Self-associated, “Distillable” Ionic Media
Abstract
:Introduction
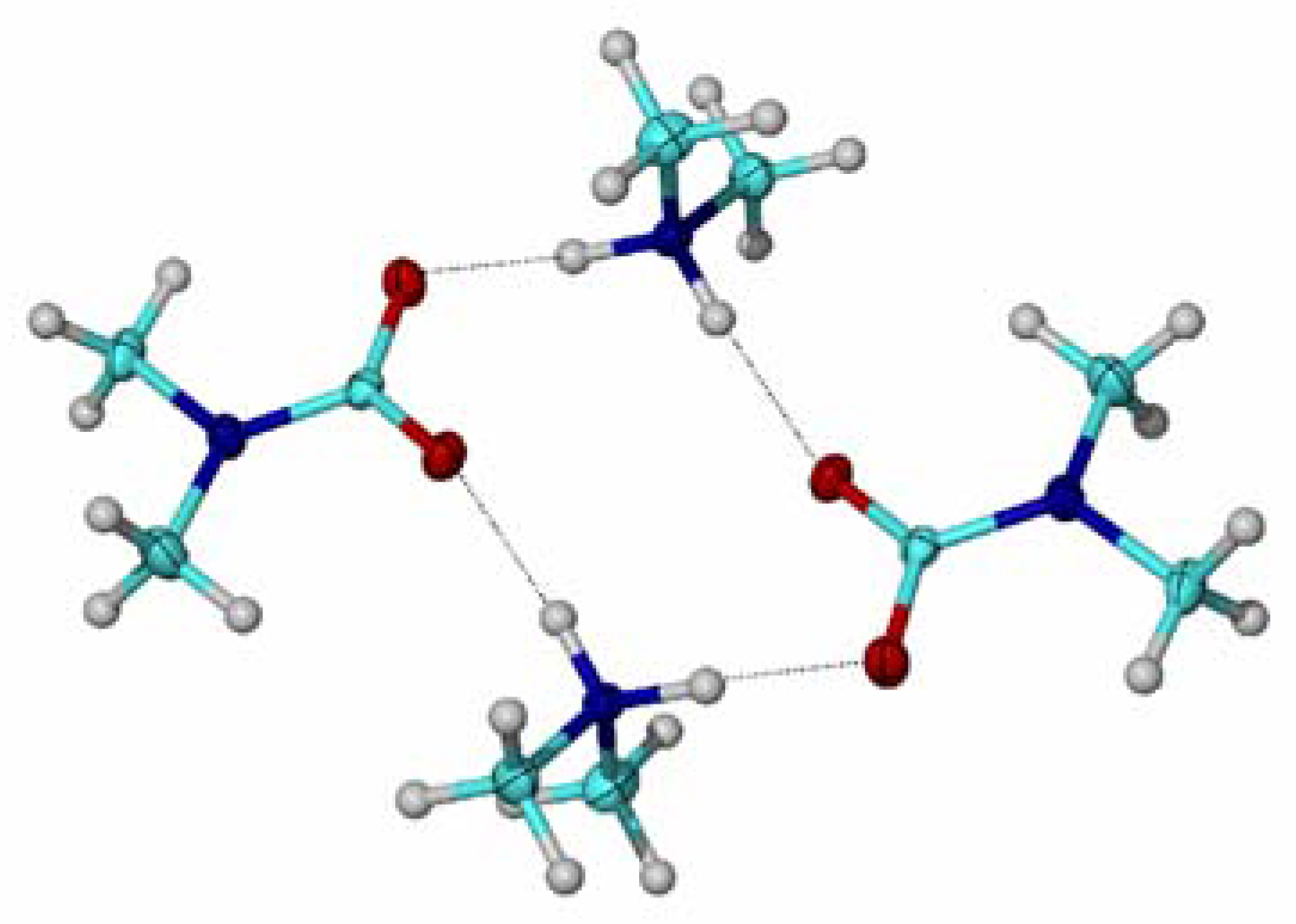
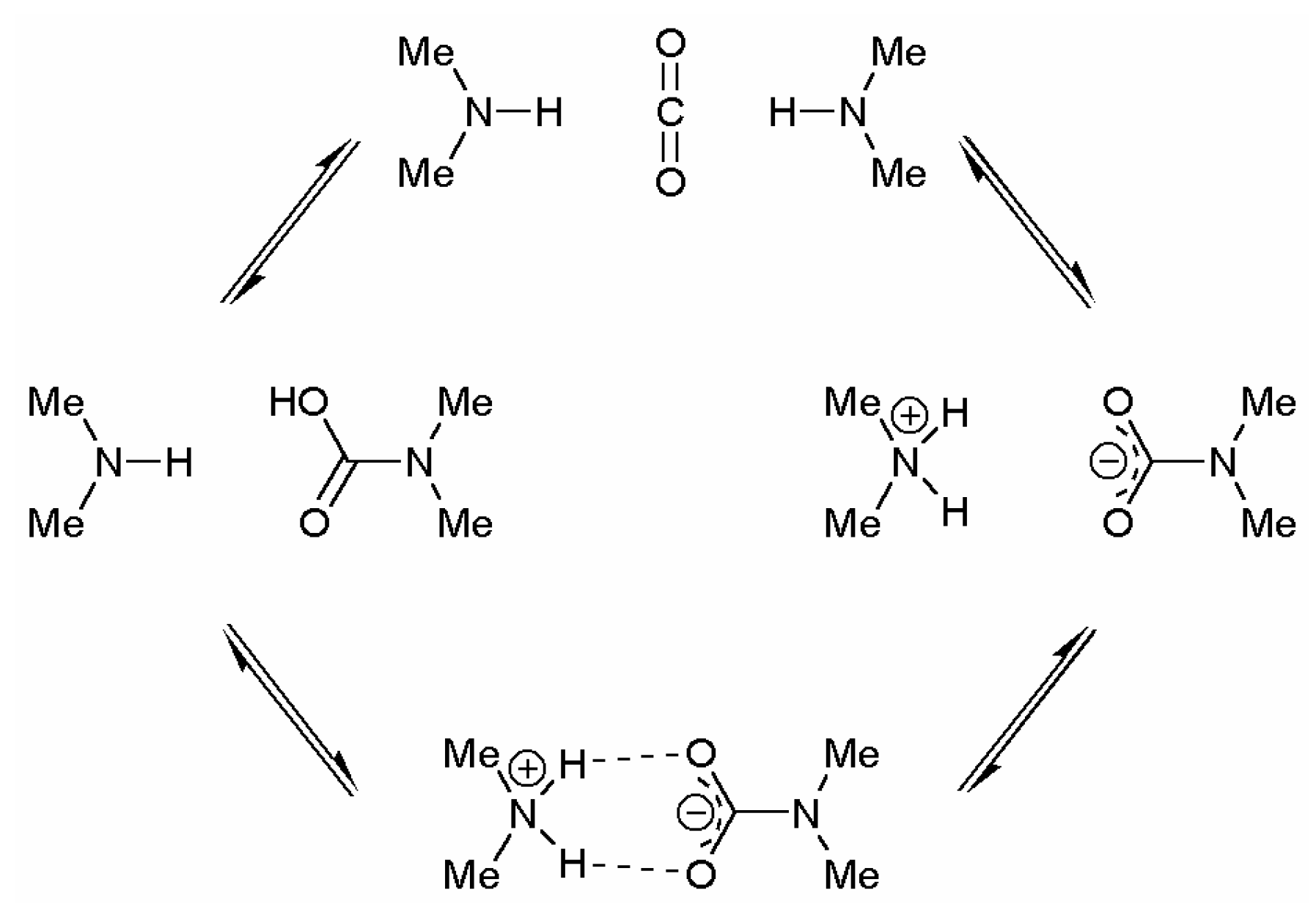
| R1 | R2 | mp range (°C) | Dissociation temperature (°C) | bp amine (°C) |
| Me | Me | 30-45 | 60-61 | 7.4 |
| Me | Et | - | 62 | 34-35 |
| Et | Et | 17-33 | 62-63 | 55 |
| Me | n-Pr | - | 70-71 | 62 |
