Stability and Folding Behavior Analysis of Zinc-Finger Using Simple Models
Abstract
:1. Introduction
2. Systems and Methods
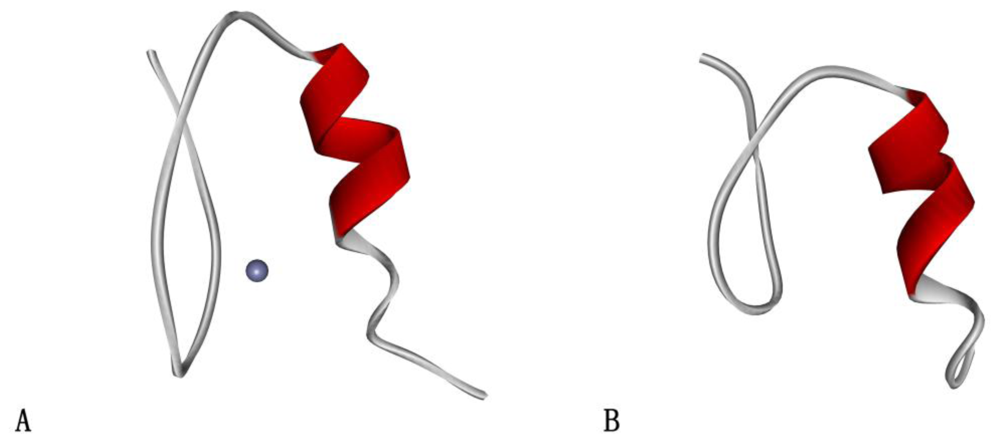
2.1. Protein Systems
2.2. The Gaussian Network Model
2.3. The Iterative Unfolding Method
- The mean-square fluctuations of the distance in all noncovalent residue pairs are calculated based on the native structure topology with Equation 7.
- The noncovalent contact in the residue pair with the largest distance fluctuation is broken. Then, a new matrix Γ is obtained, which represents a new topology during protein unfolding.
- The mean-square fluctuations of the distance in all noncovalent residue pairs are recalculated based on the new matrix Γ using Equation 7.
- The above two steps are repeated until all the noncovalent contacts are broken.
2.4. The Anisotropy Elastic Network Model
3. Results and Discussion
3.1. Force Constants for Inter-Residue Interactions
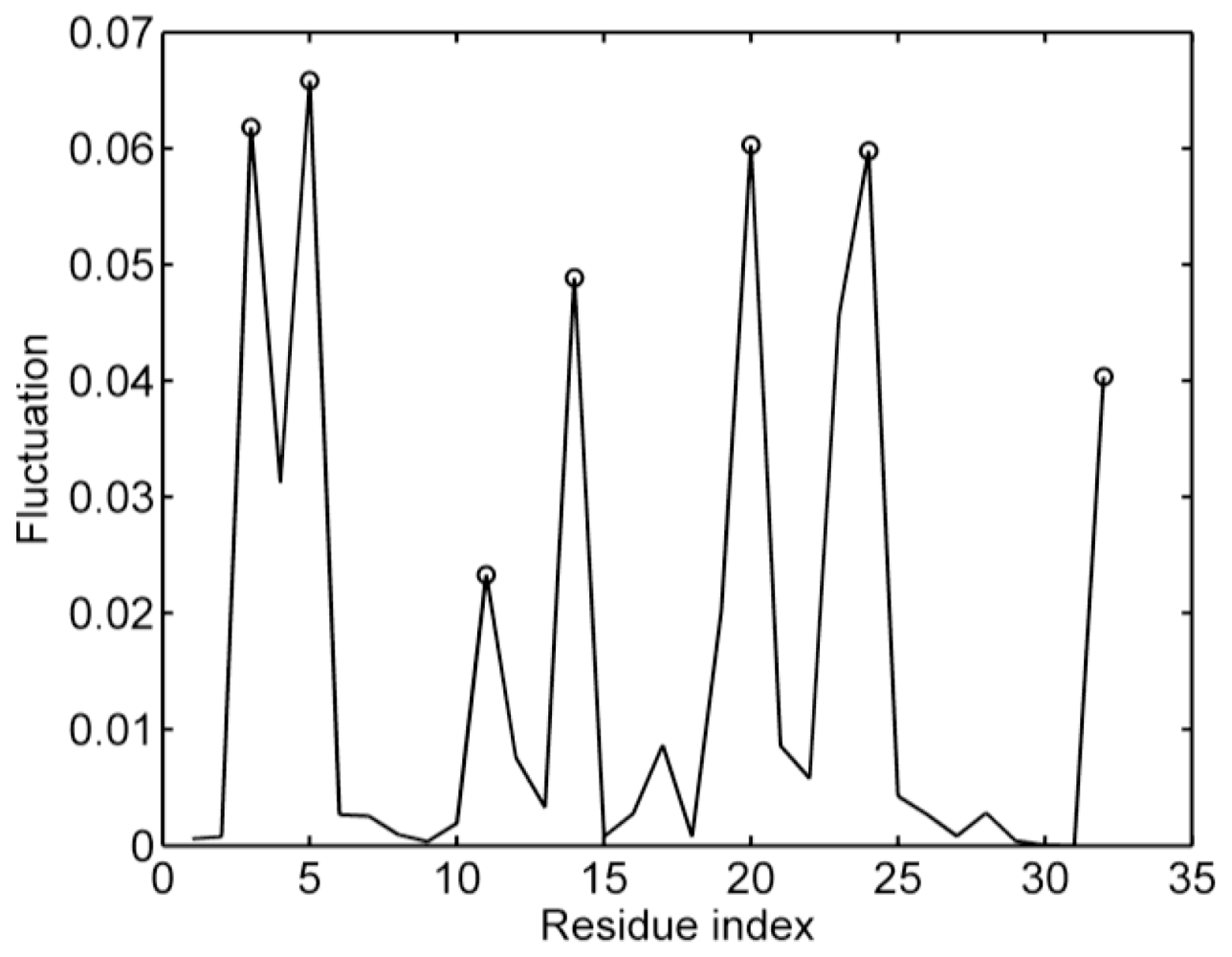
3.2. The Fast Modes of the Motions
3.3. The Slow Modes of the Motions
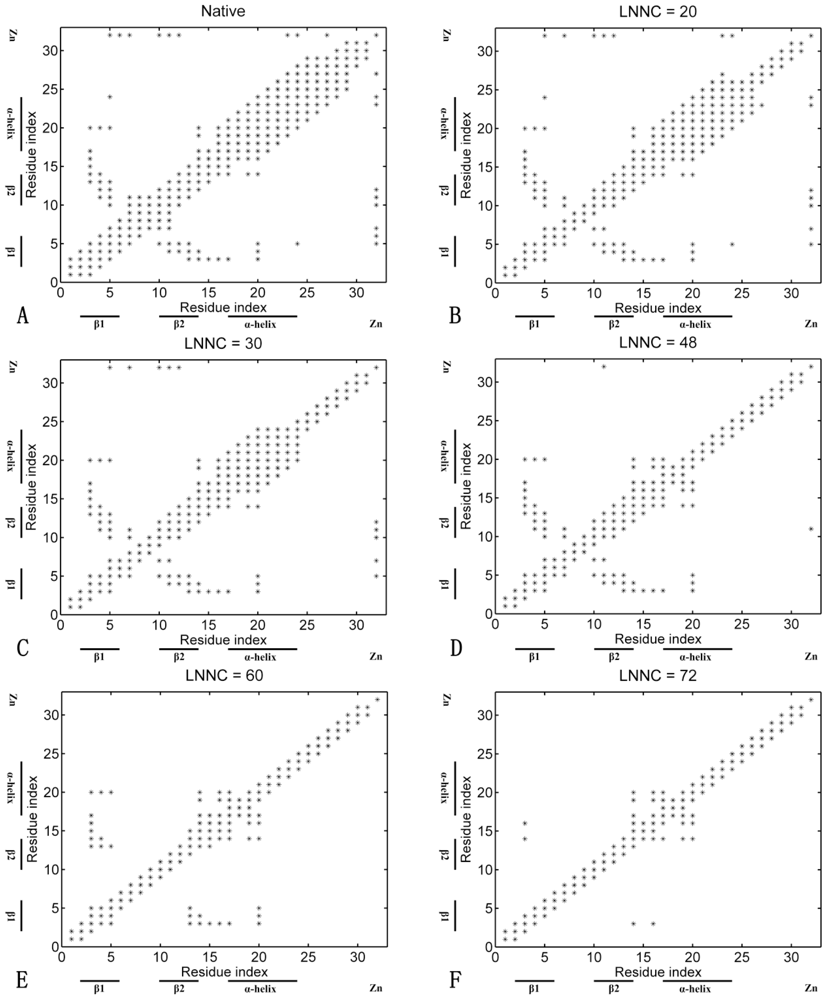
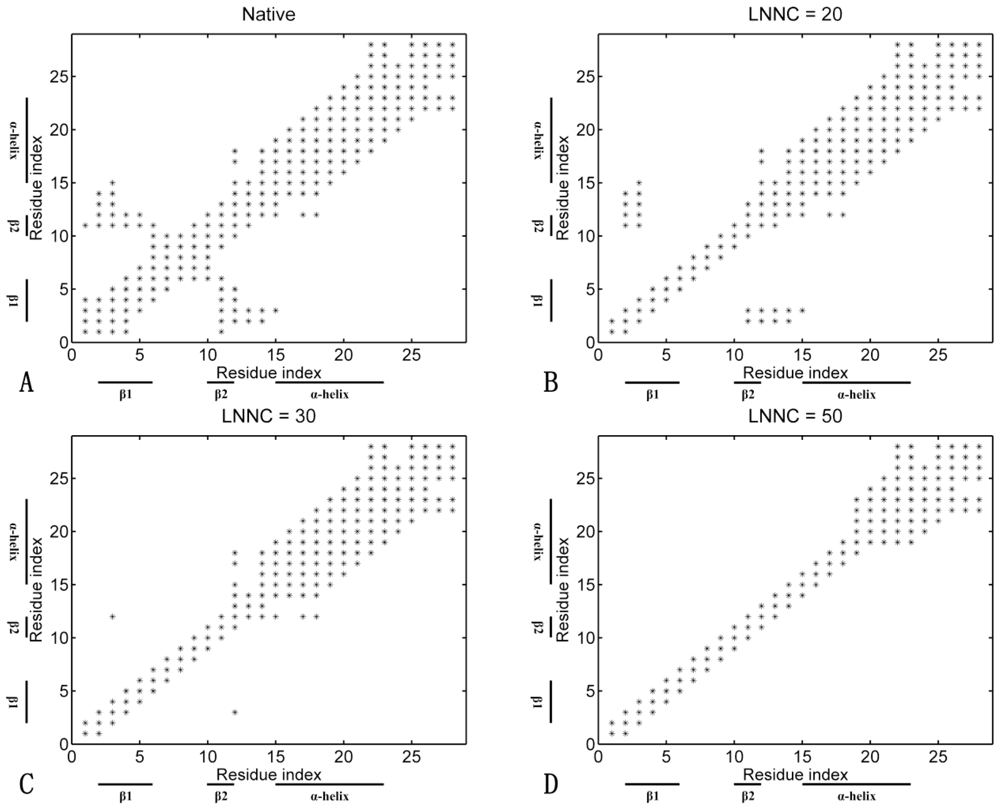
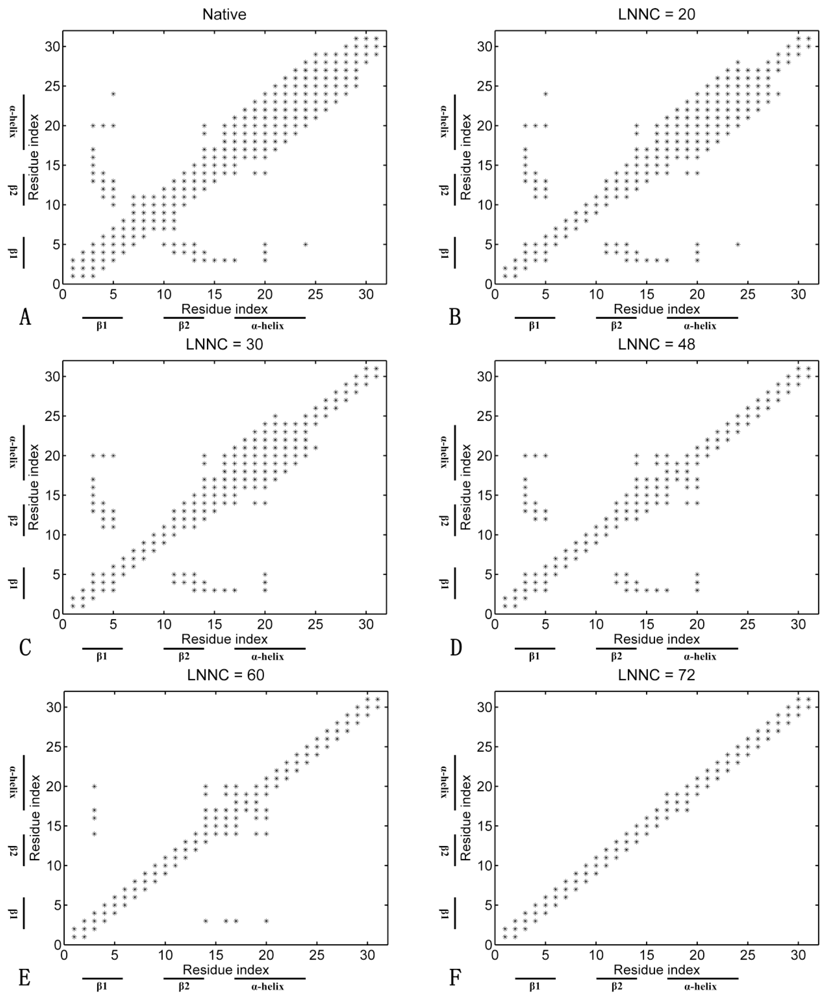
3.4. Sequences of Unfolding Events of Sp1f2 and FSD-1 Revealed by Iterative Unfolding Method
3.5. Cross-Correlation Analysis during Protein Unfolding
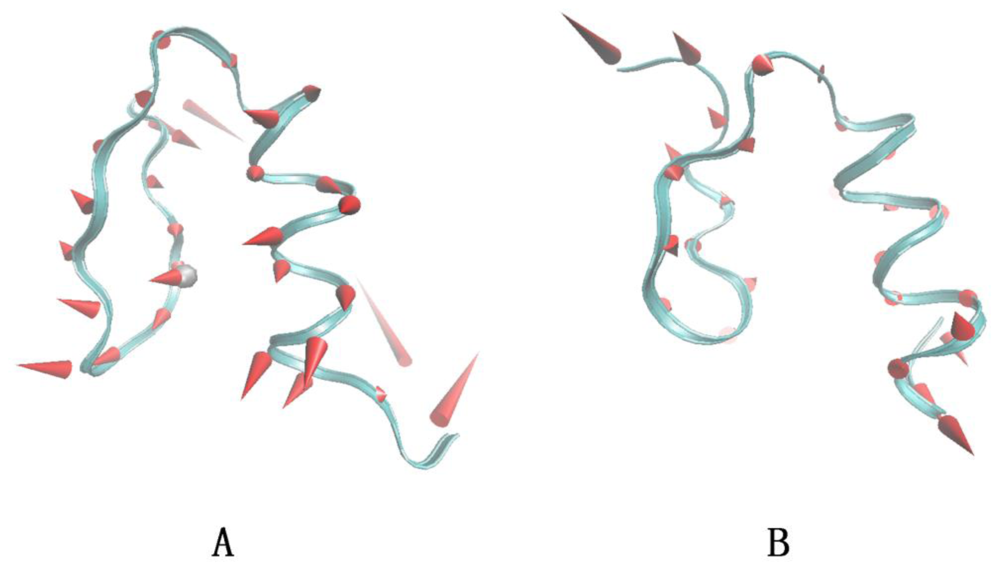
3.6. The ANM Analysis of Sp1f2 and FSD-1
3.7. The Analysis of Folding Pathways
4. Conclusions
Acknowledgements
References
- Zhang, Y. Protein structure prediction: When is it useful? Curr. Opin. Struct. Biol 2009, 19, 145–155. [Google Scholar]
