Slow Unfolding of Monomeric Proteins from Hyperthermophiles with Reversible Unfolding
Abstract
:1. Introduction
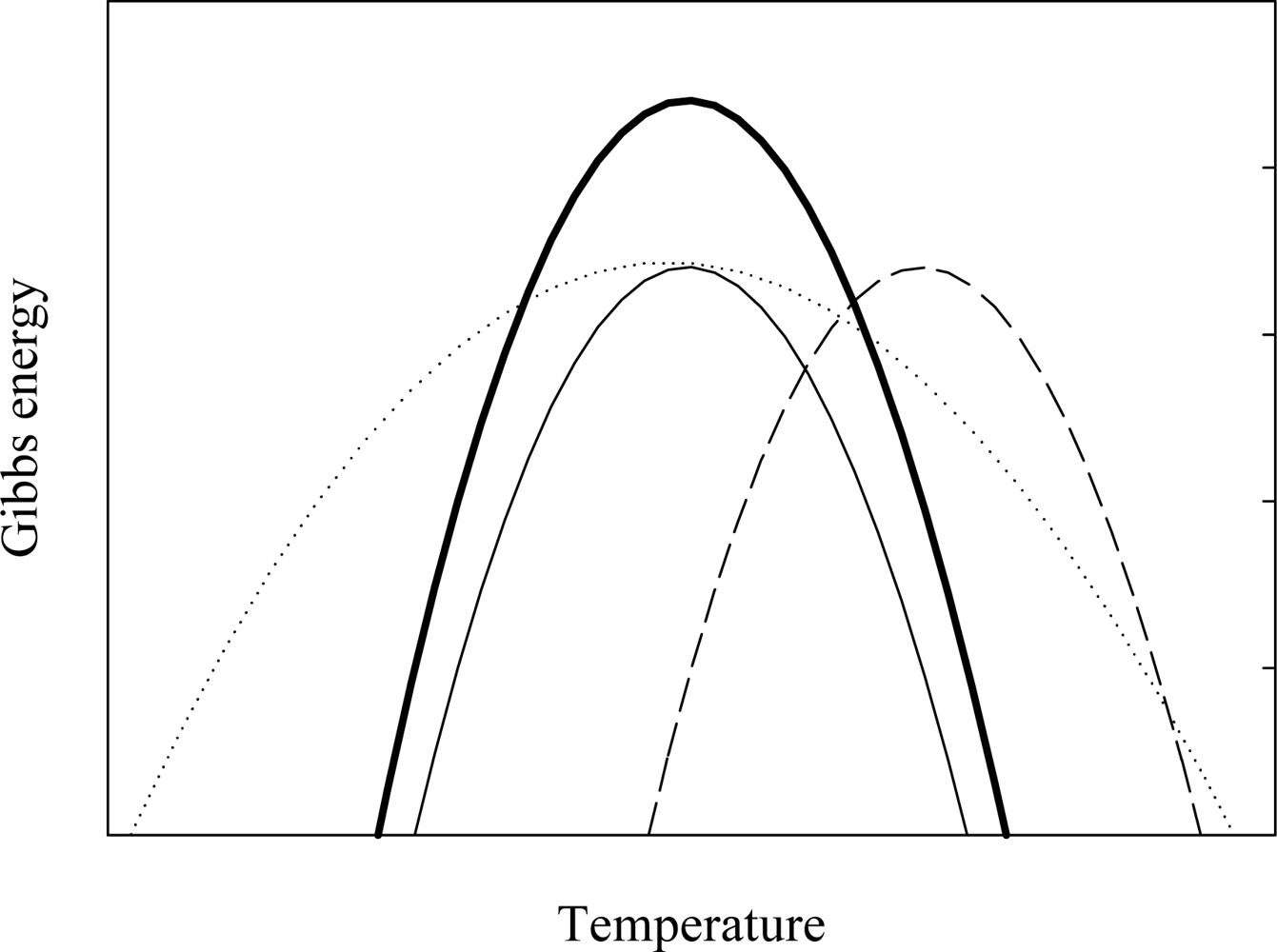
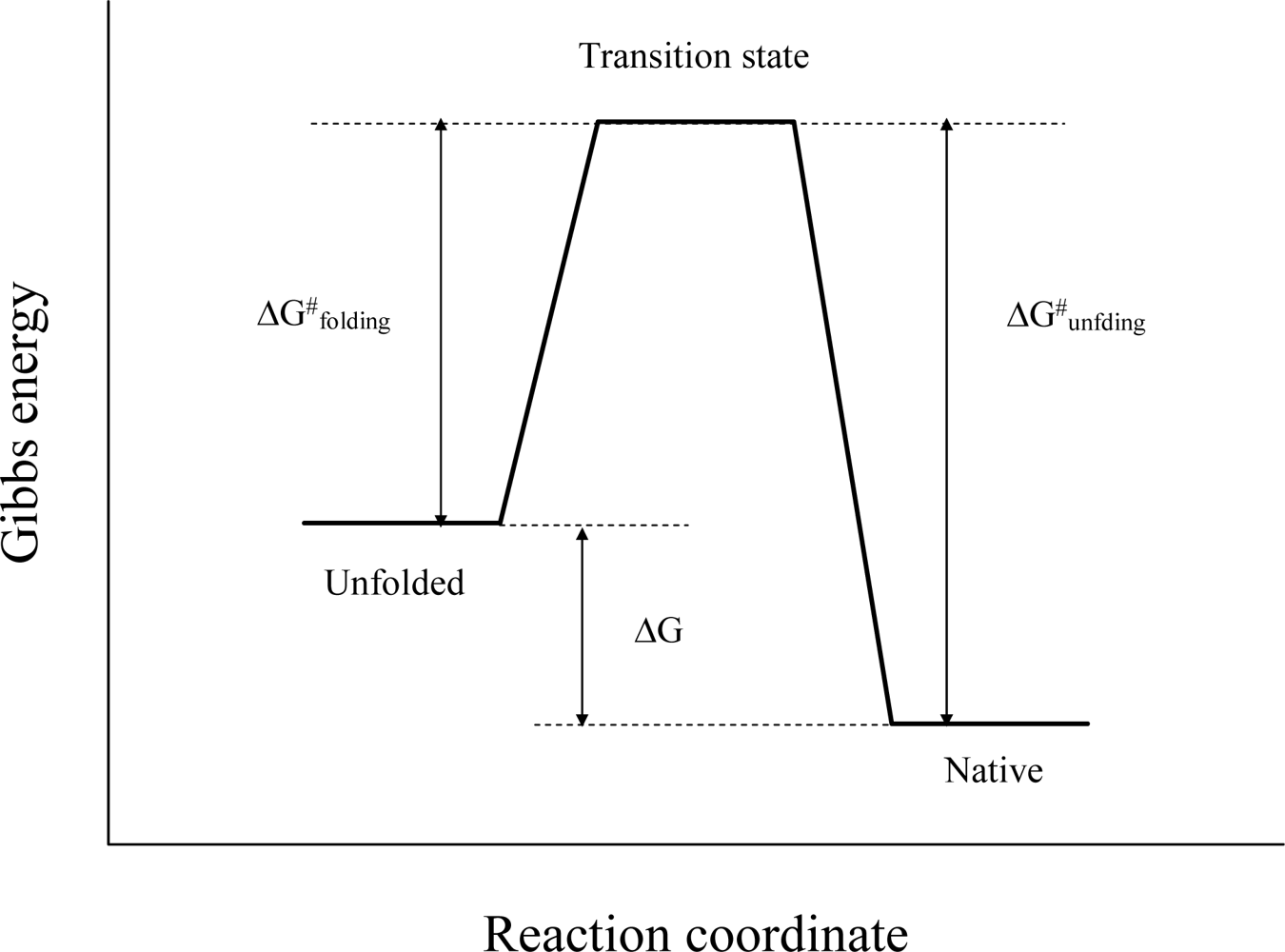
2. Characterizing the Stability and Folding of Hyperthermophilic Proteins
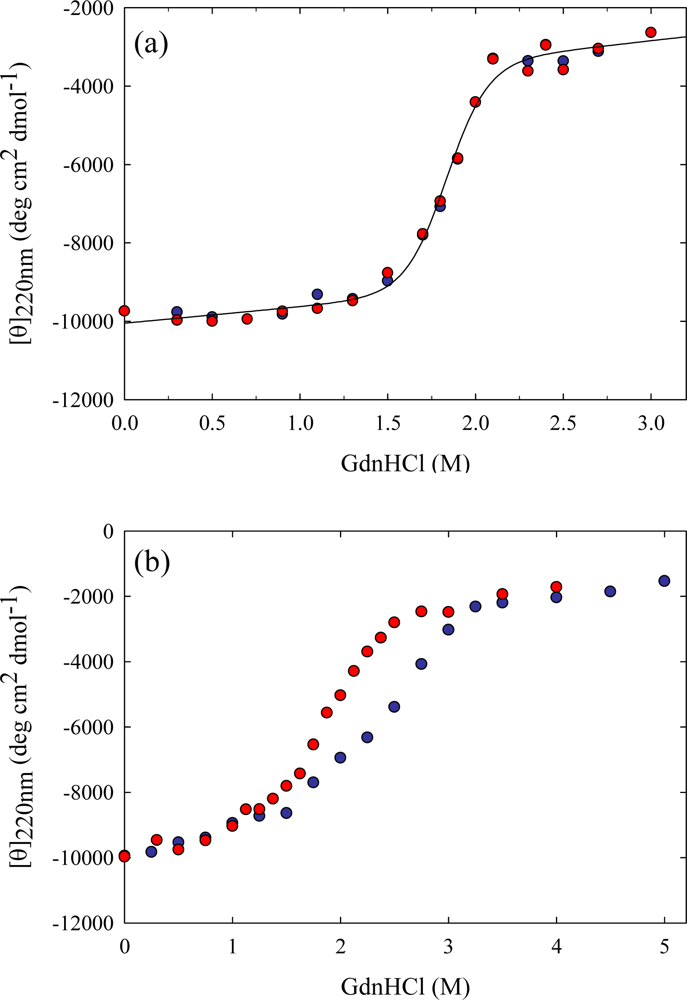
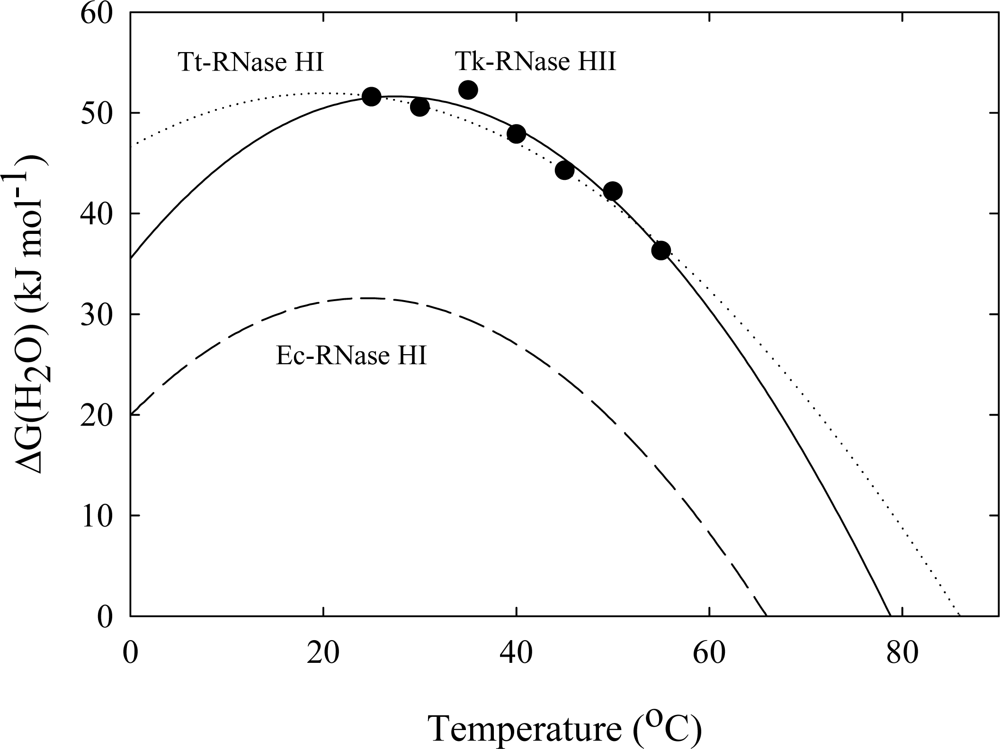
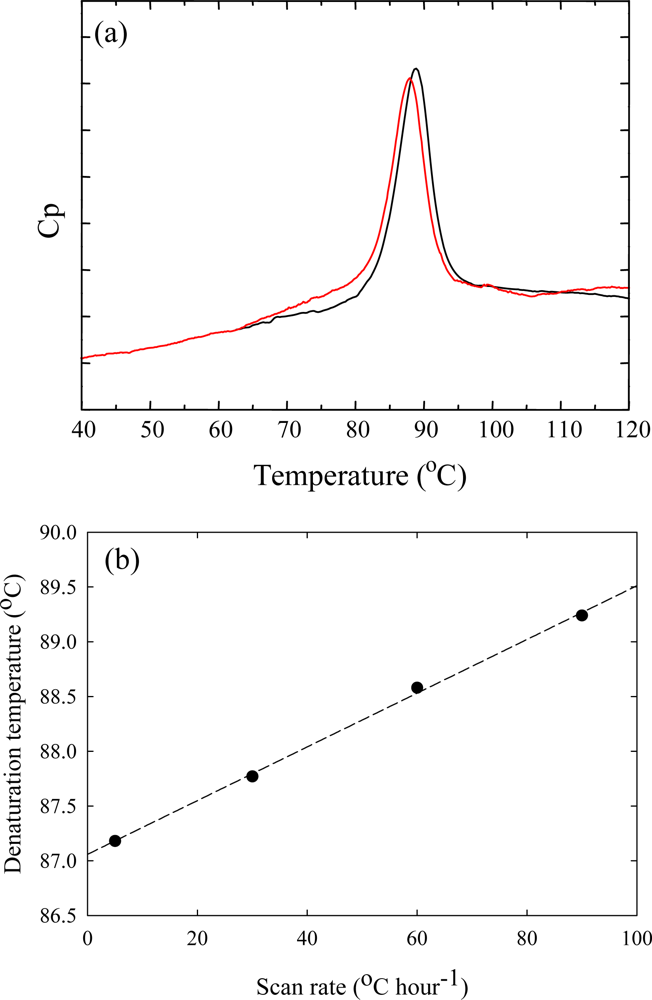
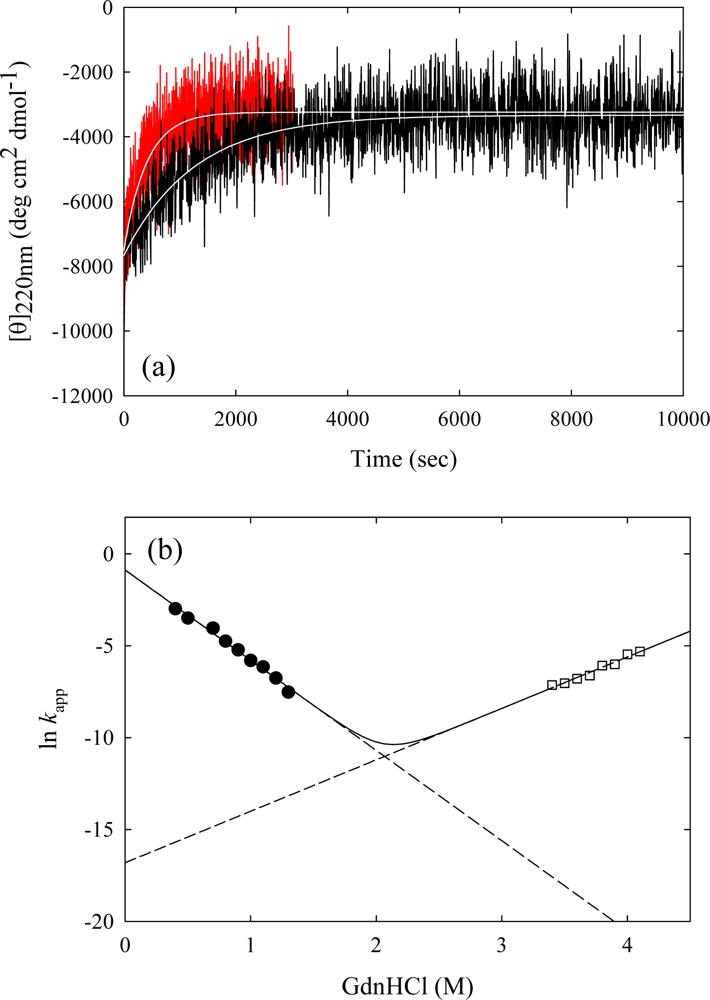
3. Ribonuclease HII from Thermococcus kodakaraensis
4. Other Proteins
4.1. Cold shock protein (Csp)
4.2. Ribosomal protein S16
5. Molecular Mechanisms of the Slow Unfolding of Hyperthermophilic Proteins
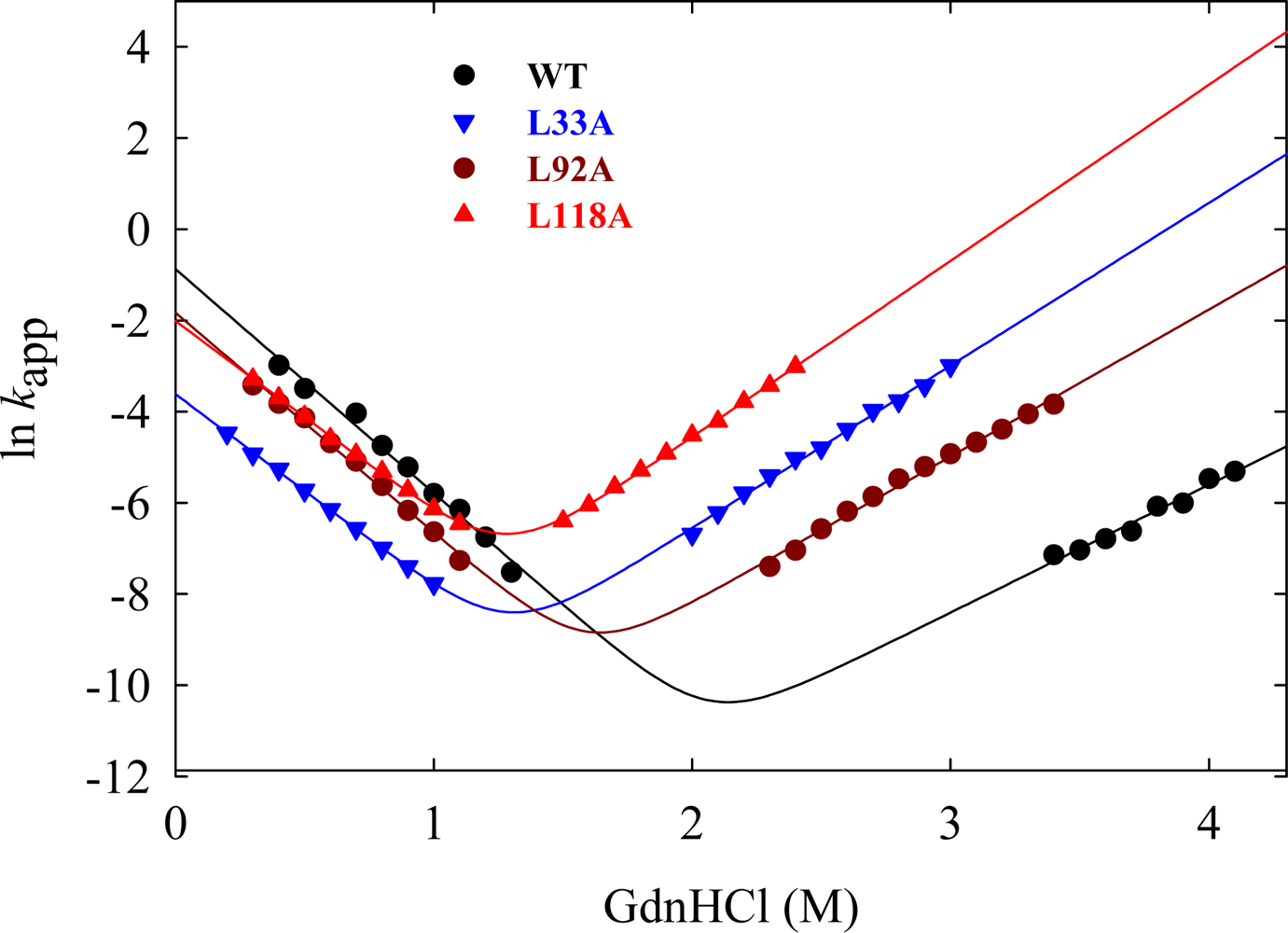
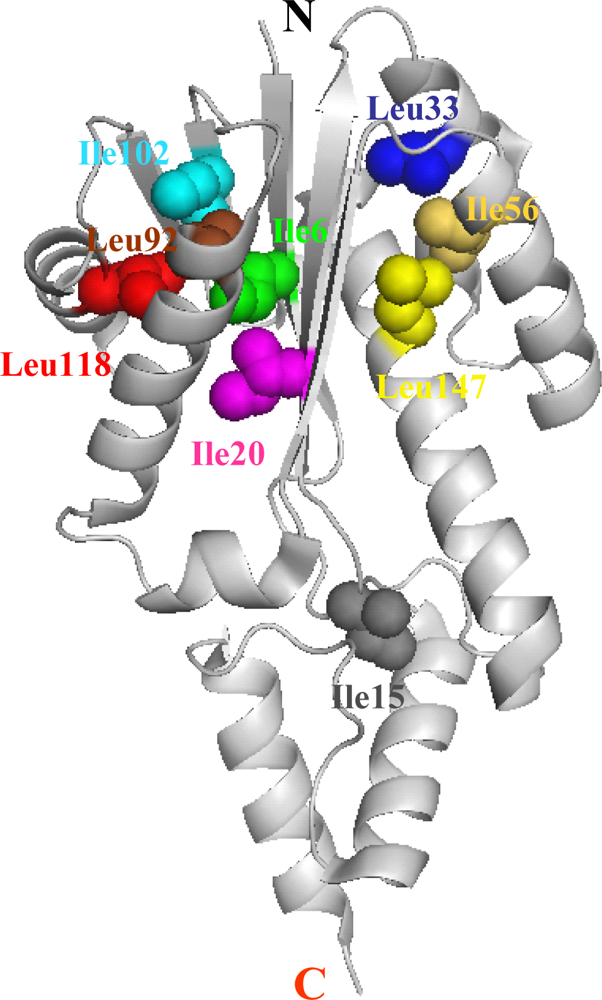
5.1. Hydrophobic interactions
5.2. The Proline Effect
5.3. The osmolyte effect
5.4. Evolutionary background of the slow unfolding of hyperthermophilic proteins
6. Conclusions
References
- Charlier, D; Droogmans, L. Microbial life at high temperature, the challenge, the strategies. Cell. Mol. Life Sci 2005, 62, 2974–2984. [Google Scholar]
- Vieille, C; Zeikus, GJ. Hyperthermophilic enzymes: sources, uses and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev 2001, 65, 1–43. [Google Scholar]
- Elcock, AH. The stability of salt bridges at high temperatures: Implications for hyper-thermophilic proteins. J. Mol. Biol 1998, 284, 489–502. [Google Scholar]
