Inactivation and Unfolding of the Hyperthermophilic Inorganic Pyrophosphatase from Thermus thermophilus by Sodium Dodecyl Sulfate
Abstract
:1. Introduction
2. Results and Discussion
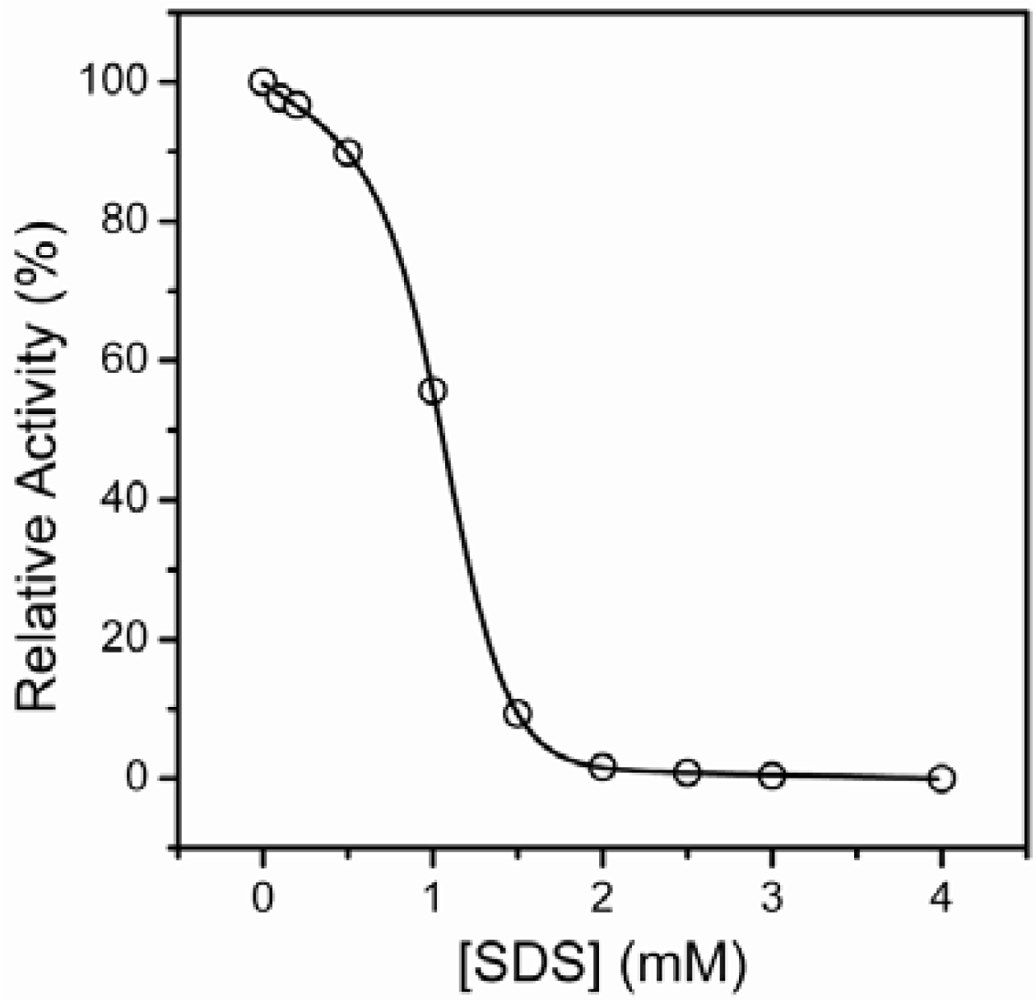
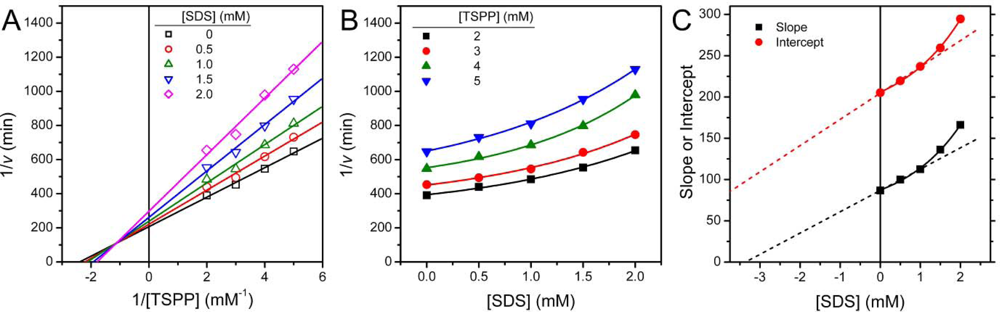
2.1. Inactivation of T-PPase by SDS
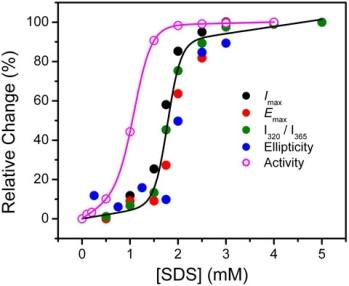
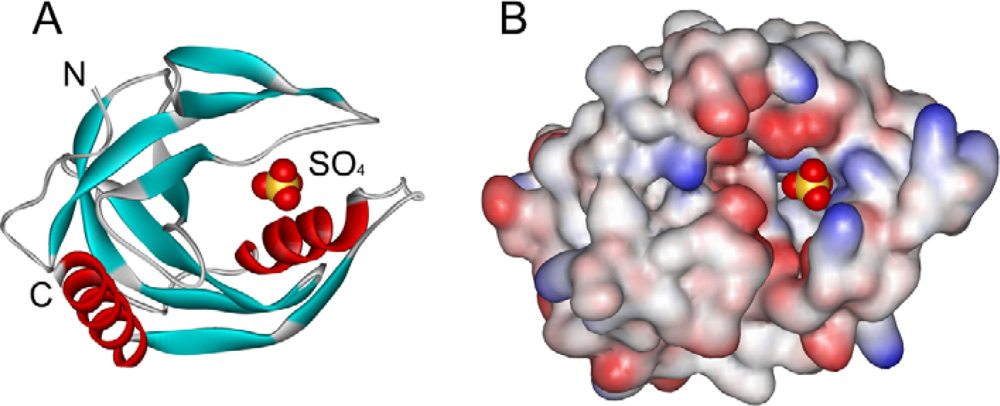
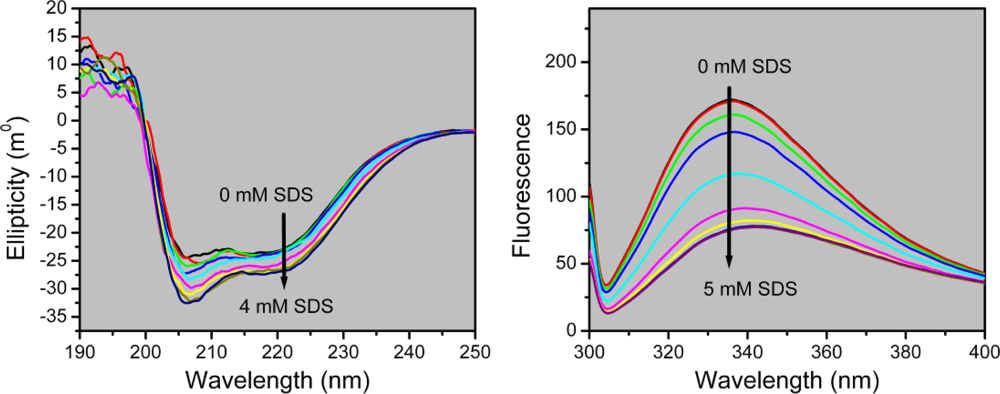
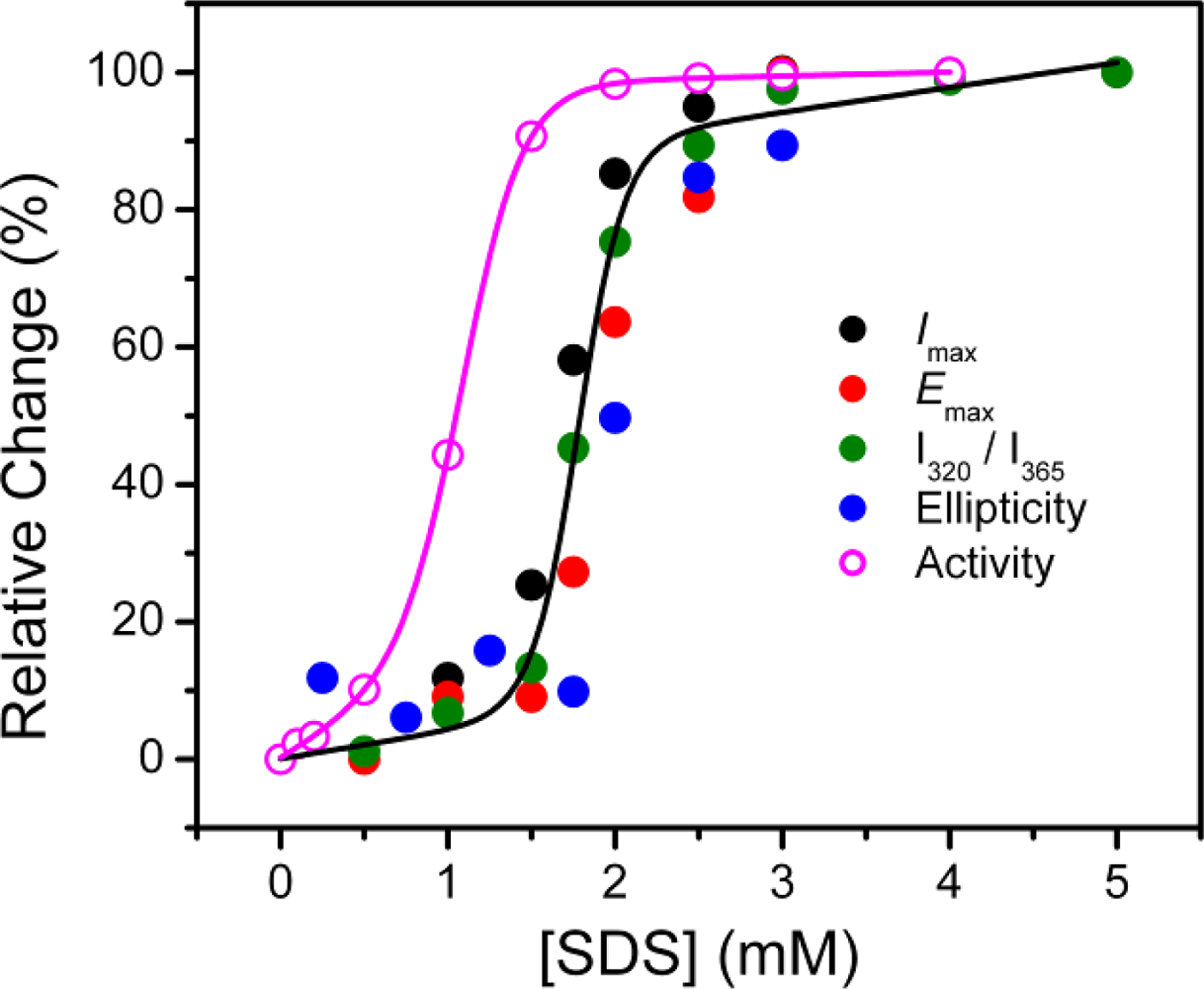
2.2. Structural Changes of PPase Induced by SDS
3. Experimental Section
3.1. Materials
3.2. Protein Expression and Purification
3.3. PPase Activity Assay
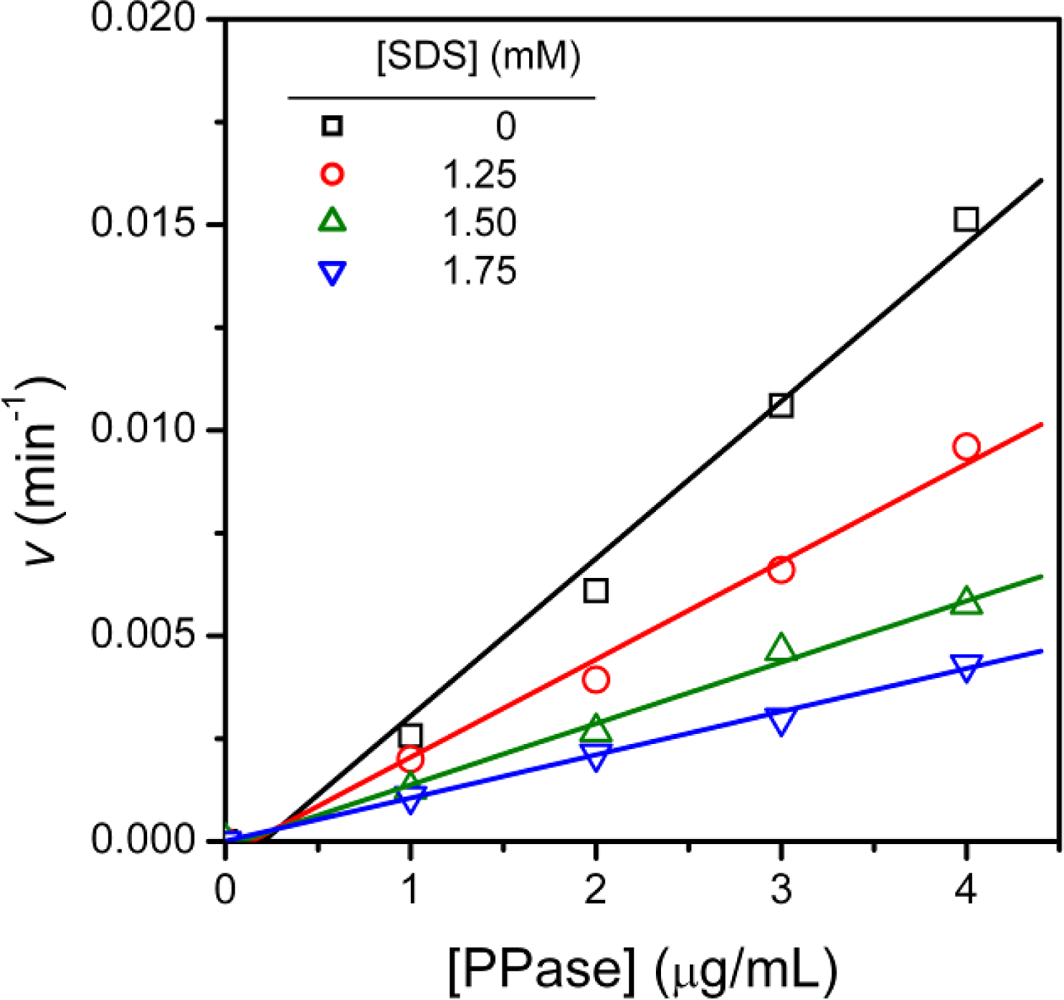
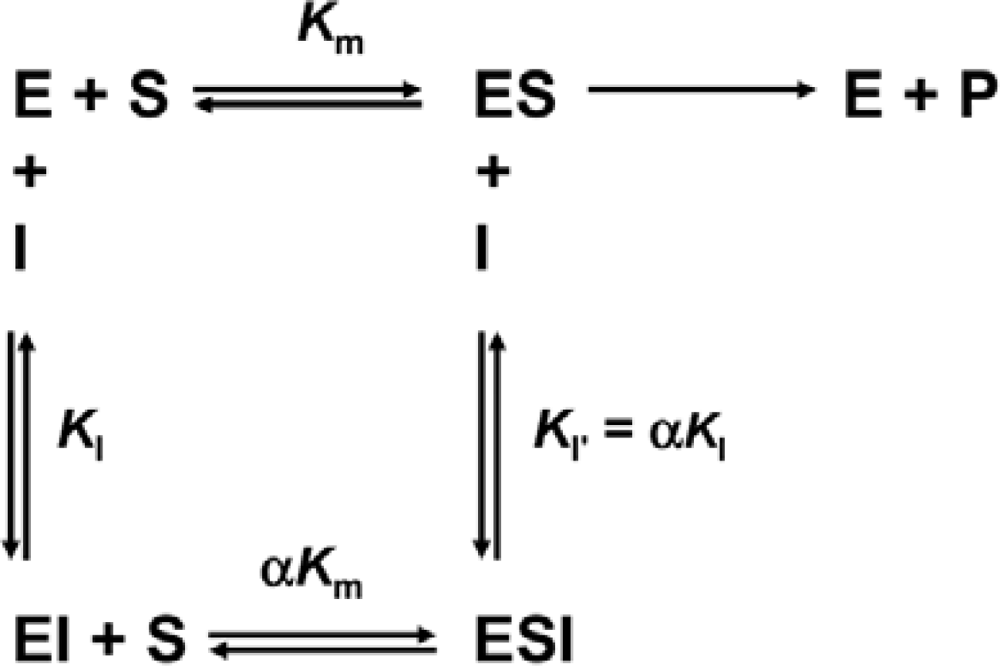
3.4. Determination of Inhibition Constants
3.5. Spectroscopy
4. Conclusions
Acknowledgments
References
- Lahti, R. Microbial inorganic pyrophosphatases. Microbiol. Rev 1983, 47, 169–178. [Google Scholar]
- Dignam, JD; Deutscher, MP. Aminoacyl-tRNA synthetase stimulatory factors and inorganic pyrophosphatase. Biochemistry 1979, 18, 3165–3170. [Google Scholar]
- Kent, RB; Guterman, SK. Pyrophosphate inhibition of rho ATPase: a mechanism of coupling to RNA polymerase activity. Proc. Natl. Acad. Sci. U.S.A 1982, 79, 3992–3996. [Google Scholar]
