 2428–2434. [Google Scholar]
- Inoue, H; Yamashita, H; Furuya, K; Nonomura, Y; Yoshioka, N; Li, S. Determination of copper(II) chlorophyllin by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1994, 679, 99–104. [Google Scholar]
- Arimoto, S; Kan-yama, K; Rai, H; Hayatsu, H. Inhibitory effect of hemin, chlorophyllin and related pyrrole pigments on the mutagenicity of benzo pyrene and its metabolites. Mutat. Res 1995, 345, 127–135. [Google Scholar]
- Liu, K; Xu, S; Xuan, W; Ling, T; Cao, Z; Huang, B; Sun, Y; Fang, L; Liu, Z; Zhao, N; et al. Carbon monoxide counteracts the inhibition of seed germination and alleviates oxidative damage caused by salt stress in Oryza sativa. Plant Sci 2007, 172, 544–555. [Google Scholar]
- Chen, M; Shen, WB; Ruan, HH; Xu, LL. Effects of Nitric Oxide on Root Growth and Its Oxidative Damage in Wheat Seedling Under Salt Stress. J. Plant Physiol. Mol. Biol 2004, 30, 569–576. [Google Scholar]
- Correa-Aragunde, N; Graziano, M; Lamattina, L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 2004, 218, 900–905. [Google Scholar]
- Correa-Aragunde, N; Graziano, M; Chevaller, C; Lamattina, L. Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J. Exp. Bot 2006, 57, 581–588. [Google Scholar]
- Durner, J; Klessig, DF. Nitric oxide as a signal in plants. Curr. Opin. Plant Biol 1999, 2, 369–374. [Google Scholar]
- Xie, YJ; Ling, TF; Han, Y; Liu, KL; Zheng, QS; Huang, LQ; Yuan, XX; He, Z; Hu, B; Fang, L; et al. Carbonmonoxide enhances salt tolerance by nitric oxide-mediated maintenance of ion homeostasis and up-regulation of antioxidant defense in wheat seedling roots. Plant Cell Environ 2008, 66, 2494–2498. [Google Scholar]
- Longo, M; Jain, V; Vedernikov, YP; Saade, GR; Goodrum, L; Facchinetti, F; Garfield, RE. Effect of nitric oxide and carbon monoxide on uterine contractility during human and rat pregnancy. Am. J. Obstet. Gynecol 1999, 181, 981–988. [Google Scholar]
- Xu, J; Xuan, W; Huang, BK; Zhou, YH; Ling, TF; Xu, S; Shen, WB. Carbon monoxide-induced adventitious rooting of hypocotyl cutting from mung bean seedling. Chin. Sci. Bull 2006, 51, 668–674. [Google Scholar]
- Shen, W; Huang, L; Shen, J; Ling, T; Xuan, W; Xu, S; Ye, M; Shao, S; Huang, B; Gu, K; et al. A Plant Growth Regulator Containing Hemin. Chin Patent 200610097240.3, 28 March 2007. [Google Scholar]
- Upadhyaya, AD; Sankhla, TD; Davis, N; Sankhla, BN; Smith, J. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J. Plant Physiol 1985, 121, 453–461. [Google Scholar]
- Beauchamp, C; Fridovich, I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem 1971, 44, 276–287. [Google Scholar]
- Pereira, GJG; Molina, SMG; Lea, PJ; Azevdo, RA. Activity of antioxidant enzymes in response to cadmium in Crotalaria juncea. Plant Soil 2002, 239, 123–132. [Google Scholar]
- Janda, T; Szalai, G; Tari, I; Paldi, E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 1999, 208, 175–180. [Google Scholar]
- Milrad de Forchetti, S; Tigier, HA. Effect of dicarboxylic acids on the peroxidase–IAA oxidase isozymes of soybean callus. Physiol. Plant 1983, 59, 355–358. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tong, M.; Zhang, L.; Wang, Y.; Jiang, H.; Ren, Y. Fe-Chlorophyllin Promotes the Growth of Wheat Roots Associated with Nitric Oxide Generation. Int. J. Mol. Sci. 2010, 11, 5246-5255. https://doi.org/10.3390/ijms11125246
Tong M, Zhang L, Wang Y, Jiang H, Ren Y. Fe-Chlorophyllin Promotes the Growth of Wheat Roots Associated with Nitric Oxide Generation. International Journal of Molecular Sciences. 2010; 11(12):5246-5255. https://doi.org/10.3390/ijms11125246
Chicago/Turabian StyleTong, Min, Liefeng Zhang, Yifan Wang, Hui Jiang, and Yong Ren. 2010. "Fe-Chlorophyllin Promotes the Growth of Wheat Roots Associated with Nitric Oxide Generation" International Journal of Molecular Sciences 11, no. 12: 5246-5255. https://doi.org/10.3390/ijms11125246