- Szilagyi, A; Zavodszky, P. Structural differences between mesophilic, moderately thermophilic and extremely thermophilic protein subunits: results of a comprehensive survey. Structure 2000, 8, 493–504. [Google Scholar]
- Cambillau, C; Claverie, JM. Structural and genomic correlates of hyperthermostability. J. Biol. Chem 2000, 275, 32383–32386. [Google Scholar]
- Ree, DC. Crystallographic analyses of hyperthermophilic proteins. Methods Enzymol 2001, 334, 423–437. [Google Scholar]
- Matsui, I; Harata, K. Implication forburied polar contacts and ion pairs in hyperthermostable enzymes. FEBS J 2007, 274, 4012–4022. [Google Scholar]
- Jaenicke, R; Böhm, G. The stability of proteins in extreme environments. Curr. Opin. Struct. Biol 1998, 8, 738–748. [Google Scholar]
- Kumar, S; Tsai, CJ; Nussinov, R. Factors enhancing protein thermostability. Protein Eng 2000, 13, 179–191. [Google Scholar]
- Kumar, S; Nussinov, R. How do thermophilic proteins deal with heat? Cell. Mol. Life Sci 2001, 58, 1216–1233. [Google Scholar]
- Razvi, A; Scholtz, JM. Lessons in stability from thermophilic proteins. Protein Sci 2006, 15, 1569–1578. [Google Scholar]