- Teplyakov, A; Obmolova, G; Wilson, KS; Ishii, K; Kaji, H; Samejima, T; Kuranova, I. Crystal structure of inorganic pyrophosphatase from Thermus thermophilus. Protein Sci 1994, 3, 1098–1107. [Google Scholar]
- Liu, B; Bartlam, M; Gao, R; Zhou, W; Pang, H; Liu, Y; Feng, Y; Rao, Z. Crystal structure of the hyperthermophilic inorganic pyrophosphatase from the archaeon Pyrococcus horikoshii. Biophys. J 2004, 86, 420–427. [Google Scholar]
- Salminen, T; Kaepylae, J; Heikinheimo, P; Kankare, J; Goldman, A; Heinonen, J; Baykov, AA; Cooperman, BS; Lahti, R. Structure and function analysis of Escherichia coli inorganic pyrophosphatase: Is a hydroxide ion the key to catalysis? Biochemistry 1995, 34, 782–791. [Google Scholar]
- Chrambach, A; Rodbard, D. Polyacrylamide gel electrophoresis. Science 1971, 172, 440–451. [Google Scholar]
- Reynolds, JA; Tanford, C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Nat Acad Sci USA 1970, 66, 1002–1007. [Google Scholar]
- Jones, MN; Wilkinson, A. The interaction between Beta-lactoglobulin and sodium N-dodecyl sulphate. Biochem. J 1976, 153, 713–718. [Google Scholar]
- Jones, MN. A microcalorimetric study of the interaction between trypsin and sodium n-dodecyl sulphate. Biochim. Biophys. Acta 1977, 491, 121–128. [Google Scholar]