- Zhang, J; Li, WF; Wang, J; Qin, M; Wu, L; Yan, ZQ; Xu, WX; Zuo, GH; Wang, W. Protein folding simulations: from coarse-grained model to all-atom model. IUBMB Life 2009, 61, 627–643. [Google Scholar]
- Chen, YW; Ding, F; Nie, HF; Serohijos, AW; Sharma, S; Wilcox, KC; Yin, SY; Dokholyan, NV. Protein folding: then and now. Arch. Biochem. Biophys 2008, 469, 4–19. [Google Scholar]
- Gruebele, M. Protein folding: the free energy surface. Curr. Opin. Struct. Biol 2002, 12, 161–168. [Google Scholar]
- Alexandrescu, AT. Amyloid accomplices and enforcers. Protein Sci 2005, 14, 1–12. [Google Scholar]
- Xiao, Y; Chen, CJ; He, Y. Folding Mechanism of Beta-Hairpin Trpzip2: Heterogeneity, Transition State and Folding Pathways. Int. J. Mol. Sci 2009, 10, 2838–2848. [Google Scholar]
- Yang, WY; Gruebele, M. Folding at the speed limit. Nature 2003, 423, 193–197. [Google Scholar]
- Anzellotti, AI; Farrell, NP. Zinc metalloproteins as medicinal targets. Chem. Soc. Rev 2008, 37, 1629–1651. [Google Scholar]
- Frankel, AD; Berg, JM; Pabo, CO. Metal-dependent folding of a single zinc finger from transcription factor IIIA. Proc. Natl. Acad. Sci. USA 1987, 84, 4841–4845. [Google Scholar]
- Laity, JH; Lee, BM; Wright, PE. Zinc finger proteins: new insights into structural and functional diversity. Curr. Opin. Struct. Biol 2001, 11, 39–46. [Google Scholar]
- Leon, O; Roth, M. Zinc fingers: DNA binding and protein-protein interactions. Biol. Res 2000, 33, 21–30. [Google Scholar]
- Wittung-Stafshede, P. Role of cofactors in folding of the blue-copper protein azurin. Inorg. Chem 2004, 43, 7926–7933. [Google Scholar]
- Wilson, CJ; Apiyo, D; Wittung-Stafshede, P. Role of cofactors in metalloprotein folding. Q. Rev. Biophys 2004, 37, 285–314. [Google Scholar]