- Ruiz-Sanz, J; Filimonov, VV; Christodoulou, E; Vorgias, CE; Mateo, PL. Thermodynamic analysis of the unfolding and stability of the dimericDNA-binding protein HU from the hyperthermophilic eubacterium Thermotoga maritima and its E34D mutant. Eur. J. Biochem 2004, 271, 1497–1507. [Google Scholar]
- Clark, AT; McCrary, BS; Edmondson, SP; Shriver, JW. Thermodynamics of core hydrophobicity and packing in the hyperthermophile proteins Sac7d and Sso7d. Biochemisty 2004, 43, 2840–2853. [Google Scholar]
- Ge, M; Xia, XY; Pan, XM. Salt bridges in the hyperthermophilic protein Ssh10b are resilient to temperature incrases. J. Bio. Chem 2008, 283, 31690–31696. [Google Scholar]
- Dams, T; Jaenicke, R. Stability and folding of dihydrofolate reductase from the hyperthermophilic bacterium Thermotoga maritima. Biochemistry 1999, 38, 9169–9178. [Google Scholar]
- Ogasahara, K; Nakamura, M; Nakura, S; Tsunasawa, S; Kato, I; Yoshimoto, T; Yutani, K. The unusually slow unfolding rate causes the high stability of pyroolidone carboxyl peptidase from a hyperthermophilile Pyrococcus furiosus: equilibrium and kinetic studies of guanidine hydrochloride-induced unfolding and refolding. Biochemistry 1998, 37, 17537–17544. [Google Scholar]
- Jaswal, SS; Sohl, JL; Dans, JH; Agard, DA. Energetic landscape of alpha-lytic protease optimizes longevity through kinetic stability. Nature 2002, 415, 343–346. [Google Scholar]