- Shi, Y; Luo, W; Tian, WX; Zhang, T; Zhou, HM. Inactivation and conformational changes of fatty acid synthase from chicken liver during unfolding by sodium dodecyl sulfate. Int. J. Biochem. Cell Biol 1998, 30, 1319–1330. [Google Scholar]
- Lu, W; Li, S; Li, GF; Gong, YD; Zhao, NM; Zhang, RX; Zhou, HM. Inactivation and dissociation of rice ribulose-1,5-bisphosphate carboxylase/oxygenase during denaturation by sodium dodecyl sulfate. Biochemistry-Moscow 2002, 67, 940–944. [Google Scholar]
- Schneider, GF; Shaw, BF; Lee, A; Carillho, E; Whitesides, GM. Pathway for unfolding of ubiquitin in sodium dodecyl sulfate, studied by capillary electrophoresis. J. Am. Chem. Soc 2008, 130, 17384–17393. [Google Scholar]
- Hansen, JH; Petersen, SV; Andersen, KK; Enghild, JJ; Damhus, T; Otzen, D. Stable intermediates determine proteins’ primary unfolding sites in the presence of surfactants. Biopolymers 2009, 91, 221–231. [Google Scholar]
- Jones, MN; Finn, A; Mosavi-Movahedi, A; Waller, BJ. The activation of Aspergillus niger catalase by sodium n-dodecyl-sulphate. Biochim. Biophys. Acta 1987, 913, 395–398. [Google Scholar]