- Li, WF; Zhang, J; Su, Y; Wang, J; Qin, M; Wang, W. Effects of zinc binding on the conformational distribution of the amyloid-beta peptide based on molecular dynamics simulations. J. Phys. Chem. B 2007, 111, 13814–13821. [Google Scholar]
- Ghosh, D; Pecoraro, VL. Probing metal-protein interactions using a de novo design approach. Curr. Opin. Chem. Biol 2005, 9, 97–103. [Google Scholar]
- Papworth, M; Kolasinska, P; Minczuk, M. Designer zinc-finger proteins and their applications. Gene 2006, 366, 27–38. [Google Scholar]
- Ghosh, D; Pecoraro, VL. Understanding metalloprotein folding using a de novo design strategy. Inorg. Chem 2004, 43, 7902–7915. [Google Scholar]
- Bombarda, E; Roques, BP; Mely, Y; Grell, E. Mechanism of zinc coordination by point-mutated structures of the distal CCHC binding motif of the HIV-1NCp7 proteins. Biochemistry 2005, 44, 7315–7325. [Google Scholar]
- Nomura, A; Sugiura, Y. Contribution of individual zinc ligands to metal binding and peptide folding of zinc finger peptides. Inorg. Chem 2002, 41, 3693–3698. [Google Scholar]
- Blasie, CA; Berg, JM. Structure-based thermodynamic analysis of a coupled metal binding-protein folding reaction involving a zinc finger peptide. Biochemistry 2002, 41, 15068–15073. [Google Scholar]
- Dudev, T; Lim, C. All-electron calculations of the nucleation structures in metal-induced zinc-finger folding: Role of the peptide backbone. J. Am. Chem. Soc 2007, 129, 12497–12504. [Google Scholar]
- Li, WF; Zhang, J; Wang, J; Wang, W. Metal-coupled folding of Cys(2)His(2) zinc-finger. J. Am. Chem. Soc 2008, 130, 892–900. [Google Scholar]