- Kaushik, JK; Ogasahara, K; Yutani, K. The unusually slow relaxation kinetics of the folding-unfolding of pyrrolidone carboxyl peptidase from a hyperthermophile, Pyrococcus furiosus. J. Mol. Biol 2002, 316, 991–1003. [Google Scholar]
- Iimura, S; Yagi, H; Ogasahara, K; Akutsu, H; Noda, Y; Segawa, S; Yutani, K. Unusually slow denaturation and refolding process of pyrrolidone carcoxyl peptidase from a hyper-thermophile are highly cooperative: Real-time NMR studies. Biochemistry 2004, 43, 11906–11915. [Google Scholar]
- Mukaiyama, A; Takano, K; Haruki, M; Morikawa, M; Kanaya, S. Kinetically robust monomeric protein from a hyperthermophile. Biochemistry 2004, 43, 13859–13866. [Google Scholar]
- Forrer, P; Chang, C; Ott, D; Wlodawer, A; Plückthun, A. Kinetic stability and crystal structure of the viral capside protein SHP. J. Mol. Biol 2004, 344, 179–193. [Google Scholar]
- Kaushik, JK; Iimura, S; Ogasahara, K; Yamagata, Y; Segawa, S; Yutani, K. Completely buried, non-ion-paired glutamic acid contributes favorably to the conformational stability of pyrrolidone carboxyl peptidases from hyperthermophiles. Biochemistry 2006, 45, 7100–7112. [Google Scholar]
- Luke, KA; Higgins, CL; Wittung-Stafshede, P. Thermodynamic stability and folding of proteins from hyperthermophilic organisms. FEBS J 2007, 274, 4023–4033. [Google Scholar]
