Improved Adhesion, Growth and Maturation of Vascular Smooth Muscle Cells on Polyethylene Grafted with Bioactive Molecules and Carbon Particles
Abstract
:1. Introduction
2. Results and Discussion
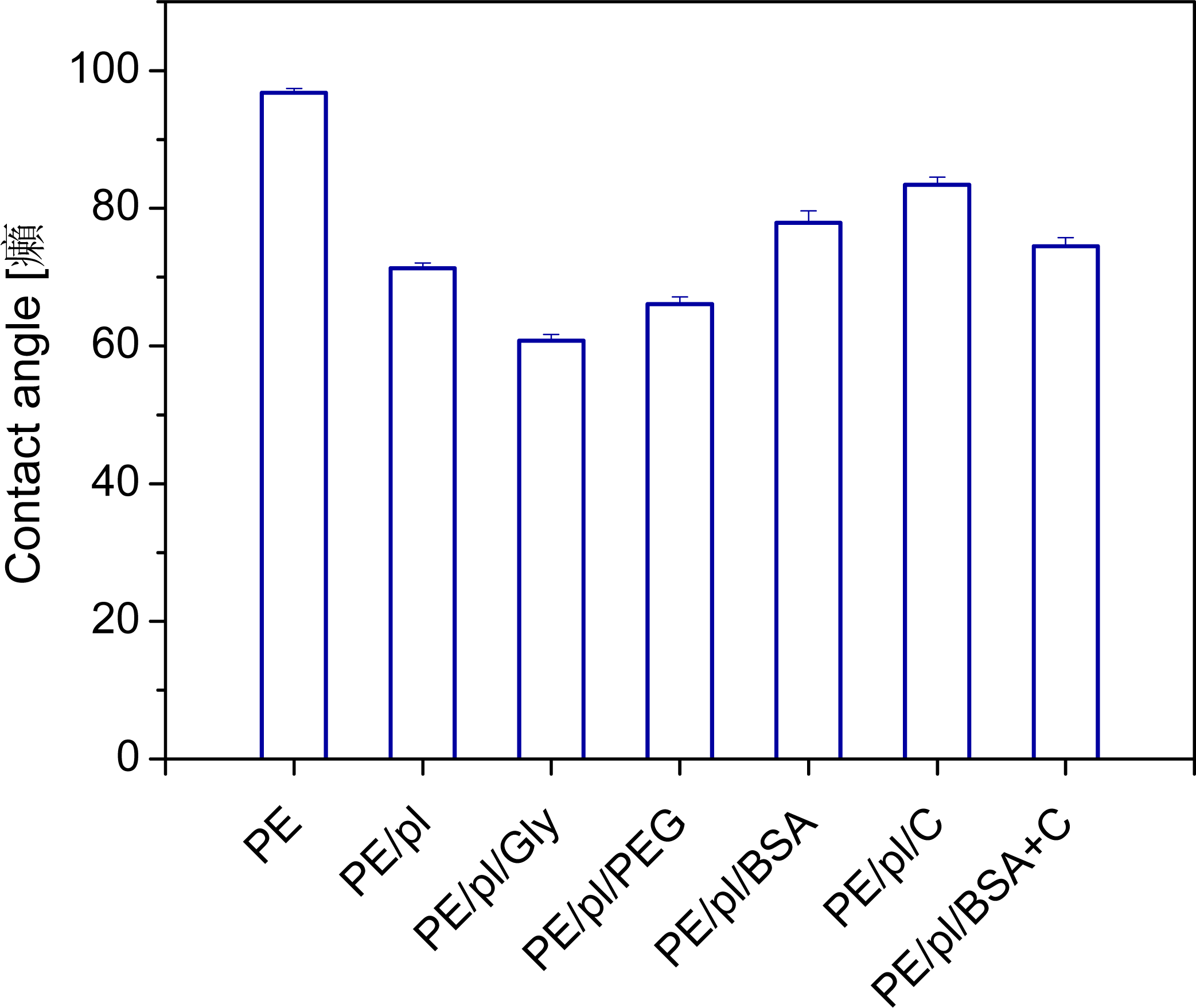
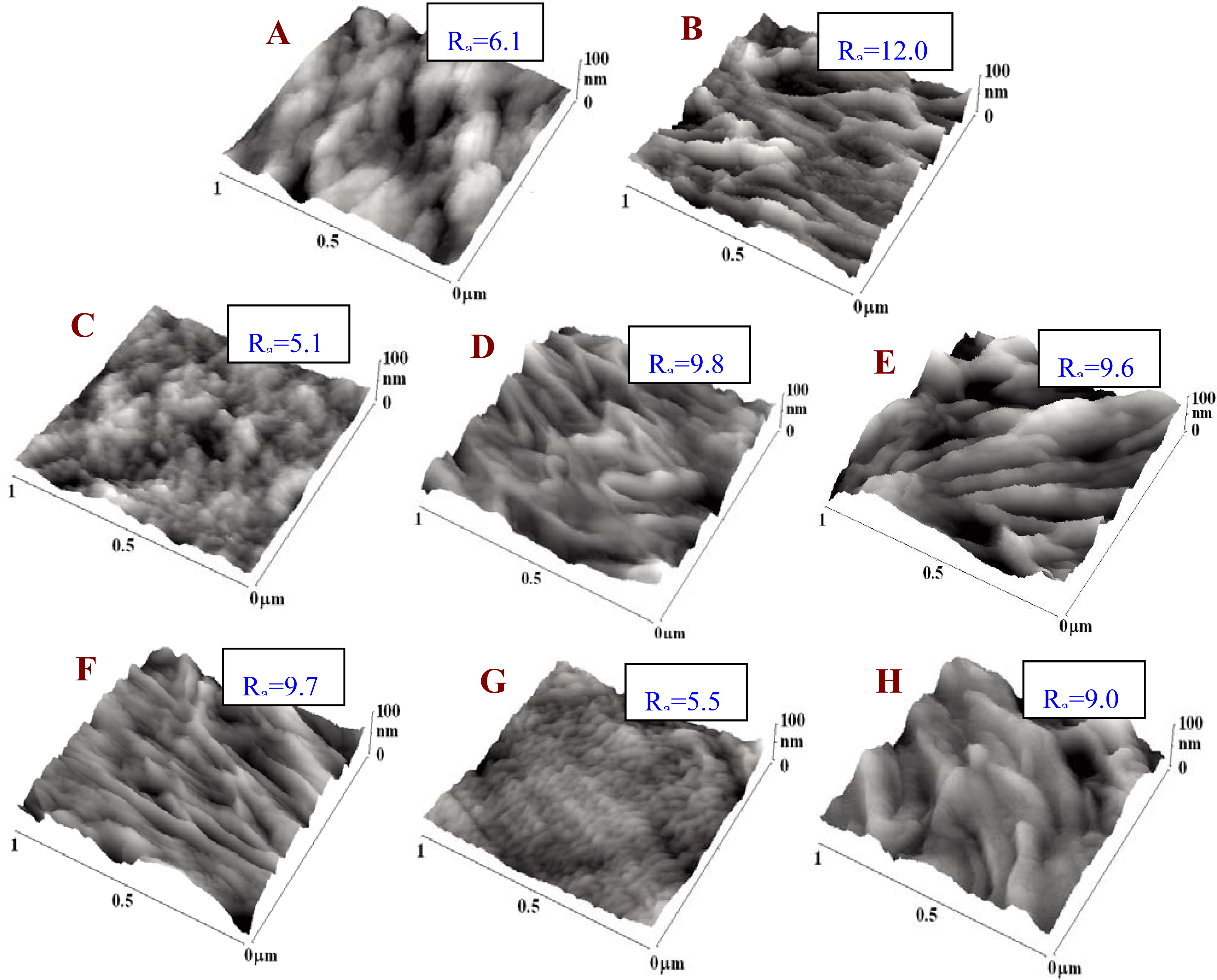
2.1. Physicochemical Properties of Polymer Samples
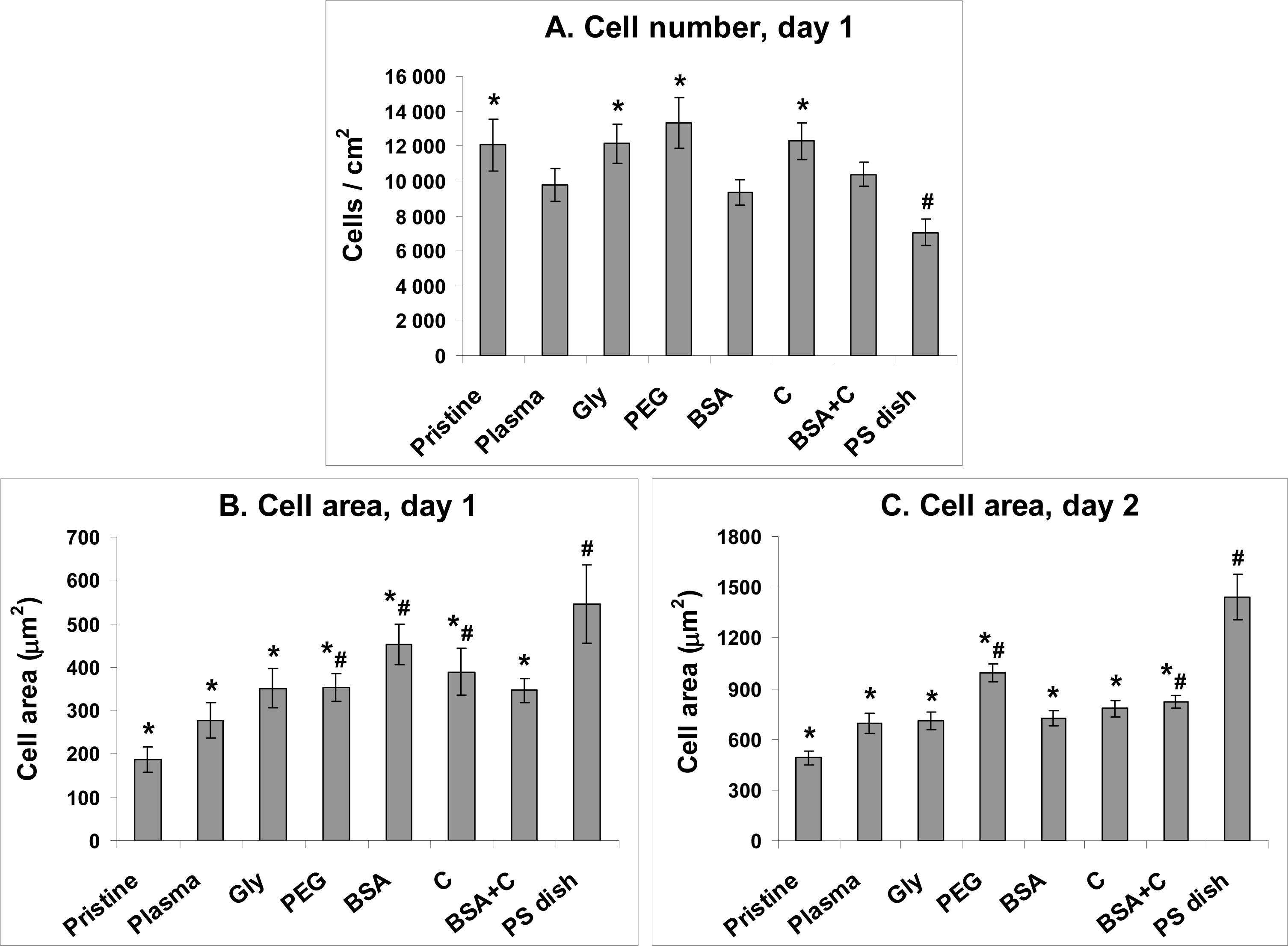
2.2. Initial Adhesion of VSMC on Polymer Samples
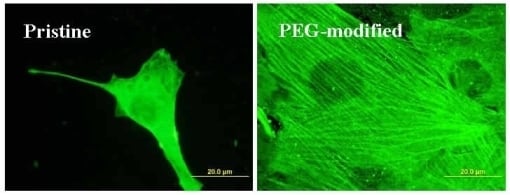
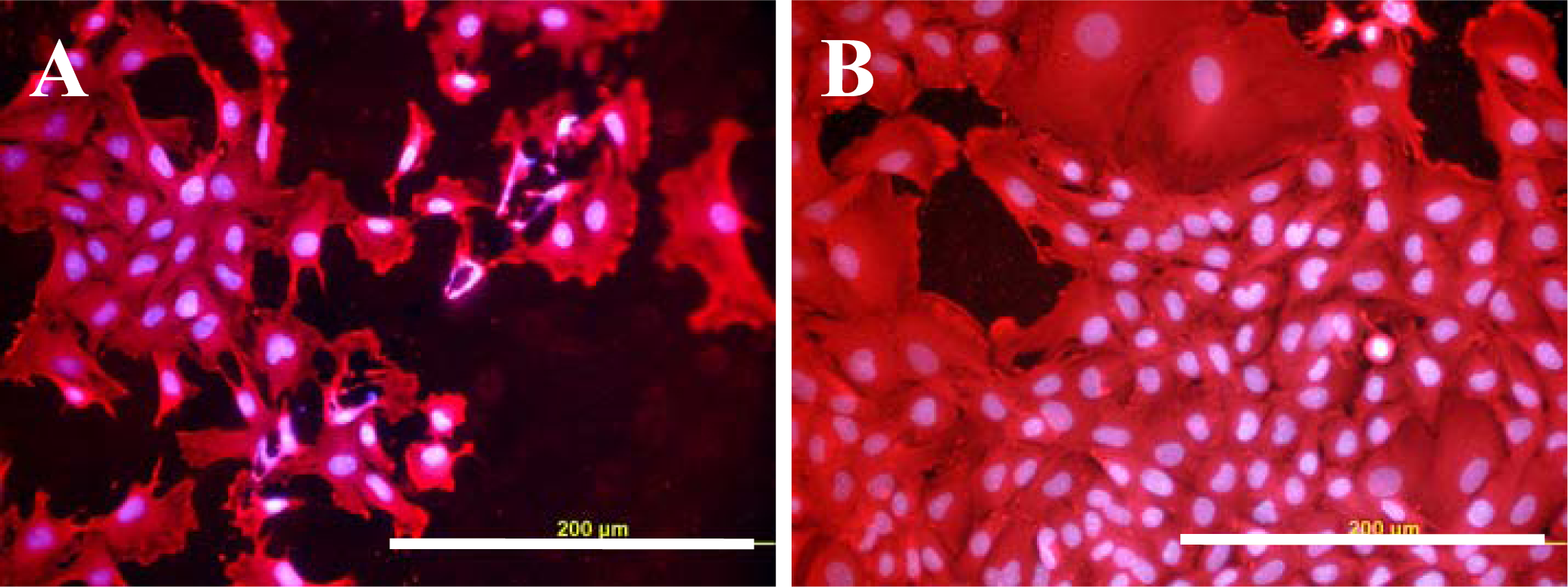
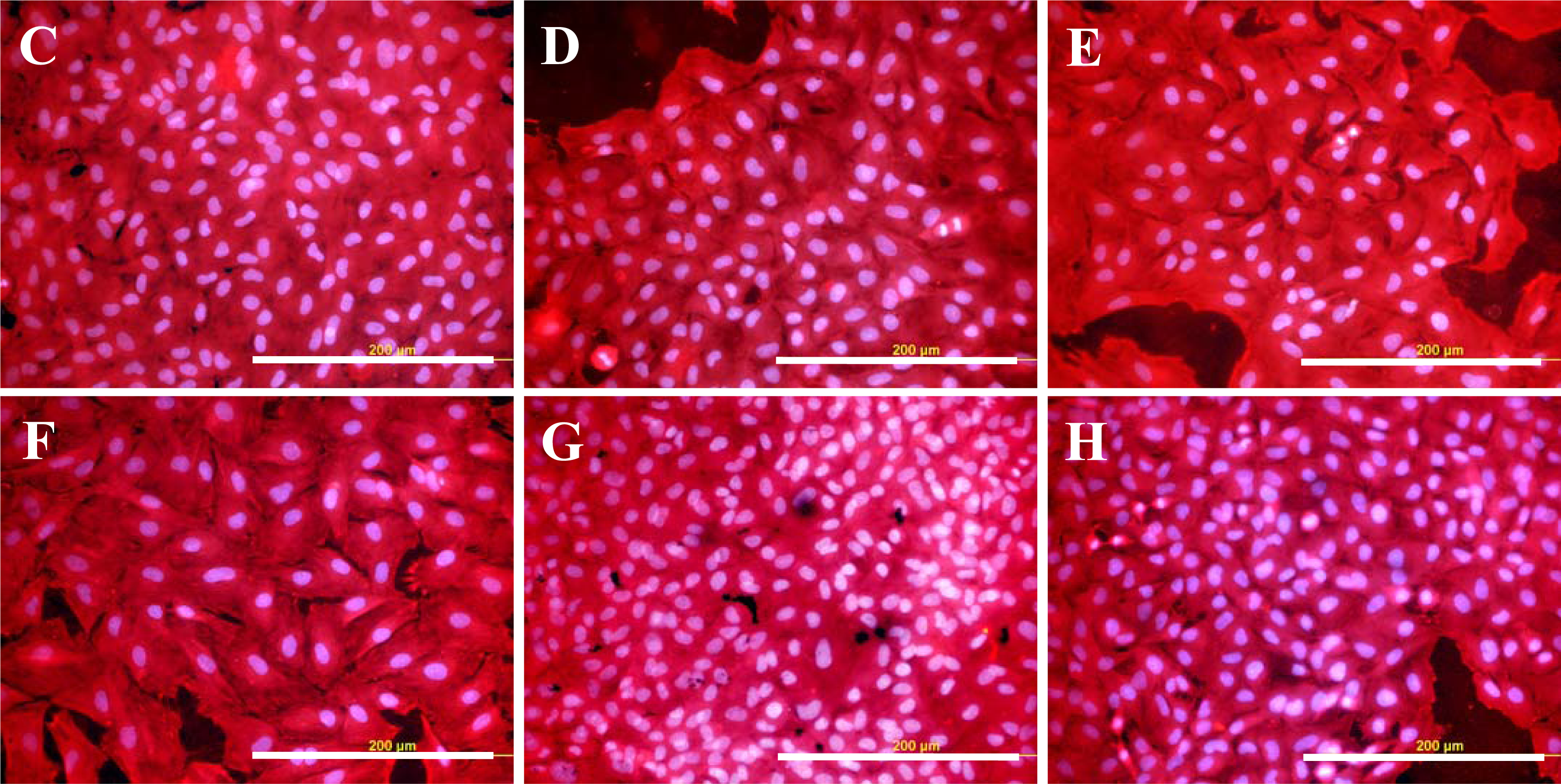
2.3. Spreading of VSMC on Polymer Samples
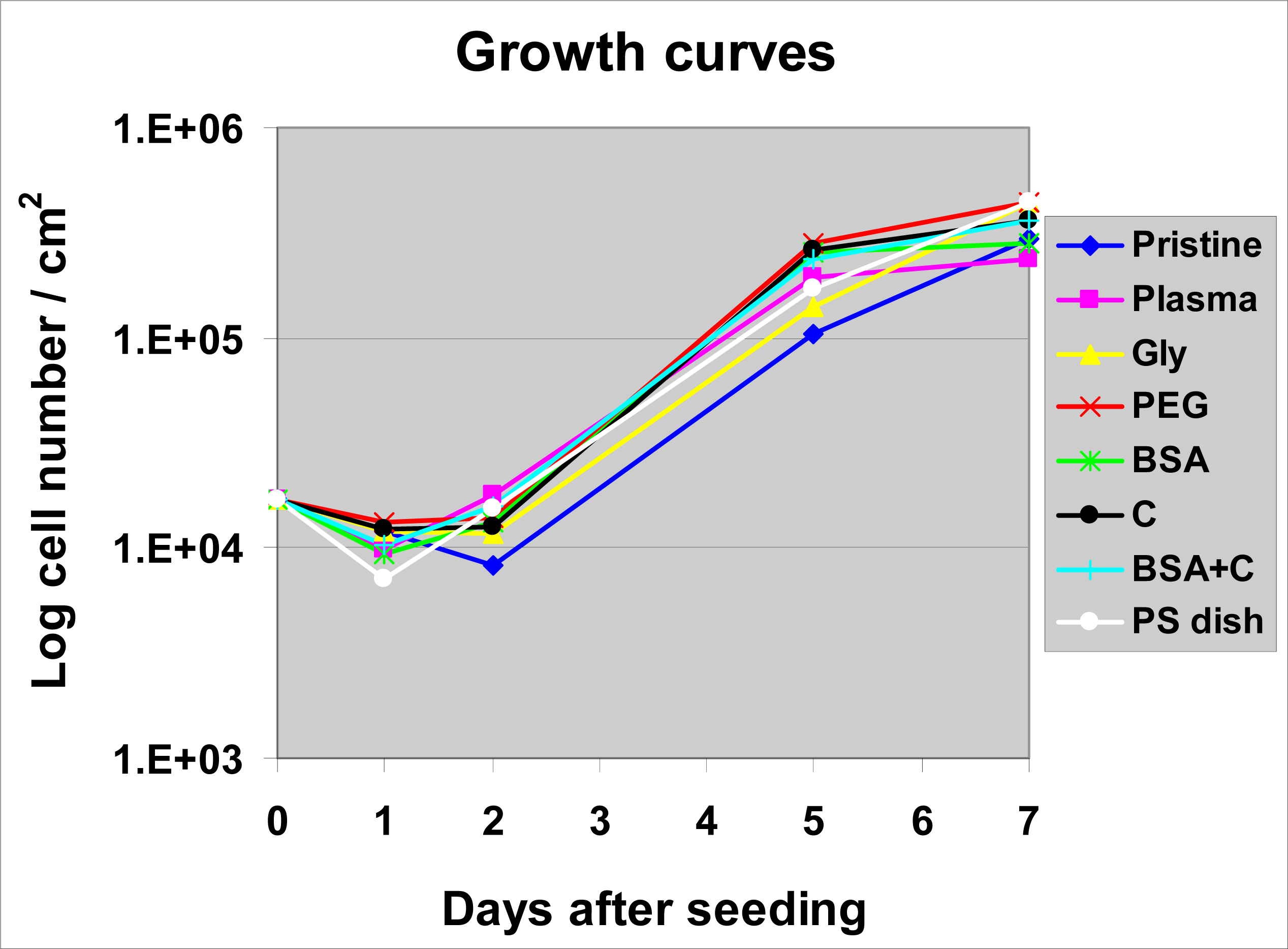
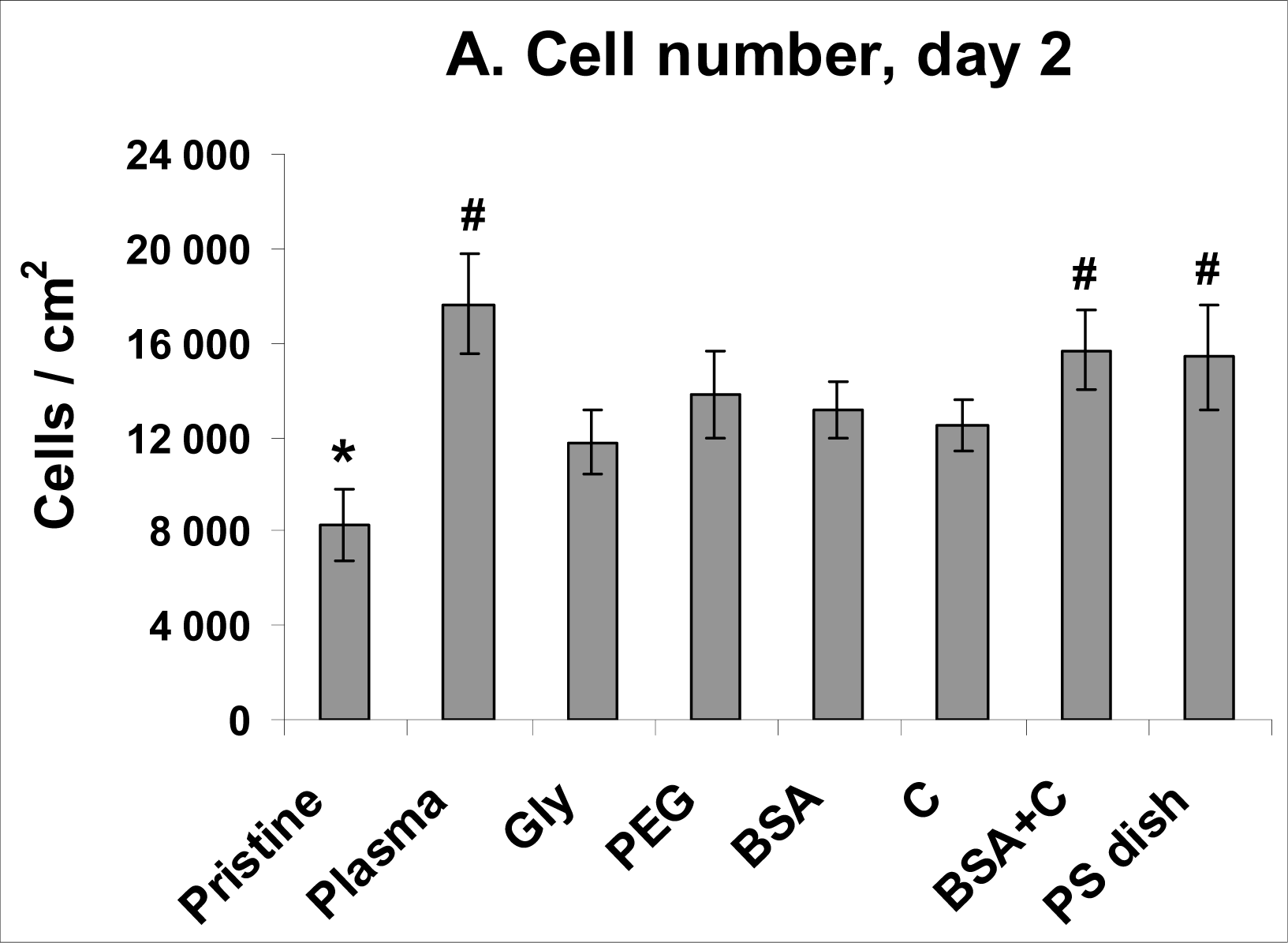
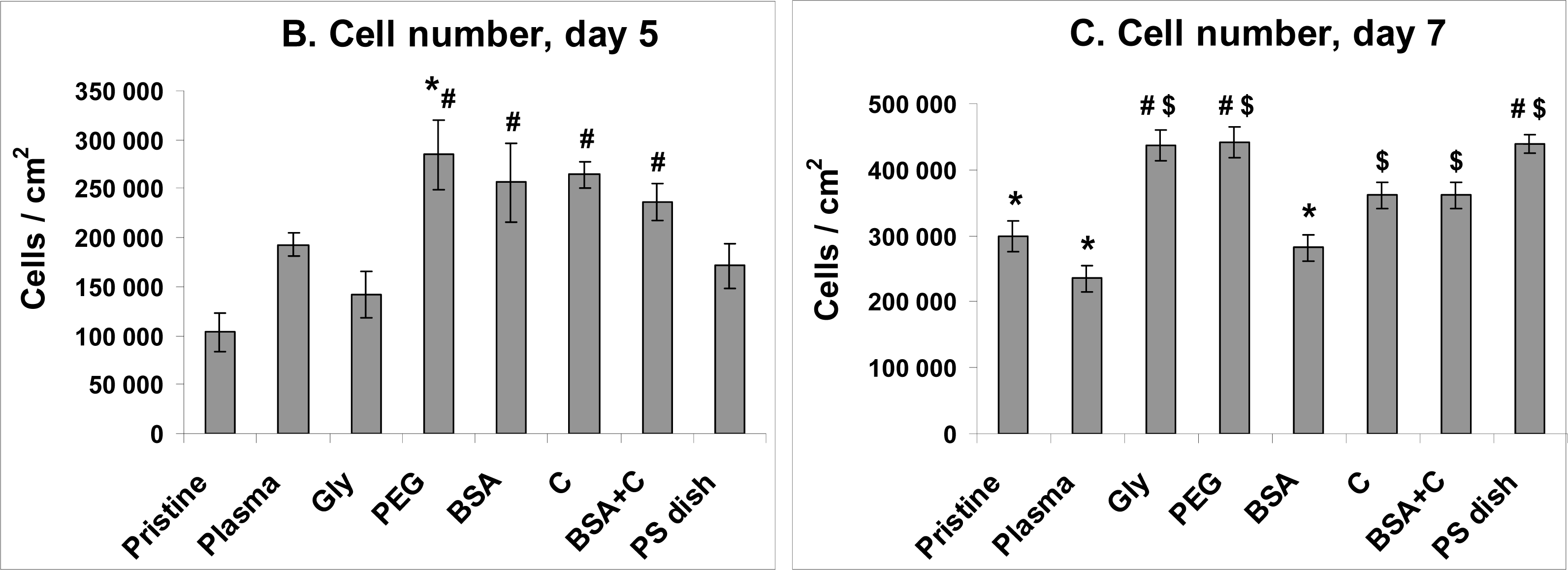
2.4. Proliferation of VSMC on Polymer Samples
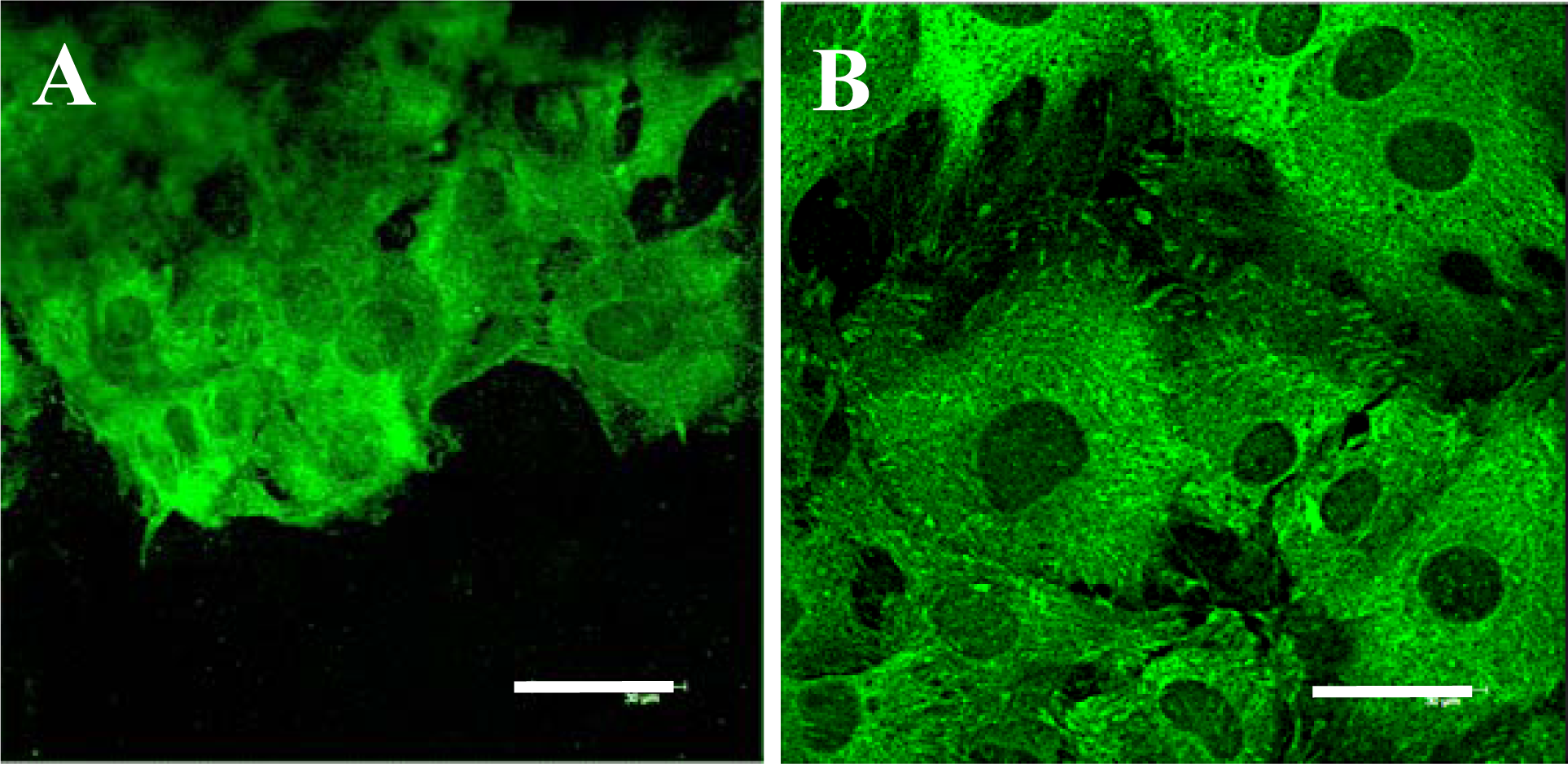
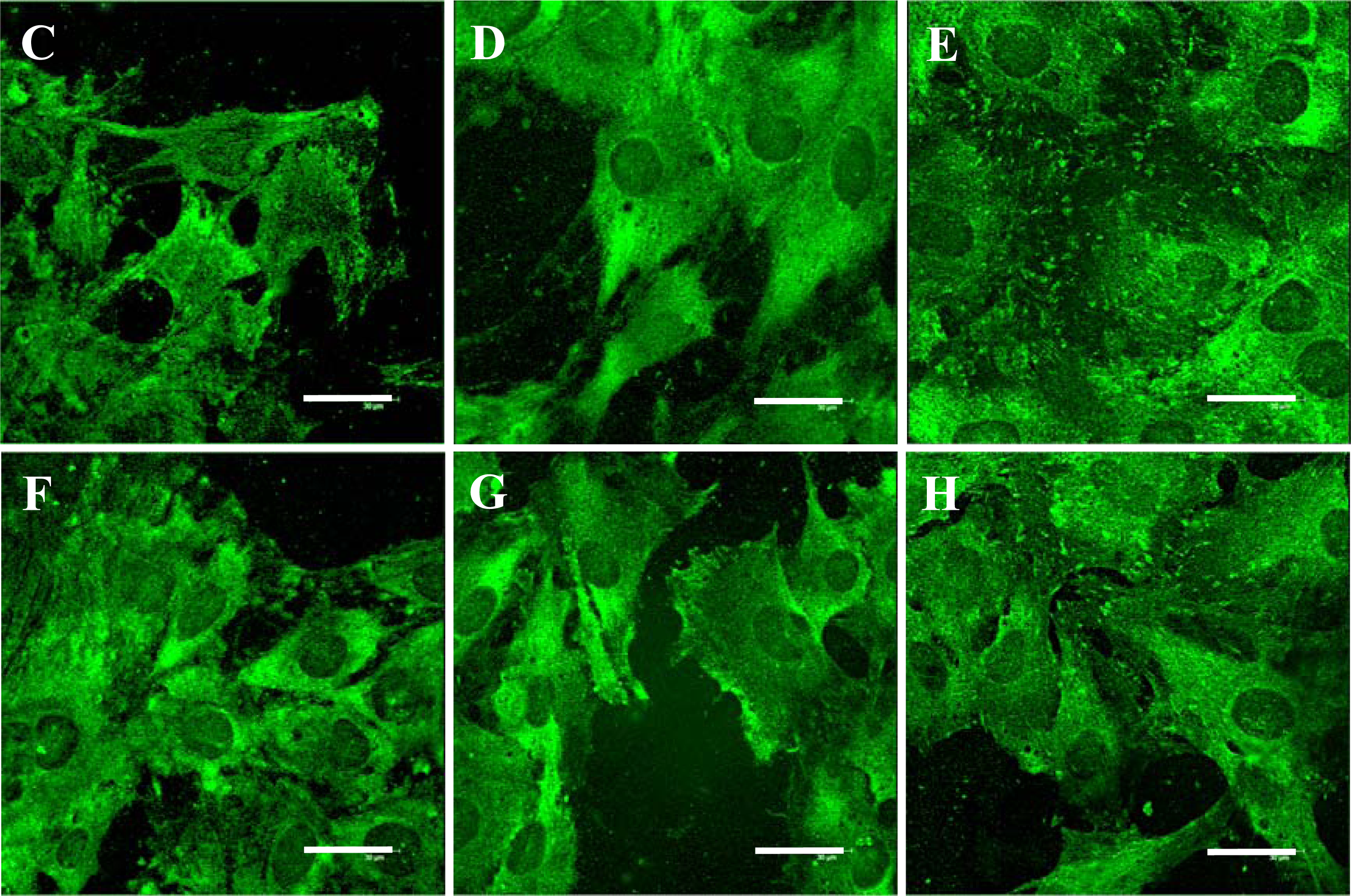
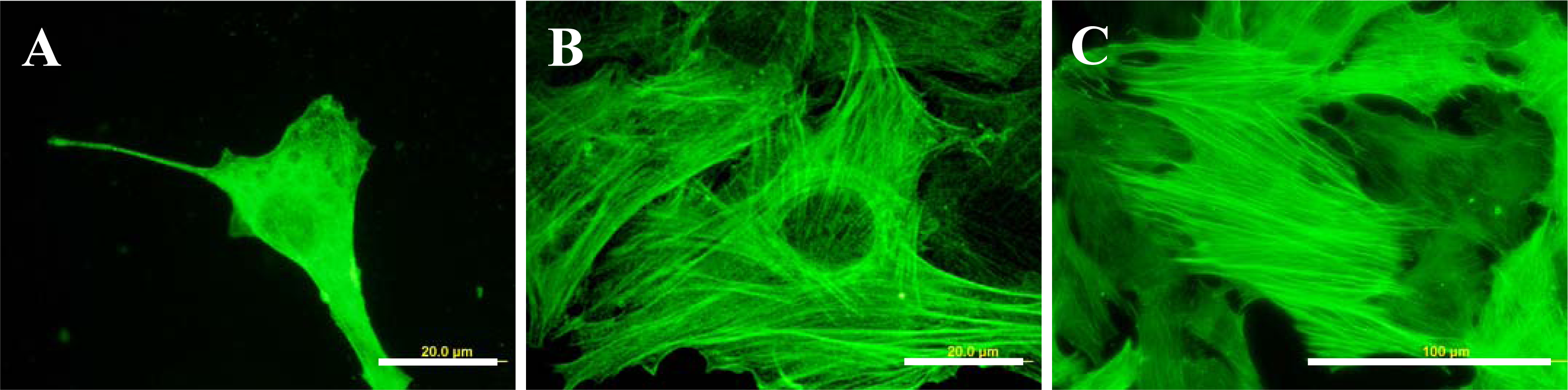
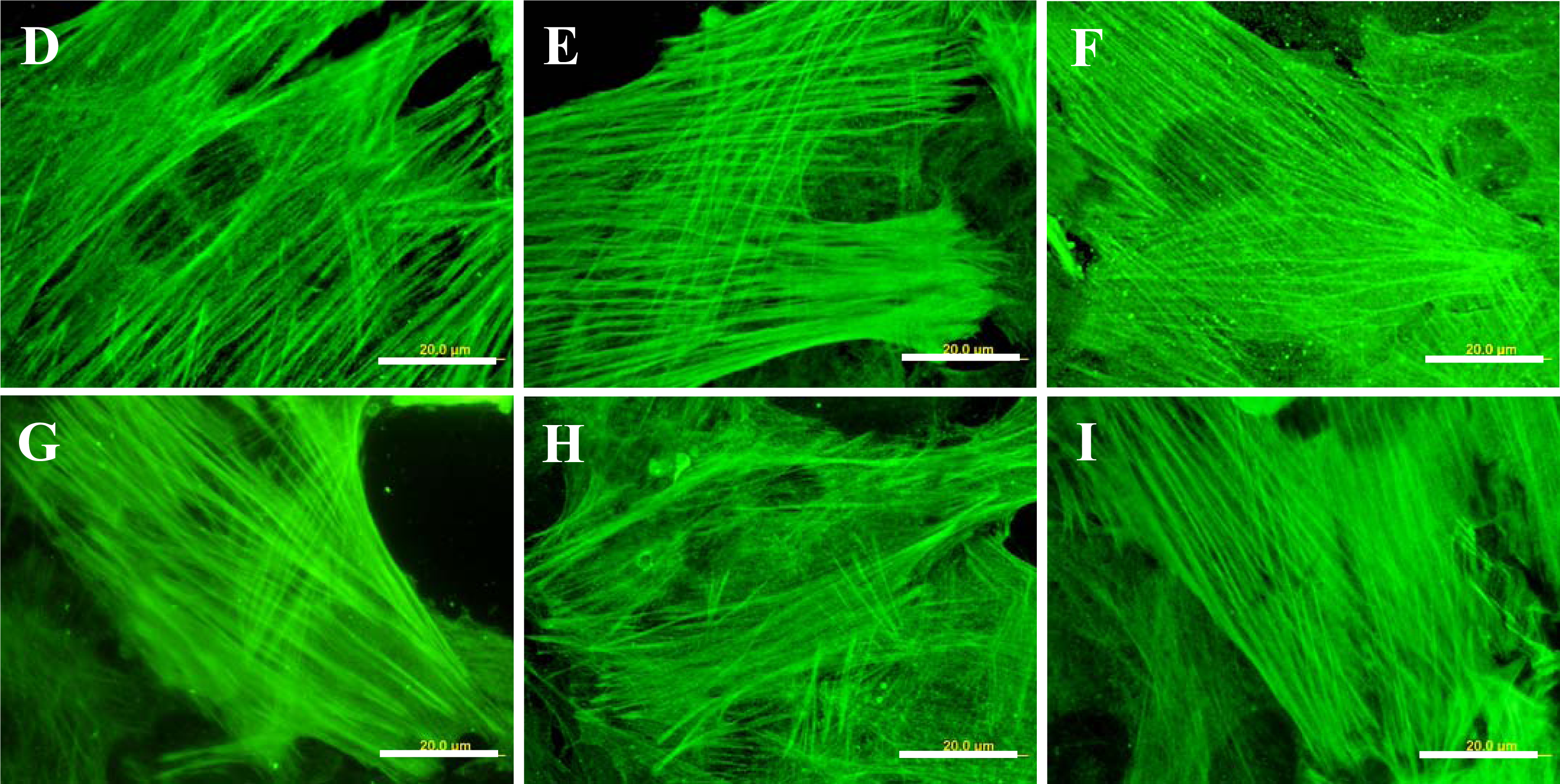
2.5. Distribution and Concentration of Molecular Markers of Adhesion and Maturation in VSMCs
3. Experimental Section
3.1. Preparation of the Polymer Samples
3.2. Evaluation of the Physical and Chemical Properties of the Polymer Samples
3.3. Cell Source and Culture Conditions
3.4. Evaluation of the Cell Number and Morphology
3.5. Immunofluorescence Staining
3.6. Enzyme-Linked Immunosorbent Assay (ELISA)
3.7. Statistics
4. Conclusions
Acknowledgments
References and Notes
- Bacakova, L; Svorcik, V; Rybka, V; Micek, I; Hnatowicz, V; Lisa, V; Kocourek, F. Adhesion and proliferation of cultured human vascular smooth muscle cells on polystyrene implanted with N+, F+ and Ar+ ions. Biomaterials 1996, 17, 1121–1126. [Google Scholar]