- Li, WF; Zhang, J; Wang, W. Understanding the folding and stability of a zinc finger-based full sequence design protein with replica exchange molecular dynamics simulations. Proteins 2007, 67, 338–349. [Google Scholar]
- Kim, SY; Lee, J; Lee, J. Folding simulations of small proteins. Biophys. Chem 2005, 115, 195–200. [Google Scholar]
- Lei, HX; Duan, Y. The role of plastic beta-hairpin and weak hydrophobic core in the stability and unfolding of a full sequence design protein. J. Chem. Phys 2004, 121, 12104–12111. [Google Scholar]
- Jang, S; Shin, S; Pak, Y. Molecular dynamics study of peptides in implicit water: ab initio folding of beta-hairpin, beta-sheet, and beta beta alpha-motif. J. Am. Chem. Soc 2002, 124, 4976–4977. [Google Scholar]
- Feng, JWA; Kao, J; Marshall, GR. A Second Look at Mini-Protein Stability: Analysis of FSD-1 Using Circular Dichroism, Differential Scanning Calorimetry, and Simulations. Biophys. J 2009, 97, 2803–2810. [Google Scholar]
- Hills, RD; Brooks, CL. Insights from Coarse-Grained Go Models for Protein Folding and Dynamics. Int. J. Mol. Sci 2009, 10, 889–905. [Google Scholar]
- Clementi, C. Coarse-grained models of protein folding: toy models or predictive tools? Curr. Opin. Struct. Biol 2008, 18, 10–15. [Google Scholar]
- Chang, S; Jiao, X; Gong, XQ; Li, CH; Chen, WZ; Wang, CX. Evolving model of amino acid networks. Phys. Rev. E 2008, 77, 061920. [Google Scholar]
- Jiao, X; Chang, S; Li, CH; Chen, WZ; Wang, CX. Construction and application of the weighted amino acid network based on energy. Phys. Rev. E 2007, 75, 051903. [Google Scholar]
- Zhang, M; Chen, CJ; He, Y; Xiao, Y. Improvement on a simplified model for protein folding simulation. Phys. Rev. E 2005, 72, 051919. [Google Scholar]