- Pace, CN. Measuring and increasing protein stability. Trends Biotechnol 1990, 8, 93–98. [Google Scholar]
- McCrary, BS; Edmondson, SP; Shriver, JW. Hyperthermophile protein folding thermodynamics: Differential scanning calorimetry and chemical denaturation of Sac7d. J. Mol. Biol 1996, 264, 784–805. [Google Scholar]
- Li, WT; Grayling, RA; Sandman, K; Edmondson, S; Shriver, JW; Reeve, JN. Thermodynamic stability of archaeal histones. Biochemistry 1998, 37, 10563–10572. [Google Scholar]
- Grättinger, M; Dankesreiter, A; Schurig, H; Jaenicke, R. TRecombinant phosphoglycerate kinase from the hyperthermophilic bacterium Thermotoga maritima: Catalytic, spectral and thermodynamic properties. J. Mol. Biol 1998, 280, 525–533. [Google Scholar]
- Shiraki, K; Nishikori, S; Fujiwara, S; Hashimoto, H; Kai, Y; Takagi, M; Imanaka, T. Comparative analyses of the conformational stability of a hyperthermophilic protein and its mesophilic counterpart. Eur. J. Biochem 2001, 268, 4144–4150. [Google Scholar]
- Deutschman, WA; Dahlquist, FW. Thermodynamic basis for the increased thermostability of CheY from the hyperthermophile Thermotoga maritima. Biochemistry 2001, 40, 13107–13113. [Google Scholar]
- Lee, CF; Allen, MD; Bycroft, M; Wong, KD. Electrostatic interactions contribute reduced heat capacity change of unfolding in a thermophilic ribosomal protein L30e. J. Mol. Biol 2005, 348, 419–431. [Google Scholar]