- Moore, BM; Flurkey, WH. Sodium dodecyl sulfate activation of a plant polyphenoloxidase. Effect of sodium dodecyl sulfate on enzymatic and physical characteristics of purified broad bean polyphenoloxidase. J. Biol. Chem 1990, 265, 4982–4988. [Google Scholar]
- Gudiksen, KL; Gitlin, I; Whitesides, GM. Differentiation of proteins based on characteristic patterns of association and denaturation in solutions of SDS. Proc. Nat. Acad. Sci. U.S.A 2006, 103, 7968–7972. [Google Scholar]
- Vieille, C; Zeikus, GJ. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev 2001, 65, 1–43. [Google Scholar]
- Segel, IH. Enzyme Kinetics; John Wiley & Sons: New York, NY, USA, 1993. [Google Scholar]
- Dixon, M; Webb, EC. Enzymes; Academic Press: London, UK, 1979. [Google Scholar]
- Kim, S-H; Zhou, H-M; Yan, Y-B. Effects of hydroxypropyl cyclodextrins on the reactivation of SDS-denatured aminoacylase. Int. J. Biol. Macromol 2007, 40, 76–82. [Google Scholar]
- He, B; Zhang, Y; Zhang, T; Wang, HR; Zhou, HM. Inactivation and unfolding of aminoacylase during denaturation in sodium dodecyl-sulfate solutions. J. Protein Chem 1995, 14, 349–357. [Google Scholar]
- Wang, ZF; Huang, MQ; Zou, XM; Zhou, HM. Unfolding, conformational change of active sites and inactivation of creatine kinase in SDS solutions. Biochim. Biophys. Acta 1995, 1251, 109–114. [Google Scholar]