- Bahar, I; Rader, AJ. Coarse-grained normal mode analysis in structural biology. Curr. Opin. Struct. Biol 2005, 15, 586–592. [Google Scholar]
- Yang, LW; Liu, X; Jursa, CJ; Holliman, M; Rader, A; Karimi, HA; Bahar, I. iGNM: a database of protein functional motions based on Gaussian Network Model. Bioinformatics 2005, 21, 2978–2987. [Google Scholar]
- Haliloglu, T; Bahar, I; Erman, B. Gaussian dynamics of folded proteins. Phys. Rev. Lett 1997, 79, 3090–3093. [Google Scholar]
- Bahar, I; Lezon, TR; Bakan, A; Shrivastava, IH. Normal Mode Analysis of Biomolecular Structures: Functional Mechanisms of Membrane Proteins. Chem. Rev 2010, 110, 1463–1497. [Google Scholar]
- Chennubhotla, C; Yang, Z; Bahar, I. Coupling between global dynamics and signal transduction pathways: a mechanism of allostery for chaperonin GroEL. Mol. Biosyst 2008, 4, 287–292. [Google Scholar]
- Atilgan, AR; Durell, SR; Jernigan, RL; Demirel, MC; Keskin, O; Bahar, I. Anisotropy of fluctuation dynamics of proteins with an elastic network model. Biophys. J 2001, 80, 505–515. [Google Scholar]
- Micheletti, C; Lattanzi, G; Maritan, A. Elastic properties of proteins: insight on the folding process and evolutionary selection of native structures. J. Mol. Biol 2002, 321, 909–921. [Google Scholar]
- Su, JG; Li, CH; Hao, R; Chen, WZ; Wang, CX. Protein unfolding behavior studied by elastic network model. Biophys. J 2008, 94, 4586–4596. [Google Scholar]
- Sulkowska, JI; Kloczkowski, A; Sen, TZ; Cieplak, M; Jernigan, RL. Predicting the order in which contacts are broken during single molecule protein stretching experiments. Proteins 2008, 71, 45–60. [Google Scholar]