- Bacakova, L; Mares, V; Bottone, MG; Pellicciari, C; Lisa, V; Svorcik, V. Fluorine-ion-implanted polystyrene improves growth and viability of vascular smooth muscle cells in culture. J. Biomed. Mater. Res 2000, 49, 369–379. [Google Scholar]
- Bacakova, L; Walachova, K; Svorcik, V; Hnatowicz, V. Adhesion and proliferation of rat vascular smooth muscle cells on polyethylene implanted with O+ and C+ ions. J. Biomater. Sci.—Polym. Ed 2001, 12, 817–834. [Google Scholar]
- Bacakova, L; Filova, E; Rypacek, F; Svorcik, V; Stary, V. Cell adhesion on artificial materials for tissue engineering. Physiol. Res 2004, 53, S35–S45. [Google Scholar]
- Bacakova, L; Svorcik, V. Cell colonization control by physical and chemical modification of materials. In Cell Growth Processes: New Research; Kimura, D, Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2008; pp. 5–56. [Google Scholar]
- Heitz, J; Svorcik, V; Bacakova, L; Rockova, K; Ratajova, E; Gumpenberger, T; Bauerle, D; Dvorankova, B; Kahr, H; Graz, I; Romanin, C. Cell adhesion on polytetrafluoroethylene modified by UV-irradiation in an ammonia atmosphere. J. Biomed. Mater. Res. A 2003, 67, 130–137. [Google Scholar]
- Svorcik, V; Rockova, K; Ratajova, E; Heitz, J; Huber, N; Bäuerle, D; Bacakova, L; Dvorankova, B; Hnatowicz, V. Cell proliferation on UV-excimer lamp modified and grafted polytetrafluoroethylene. Nucl. Instr. Meth. B 2004, 217, 307–313. [Google Scholar]
- Walachova, K; Svorcik, V; Bacakova, L; Hnatowicz, V. Smooth muscle cell interaction with modified polyethylene. Biomaterials 2002, 23, 2989–2996. [Google Scholar]