- Razvi, A; Scholtz, JM. A thermodynamic comparison of HPr proteins from extremophilic organisms. Biochemistry 2006, 45, 4084–4092. [Google Scholar]
- Gianni, S; Ivarsson, Y; Jemth, P; Brunori, M; Travaglini-Allocatelli, C. Identification and characterization of protein folding intermediates. Biophys. Chem 2007, 128, 105–113. [Google Scholar]
- Mukaiyama, A; Haruki, M; Ota, M; Koga, Y; Takano, K; Kanaya, S. A hyperthermophilic protein acquires function at the cost of stability. Biochemistry 2006, 45, 12673–12679. [Google Scholar]
- Dong, H; Mukaiyama, A; Tadokoro, T; Koga, Y; Takano, K; Kanaya, S. Hydrophobic effect on the stability and folding of a hyperthermophilic protein. J. Mol. Biol 2008, 378, 264–272. [Google Scholar]
- Takano, K; Higashi, R; Okada, J; Mukaiyama, A; Tadokoro, T; Koga, Y; Kanaya, S. Proline effect on the thermostability and slow unfolding of a hyperthermophilic protein. J. Biochem 2009, 145, 79–85. [Google Scholar]
- Mukaiyama, A; Koga, Y; Takano, K; Kanaya, S. Osmolyte effect on the stability and folding of a hyperthermophilic protein. Proteins: Struct., Funct., Bioinf 2008, 71, 110–118. [Google Scholar]
- Crouch, RJ; Dirksen, ML; Ribonuclease, H; Linn, SM; Robert, RJ (Eds.) Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1982; pp. 211–241.