- Li, S; Wang, LT; Zhou, HM. SDS-induced conformational changes and inactivation of the bacterial chaperonin GroEL. J. Protein Chem 1999, 18, 653–657. [Google Scholar]
- Parker, W; Song, PS. Protein structures in SDS micelle-protein complexes. Biophys J 1992, 61, 1435–1439. [Google Scholar]
- Reshetnyak, YK; Koshevnik, Y; Burstein, EA. Decomposition of protein tryptophan fluorescence spectra into log-normal components. III. Correlation between fluorescence and microenvironment parameters of individual tryptophan residues. Biophys. J 2001, 81, 1735–1758. [Google Scholar]
- Turoverov, KK; Haitlina, SY; Pinaev, GP. Ultra-violet fluorescence of actin. Determination of native actin content in actin preparations. FEBS Lett 1976, 62, 4–6. [Google Scholar]
- Tsou, C-L. Location of the active sites of some enzymes in limited and flexible molecular regions. Trends Biochem. Sci 1986, 11, 427–429. [Google Scholar]
- Tsou, CL. Conformational flexibility of enzyme active sites. Science 1993, 262, 380–381. [Google Scholar]
- Zhou, Y; Lau, FW; Nauli, S; Yang, D; Bowie, JU. Inactivation mechanism of the membrane protein diacylglycerol kinase in detergent solution. Protein Sci 2001, 10, 378–383. [Google Scholar]