- Turos, A; Jagielski, J; Piatkowska, A; Bielinski, D; Slusarski, L; Madi, NK. Ion beam modification of surface properties of polyethylene. Vacuum 2003, 70, 201–206. [Google Scholar]
- Wang, Y; Lu, L; Zhang, Y; Chen, X. Improvement in hydrophilicity of PHBV films by plasma treatment. J. Biomed. Mater. Res. A 2006, 76, 589–595. [Google Scholar]
- Kasalkova, N; Kolarova, K; Bacakova, L; Parizek, M; Svorcik, V. Cell adhesion and proliferation on modified PE. Mater Sci Forum 2007, 567–568, 269–272. [Google Scholar]
- Svorcik, V; Kasalkova, N; Slepicka, P; Zaruba, K; Bacakova, L; Parizek, M; Lisa, V; Ruml, T; Gbelcova, H; Rimpelova, S; Mackova, A. Cytocompatibility of Ar+ plasma-treated and Au nanoparticle-grafted PE. Nucl. Instr. Meth. B 2009, 267, 1904–1910. [Google Scholar]
- Mikulikova, R; Moritz, S; Gumpenberger, T; Olbrich, M; Romanin, C; Bacakova, L; Svorcik, V; Heitz, J. Cell microarrays on photochemically modified polytetrafluoroethylene. Biomaterials 2005, 26/27, 5572–5580. [Google Scholar]
- Rockova, K; Svorcik, V; Bacakova, L; Dvorankova, B; Heitz, J. Biocompatibility of ion beam-modified and RGD-grafted polyethylene. Nucl. Instrum.Meth. B 2004, 225, 275–279. [Google Scholar]
- Kotal, V; Svorcik, V; Slepička, P; Blahova, O; Sutta, P; Hnatowicz, V. Gold coating of PET modified by argon plasma. Plasma Proc. Polym 2007, 4, 69–75. [Google Scholar]
- Svorcik, V; Kotal, V; Siegel, J; Sajdl, P; Mackova, A; Hnatowicz, V. Ablation and water etching of poly(ethylene) modified by Ar plasma. Polym. Degr. Stab 2007, 92, 1645–1651. [Google Scholar]