- Narayan, VA; Kriwacki, RW; Caradonna, JP. Structures of zinc finger domains from transcription factor Sp1. Insights into sequence-specific protein-DNA recognition. J. Biol. Chem 1997, 272, 7801–7809. [Google Scholar]
- Dahiyat, BI; Mayo, SL. De novo protein design: fully automated sequence selection. Science 1997, 278, 82–87. [Google Scholar]
- Erman, B. The Gaussian network model: precise predictions of residue fluctuations and application to binding problems. Biophys. J 2006, 91, 3589–3599. [Google Scholar]
- Kloczkowski, A; Mark, JE; Erman, B. Chain dimensions and fluctuations in random elastomeric networks. Phantom Gaussian networks in the undeformed state. Macromolecules 1989, 22, 1423–1432. [Google Scholar]
- Bahar, I; Atilgan, AR; Erman, B. Direct evaluation of thermal fluctuations in proteins using a single-parameter harmonic potential. Fold. Des 1997, 2, 173–181. [Google Scholar]
- Haliloglu, T; Keskin, O; Ma, BY; Nussinov, R. How similar are protein folding and protein binding nuclei? Examination of vibrational motions of energy hot spots and conserved residues. Biophys. J 2005, 88, 1552–1559. [Google Scholar]
- Bahar, I; Atilgan, AR; Demirel, MC; Erman, B. Vibrational dynamics of folded proteins: significance of slow and fast motions in relation to function and stability. Phys. Rev. Lett 1998, 80, 2733. [Google Scholar]
- Jasanoff, A; Weiss, MA. Aromatic histidine interactions in the zinc finger motif: structural inequivalence of phenylalanine and tyrosine in the hydrophobic core. Biochemistry 1993, 32, 1423–1432. [Google Scholar]
- Jasanoff, A; Kochoyan, M; Fraenkel, E; Lee, JP; Weiss, MA. Aromatic aromatic interactions in the zinc finger motif: Analysis of the 2-dimensional nuclear magnetic resonance structure of a mutant domain. J. Mol. Biol 1992, 225, 1035–1047. [Google Scholar]