- Ohtani, N; Haruki, M; Morikawa, M; Kanaya, S. Molecular diversities of RNase H. J. Biosci. Bioeng 1999, 88, 12–19. [Google Scholar]
- Muroya, A; Tsuchiya, D; Ishikawa, M; Haruki, M; Morikawa, M; Kanaya, S; Morikawa, K. catalytic center of an archaeal type2 ribonuclease H as revealed by X-ray crystallographic and mutational analyses. Protein Sci 2001, 10, 707–714. [Google Scholar]
- Takano, K; Endo, S; Mukaiyama, A; Chon, H; Matsumura, H; Koga, Y; Kanaya, S. Structure of amyloid beta fragments in aqueous environments. FEBS J 2006, 273, 150–158. [Google Scholar]
- Takano, K; Katagiri, Y; Mukaiyama, A; Chon, H; Matsumura, H; Koga, Y; Kanaya, S. Conformational contagion in a protein: structural properties of a chameleon sequence. Proteins 2007, 68, 617–625. [Google Scholar]
- Hollien, J; Marqusee, S. A thermodynamic comparison of mesophilic and thermophilic ribonucleases H. Biochemistry 1999, 38, 3831–3836. [Google Scholar]
- Tanford, C. Protein folding. Part C. Adv. Protein Chem 1970, 24, 1–95. [Google Scholar]
- Raschke, TM; Kho, J; Marqusee, S. Confirmation of the hierarchical folding of RNase H: A protein engineering study. Nature Struct. Biol 1999, 6, 825–831. [Google Scholar]
- Hollien, J; Marqusee, S. Comparison of the folding processes of T. thermophilus and E. coli ribonucleases H. J. Mol. Biol 2002, 316, 327–340. [Google Scholar]
- Perl, D; Welker, C; Schindler, T; Schröder, K; Marahiel, MA; Janicke, R; Schmid, FX. Conservation of rapid two-state folding in mesophilic, thermophilic and hyperthermophilic cold shock proteins. Nature Struct. Biol 1998, 5, 229–235. [Google Scholar]
- Dominy, BN; Perl, D; Schmid, FX; Brooks, CL, III. The effect of ionic strength on protein stability: The cold shock protein family. J. Mol. Biol 2002, 319, 541–554. [Google Scholar]
- Schuler, B; Kremer, W; Kalbitzer, HR; Jaenicke, R. Role of entropy in protein thermostability: folding kinetics of a hyperthermophilic cold shock protein at high temperatures using 19F NMR. Biochemistry 2002, 41, 11670–11680. [Google Scholar]
- Wallgren, M; Åden, J; Pylypenko, O; Mikaelsson, T; Johansson, LB-Å; Rak, A; Wolf-Watz, M. Extreme temperature tolerance of a hyperthermophilic protein coupled to residual structure in the unfolded state. J. Mol. Biol 2008, 379, 845–858. [Google Scholar]
- Pace, CN. Contribution of the hydrophobic effect to globular protein stability. J. Mol. Biol 1992, 226, 29–35. [Google Scholar]
- Takano, K; Ogasahara, K; Kaneda, H; Yamagata, Y; Fujii, S; Kanaya, E; Kikuchi, M; Oobatake, M; Yutani, K. Contribution of hydrophobic residues to the stability of human lysozyme: calorimetric studies and X-ray structural analyses of the five isoleucine to valine mutans. J. Mol. Biol 1995, 254, 62–76. [Google Scholar]
- Takano, K; Yamagata, Y; Fujii, S; Yutani, K. Contribution of hydrophobic effect to the stability of human lysozyme: calorimetric studies and X-ray structural analysis of the nine valine to alanine mutans. Biochemistry 1997, 36, 688–698. [Google Scholar]
- Takano, K; Funahashi, J; Yamagata, Y; Fujii, S; Yutani, K. Contribution of water molecules in the interior of a protein to the conformational stability. J. Mol. Biol 1997, 274, 132–142. [Google Scholar]
- Takano, K; Yamagata, Y; Yutani, K. A general rule for the relationship between hydrophobic effect and conformational stability of a protein: Stability and structure of a series of hydrophobic mutants of human lysozyme. J. Mol. Biol 1998, 280, 749–761. [Google Scholar]
- Matthews, BW; Nicholson, H; Beckel, WJ. Enhanced protein thermostability from site-directed mutations that decrease the entropy of unfolding. Proc. Natl. Acad. Sci. USA 1987, 84, 6663–6667. [Google Scholar]