- Chu, PK; Chen, JY; Wang, LP; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng 2002, R36, 143–206. [Google Scholar]
- Siegel, J; Reznickova, A; Chaloupka, A; Slepicka, P; Svorcik, V. Ablation and water etching of plasma treated polymers. Rad. Eff. Def. Sol 2008, 163, 779–785. [Google Scholar]
- Švorčík, V; Rybka, V; Stibor, I; Hnatowicz, V; Vacik, J; Stopka, P. Synthesis of grafted polyethylene by ion beam modification. Polym. Deg. Stab 1997, 58, 143–147. [Google Scholar]
- Švorčík, V; Hnatowicz, V; Stopka, P; Bačáková, L; Heitz, J; Ochsner, R; Ryssel, H. Aminoacids grafting of Ar ions modified PE. Rad. Phys. Chem 2001, 60, 89–94. [Google Scholar]
- Svorcik, V; Kolarova, K; Slepicka, P; Mackova, A; Novotna, M; Hnatowicz, V. Modification of surface properties of high and low density PE by Ar plasma discharge. Polym. Degr. Stab 2006, 91, 1219–1225. [Google Scholar]
- Orlandi, A; Ropraz, P; Gabbiani, G. Proliferative activity and alpha-smooth muscle actin expression in cultured rat aortic smooth muscle cells are differently modulated by transforming growth factor-beta 1 and heparin. Exp. Cell. Res 1994, 214, 528–536. [Google Scholar]
- Webster, TJ; Ergun, C; Doremus, RH; Siegel, RW; Bizios, R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J. Biomed. Mater. Res 2000, 51, 475–483. [Google Scholar]
- .
- Bacakova, L; Filova, E; Kubies, D; Machova, L; Proks, V; Malinova, V; Lisa, V; Rypacek, F. Adhesion and growth of vascular smooth muscle cells in cultures on bioactive RGD peptide-carrying polylactides. J. Mater. Sci. Mater. Med 2007, 18, 1317–1323. [Google Scholar]