- Miura, T; Satoh, T; Takeuchi, H. Role of metal-ligand coordination in the folding pathway of zinc finger peptides. Biochim. Biophys. Acta. Protein. Struct. Mol. Enzymol 1998, 1384, 171–179. [Google Scholar]
- Shi, YG; Beger, RD; Berg, JM. Metal-binding properties of single amino-acid deletion mutants of zinc finger peptides: studies using cobalt(II) as a spectroscopic probe. Biophys. J 1993, 64, 749–753. [Google Scholar]
- Wu, L; Li, WF; Liu, F; Zhang, J; Wang, J; Wang, W. Understanding protein folding cooperativity based on topological consideration. J. Chem. Phys 2009, 131, 065105. [Google Scholar]
- Su, JG; Jiao, X; Sun, TG; Li, CH; Chen, WZ; Wang, CX. Analysis of domain movements in glutamine-binding protein with simple models. Biophys. J 2007, 92, 1326–1335. [Google Scholar]





© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chang, S.; Jiao, X.; Hu, J.-P.; Chen, Y.; Tian, X.-H. Stability and Folding Behavior Analysis of Zinc-Finger Using Simple Models. Int. J. Mol. Sci. 2010, 11, 4014-4034. https://doi.org/10.3390/ijms11104014
Chang S, Jiao X, Hu J-P, Chen Y, Tian X-H. Stability and Folding Behavior Analysis of Zinc-Finger Using Simple Models. International Journal of Molecular Sciences. 2010; 11(10):4014-4034. https://doi.org/10.3390/ijms11104014
Chicago/Turabian StyleChang, Shan, Xiong Jiao, Jian-Ping Hu, Yan Chen, and Xu-Hong Tian. 2010. "Stability and Folding Behavior Analysis of Zinc-Finger Using Simple Models" International Journal of Molecular Sciences 11, no. 10: 4014-4034. https://doi.org/10.3390/ijms11104014