- Heinonen, JK; Lahti, RJ. A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal. Biochem 1981, 113, 313–317. [Google Scholar]
- Hoe, HS; Kim, HK; Kwon, ST. Expression in Escherichia coli of the thermostable inorganic pyrophosphatase from the Aquifex aeolicus and purification and characterization of the recombinant enzyme. Protein Expr. Purif 2001, 23, 242–248. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mu, H.; Zhou, S.-M.; Xia, Y.; Zou, H.; Meng, F.; Yan, Y.-B. Inactivation and Unfolding of the Hyperthermophilic Inorganic Pyrophosphatase from Thermus thermophilus by Sodium Dodecyl Sulfate. Int. J. Mol. Sci. 2009, 10, 2849-2859. https://doi.org/10.3390/ijms10062849
Mu H, Zhou S-M, Xia Y, Zou H, Meng F, Yan Y-B. Inactivation and Unfolding of the Hyperthermophilic Inorganic Pyrophosphatase from Thermus thermophilus by Sodium Dodecyl Sulfate. International Journal of Molecular Sciences. 2009; 10(6):2849-2859. https://doi.org/10.3390/ijms10062849
Chicago/Turabian StyleMu, Hang, Sheng-Mei Zhou, Yong Xia, Hechang Zou, Fanguo Meng, and Yong-Bin Yan. 2009. "Inactivation and Unfolding of the Hyperthermophilic Inorganic Pyrophosphatase from Thermus thermophilus by Sodium Dodecyl Sulfate" International Journal of Molecular Sciences 10, no. 6: 2849-2859. https://doi.org/10.3390/ijms10062849