- Filova, E; Bacakova, L; Lisa, V; Kubies, D; Machova, L; Lapcikova, M; Rypacek, F. Adhesion and proliferation of vascular smooth muscle cells on polylactide-polyethylene oxide copolymers with different content and length of polyethylene oxide chains. Eng. Biomater 2004, 7, 19–21. [Google Scholar]
- Horbett, TA. Principles underlying the role of adsorbed plasma proteins in blood interactions with foreign materials. Cardiovasc. Pathol 1993, 2, 137S–148S. [Google Scholar]
- Koenig, AL; Gambillara, V; Grainger, DW. Correlating fibronectin adsorption with endothelial cell adhesion and signaling on polymer substrates. J. Biomed. Mater. Res. A 2003, 64, 20–37. [Google Scholar]
- Koblinski, JE; Wu, M; Demeler, B; Jacob, K; Kleinman, HK. Matrix cell adhesion activation by non-adhesion proteins. J. Cell Sci 2005, 118, 2965–2974. [Google Scholar]
- Pignataro, B; Conte, E; Scandurra, A; Marletta, G. Improved cell adhesion to ion beam-irradiated polymer surfaces. Biomaterials 1997, 18, 1461–1470. [Google Scholar]
- Kubova, O; Svorcik, V; Heitz, J; Moritz, S; Romanin, C; Matejka, P; Mackova, A. Characterization and cytocompatibility of carbon layers prepared by photo-induced chemical vapor deposition. Thin Solid Films 2007, 515, 6765–6772. [Google Scholar]
- Bacakova, L; Grausova, L; Vacik, J; Fraczek, A; Blazewicz, S; Kromka, A; Vanecek, M; Svorcik, V. Improved adhesion and growth of human osteoblast-like MG 63 cells on biomaterials modified with carbon nanoparticles. Diamond Relat. Mater 2007, 16, 2133–2140. [Google Scholar]