- Melchionna, S; Sinibaldi, R; Briganti, G. Explanation of the stability of thermophilic proteins based on unique micromorphology. Biophys. J 2006, 90, 4204–4212. [Google Scholar]
- Sterpone, F; Bertonati, C; Briganti, G; Melchionna, S. Key role of proximal water in regulating thermostable proteins. J. Phys. Chem. B 2009, 113, 131–137. [Google Scholar]
- Watanabe, K; Suzuki, Y. Protein thermostabilization by proline substitutions. J. Mol. Catal. B Enzyme 1998, 4, 167–180. [Google Scholar]
- Richardson, JS; Richardson, DC. Amino acid preferences for specific locations at the ends of alpha helices. Science 1988, 240, 1648–1652. [Google Scholar]
- Yancey, PH; Somero, GN. Counteraction of urea destabilization of protein structure by methylamine osmoregulatory compounds of elasmobranch fishes. Biochem. J 1979, 18, 317–323. [Google Scholar]
- Yancey, PH; Clark, ME; Hand, SC; Bowlus, RD; Somero, GN. Living with water stress: Evolution of osmolyte systems. Science 1982, 217, 1214–1222. [Google Scholar]
- Frye, KJ; Royer, CA. The kinetic basis for the stabilization of staphylococcal nuclease by xylose. Protein Sci 1997, 6, 789–793. [Google Scholar]
- Russo, AT; Rösgen, J; Bolen, DW. Osmolyte effects on kinetics of FKBP12 C22A folding coupled with prolyl isomerization. J. Mol. Biol 2003, 330, 851–866. [Google Scholar]
- Atomi, H; Fukui, T; Kanai, T; Morikawa, M; Imanaka, T. Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 2004, 1, 263–267. [Google Scholar]
- Deckert, G; Warren, PV; Gaasterland, T; Young, WG; Lenox, AL; Graham, DE; Overbeek, R; Snead, MA; Keller, M; Aujay, M. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature 1998, 392, 353–358. [Google Scholar]
- Nelson, KE; Clayton, RA; Gill, SR; Gwinn, ML; Dodson, RJ; Haft, DH; Hickey, EK; Peterson, JD; Nelson, WC; Ketchum, KA. Evidence for lateral gene transfer between Archaea and bacteria from genome sequence of Thermotoga maritima. Nature 1999, 399, 323–329. [Google Scholar]
- Delong, EF. A phylogenetic perspective on hyperthermophilic microorganisms. Methods Enzymol 2001, 330, 3–11. [Google Scholar]
- Huber, R; Stetter, KO. Discovery of hyperthermophilic miceoorganisms. Methods Enzymol 2001, 330, 11–24. [Google Scholar]
- Berezovsky, IN; Shakhnovich, EI. Physics and evolution of thermophilic adaptation. Proc. Natl. Acad. Sci. USA 2005, 102, 12742–12747. [Google Scholar]
| Protein | Organism | G(H2O) (kJ mol−1) | kref(H2O) (s−1) | kref(H2O) (s−1) |
|---|---|---|---|---|
| RNase HII | Thermococcus | 43.6 (50 °C) | 5.0 × 10−8 (50°C) | 7.8 × 10−1 (50 °C) |
| kodakaraensis | 48.3 (25 °C) | 6.0 × 10−10 (25 °C) | 0.4 × 10−1 (25 °C) | |
| Cold shock protein | Thermotoga maritima | 26.2 (25 °C) | 1.8 × 10−2 (25 °C) | 5.7 × 102 (25 °C) |
| Ribosomal protein S16 | Aquifex aeolicus | 24.8 (25 °C) | 1.6 × 10−2 (25 °C) | 3.8 × 10 (25 °C) |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/). This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mukaiyama, A.; Takano, K. Slow Unfolding of Monomeric Proteins from Hyperthermophiles with Reversible Unfolding. Int. J. Mol. Sci. 2009, 10, 1369-1385. https://doi.org/10.3390/ijms10031369
Mukaiyama A, Takano K. Slow Unfolding of Monomeric Proteins from Hyperthermophiles with Reversible Unfolding. International Journal of Molecular Sciences. 2009; 10(3):1369-1385. https://doi.org/10.3390/ijms10031369
Chicago/Turabian StyleMukaiyama, Atsushi, and Kazufumi Takano. 2009. "Slow Unfolding of Monomeric Proteins from Hyperthermophiles with Reversible Unfolding" International Journal of Molecular Sciences 10, no. 3: 1369-1385. https://doi.org/10.3390/ijms10031369