- Lesny, P; Pradny, M; Jendelova, P; Michalek, J; Vacik, J; Sykova, E. Macroporous hydrogels based on 2-hydroxyethyl methacrylate. Part 4: Growth of rat bone marrow stromal cells in three-dimensional hydrogels with positive and negative surface charges and in polyelectrolyte complexes. J. Mater. Sci. Mater. Med 2006, 17, 829–833. [Google Scholar]
- Han, M; Wen, JK; Zheng, B; Cheng, Y; Zhang, C. Serum deprivation results in redifferentiation of human umbilical vascular smooth muscle cells. Am. J. Physiol. Cell. Physiol 2006, J291, C50–C58. [Google Scholar]
- Ezzell, RM; Goldmann, WH; Wang, N; Parashurama, N; Ingber, DE. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp. Cell Res 1997, 231, 14–26. [Google Scholar]
- Sawada, Y; Sheetz, MP. Force transduction by Triton cytoskeletons. J. Cell Biol 2002, 156, 609–615. [Google Scholar]
- Van Amerongen, A; Wichers, JH; Berendsen, LBJM; Timmermans, AJM; Keizer, GD; Van Doorn, AWJ; Bantjes, A; van Gelder, WMJ. Colloidal carbon particles as a new label for rapid immunochemical test methods—Quantitative computer image-analysis of results. J. Biotechnol 1993, 30, 185–195. [Google Scholar]
- Lowry, OH; Rosebrough, NJ; Farr, AL; Randall, RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem 1951, 193, 265–275. [Google Scholar]
- Filova, E; Brynda, E; Riedel, T; Bacakova, L; Chlupac, J; Lisa, V; Houska, M; Dyr, JE. Vascular endothelial cells on two- and three-dimensional fibrin assemblies for biomaterial coatings. J. Biomed. Mater. Res. A 2009, 90, 55–69. [Google Scholar]
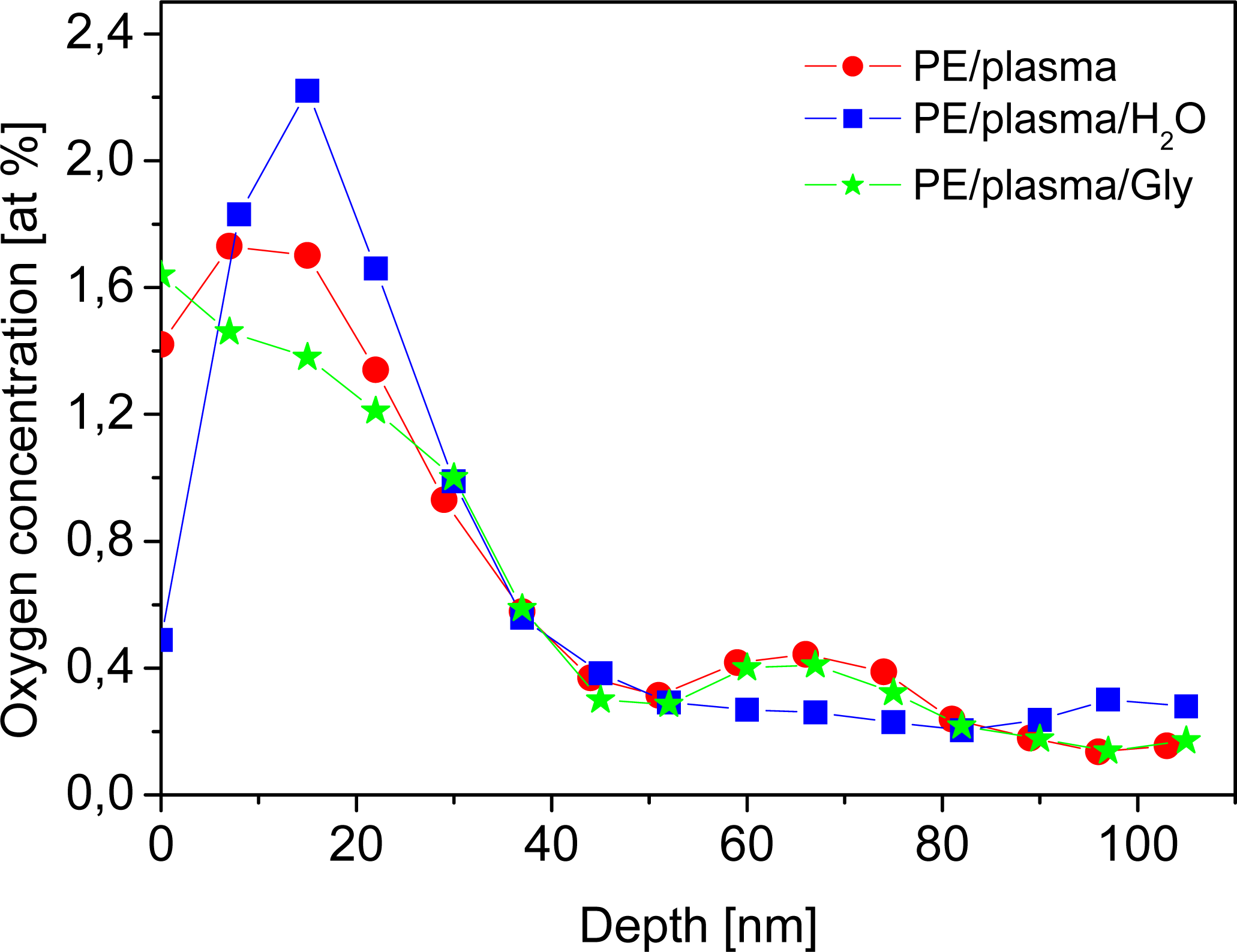
| Sample | O concentration (1016 at cm−2) |
|---|---|
| PE/plasma | 2.63 |
| PE/plasma/H2O | 1.23 |
| PE/plasma/PEG | 2.19 |
| Protein/PE modification | Talin | Vinculin | Paxillin | α-Actinin | β-Actin | α-Actin | SM1 SM2 myosins |
|---|---|---|---|---|---|---|---|
| Pristine | 100 ± 9 | 100 ± 14 | 100 ± 28 | 100 ± 15 | 100 ± 8 | 100 ± 4 | 100 ± 16 |
| Plasma | 134 ± 12 | 154 ± 17 | 98 ± 2 | 129 ± 28 | 77 ± 10 | 91 ± 10 | 69 ± 18 |
| Gly | 109 ± 12 | 140 ± 18 | 136 ± 23 | 50 ± 8$ | 68 ± 3 | 119 ± 6C, * | 78 ± 15 |
| PEG | 202 ± 16#, $ | 123 ± 8 | 152 ± 30 | 52 ± 10$ | 107 ± 8* | 123 ± 6$, C, * | 128 ± 14 |
| BSA | 127 ± 10 | 151 ± 13 | 143 ± 27 | 37 ± 9$ | 95 ± 7* | 115 ± 7 | 73 ± 17 |
| C | 112 ± 4 | 133 ± 9 | 104 ± 15 | 89 ± 8 | 111 ± 17* | 87 ± 3 | 87 ± 16 |
| BSA + C | 201 ± 14#, * | 185 ± 14#, * | 114 ± 18 | 72 ± 12 | 110 ± 12* | 111 ± 7 | 108 ± 13 |
| PS dish | 155 ± 29 | 113 ± 12 | 175 ± 37 | 93 ± 7 | 55 ± 9 | 89 ± 10 | 103 ± 16 |
| Antibody against | Developed in, type | Company, Cat. No. | Dilution | Incubation |
|---|---|---|---|---|
| Chicken talin | Mouse, monoclonal | Sigma a T 3287 | Immf.: 1:200 ELISA: 1:500 | Immf.: Overnight, 4 °C ELISA: 60 min, RT c |
| Human vinculin | Mouse, monoclonal | Sigma a V9131 | Immf.: 1:200 ELISA: 1:400 | Immf.: Overnight, 4 °C ELISA: 60 min, RT c |
| Recombinant human paxilin | Rabbit polyclonal | Chemicon b P1093 | Immf.: 1:200 ELISA: 1:400 | Immf.: Overnight, 4 °C ELISA: 60 min, RT c |
| Chicken α-actinin | Rabbit, polyclonal | Sigma a A2543 | Immf.: 1:200 ELISA: 1:500 | Immf.: Overnight, 4 °C ELISA: 60 min, RT c |
| Synthetic peptide of α-smooth muscle actin | Mouse, monoclonal | Sigma a A2547 | Immf.: 1:200 ELISA: 1:400 | Immf.: Overnight, 4 °C ELISA: 60 min, RT c |
| Synthetic peptide of β-actin | Mouse, monoclonal | Sigma a A 5441 | Immf.: 1:200 ELISA: 1:400 | Immf.: Overnight, 4 °C ELISA: 60 min, RT c |
| Human smooth muscle myosin SM1 and SM2 | Mouse, monoclonal | Sigma a M7786 | Immf.: 1:200 ELISA: 1:500 | Immf.: Overnight, 4 °C ELISA: 60 min, RT c |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Parizek, M.; Kasalkova, N.; Bacakova, L.; Slepicka, P.; Lisa, V.; Blazkova, M.; Svorcik, V. Improved Adhesion, Growth and Maturation of Vascular Smooth Muscle Cells on Polyethylene Grafted with Bioactive Molecules and Carbon Particles. Int. J. Mol. Sci. 2009, 10, 4352-4374. https://doi.org/10.3390/ijms10104352
Parizek M, Kasalkova N, Bacakova L, Slepicka P, Lisa V, Blazkova M, Svorcik V. Improved Adhesion, Growth and Maturation of Vascular Smooth Muscle Cells on Polyethylene Grafted with Bioactive Molecules and Carbon Particles. International Journal of Molecular Sciences. 2009; 10(10):4352-4374. https://doi.org/10.3390/ijms10104352
Chicago/Turabian StyleParizek, Martin, Nikola Kasalkova, Lucie Bacakova, Petr Slepicka, Vera Lisa, Martina Blazkova, and Vaclav Svorcik. 2009. "Improved Adhesion, Growth and Maturation of Vascular Smooth Muscle Cells on Polyethylene Grafted with Bioactive Molecules and Carbon Particles" International Journal of Molecular Sciences 10, no. 10: 4352-4374. https://doi.org/10.3390/ijms10104352