| -(CH2)4- | - | 55-80 | 105 | 87-88 |
| -(CH2)5- | - | 48-63 | - | 106 |
| Me | H | 99-133 | - | -6.5 |
Results and Discussion

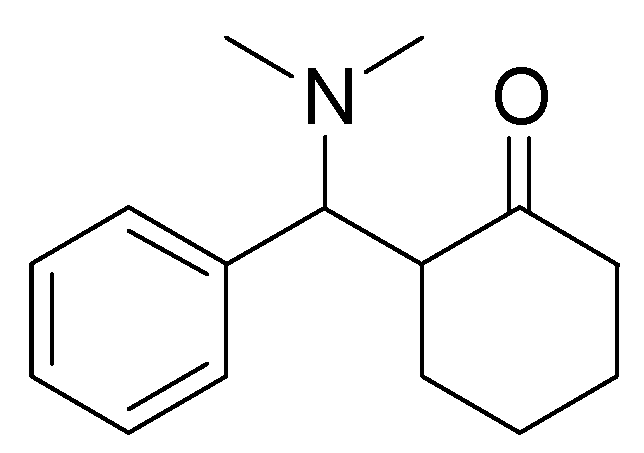
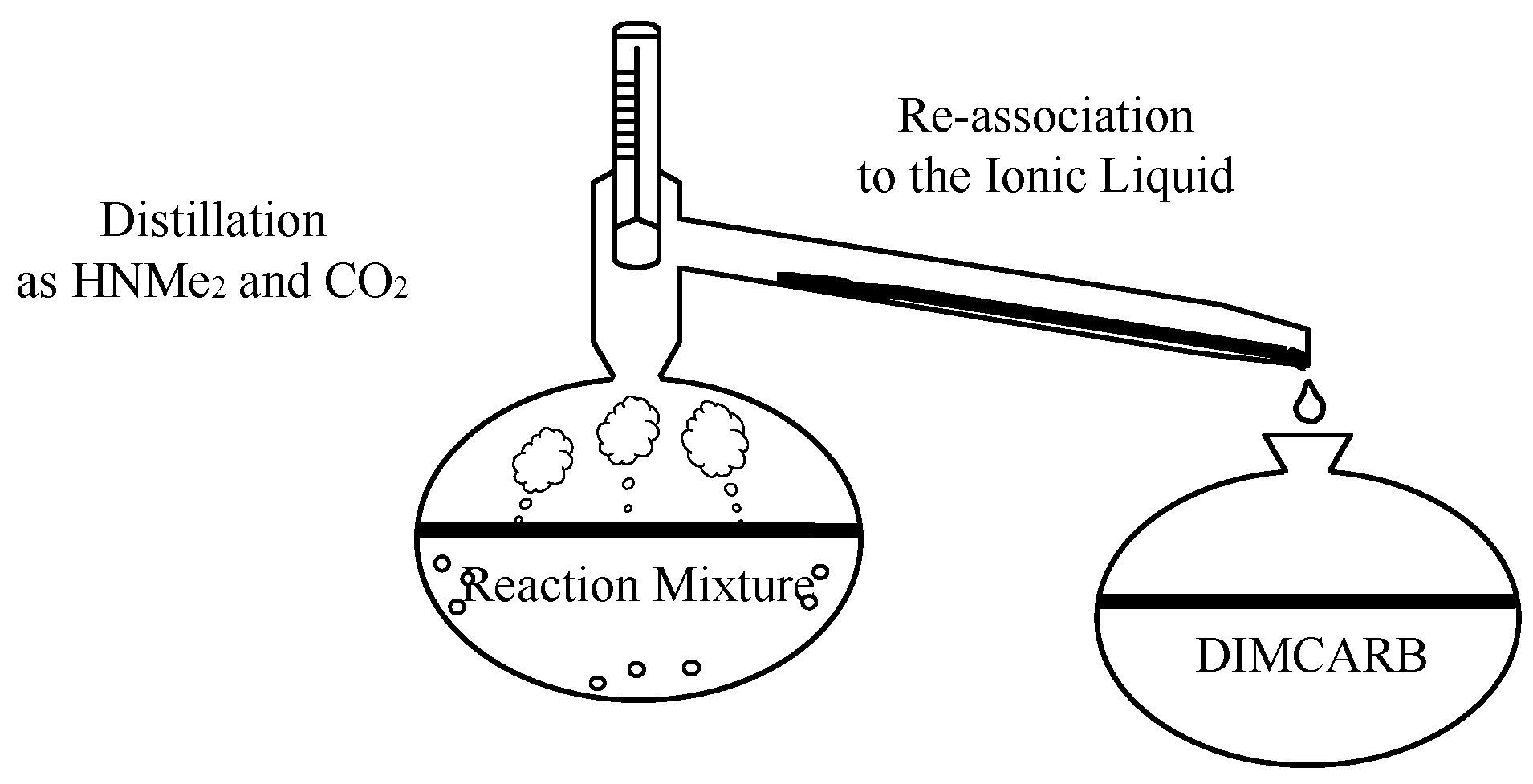
Conclusions
Experimental
General Method for Performing Aldol Condensations in DIMCARB
References and Notes
- EPA Office of Compliance Sector Notebook Project ‘Profile of the Organic Chemical Industry’ 1995, page 35 (to be found at www.epa.gov/oeca/sector).
- For reviews on reactions in water see: (a) Li, C. -J. Aqueous Barbier-Grignard type reaction: scope, mechanism, and synthetic applications. Tetrahedron 1996, 52, 5463–68. [Google Scholar] [CrossRef]; (b) Lubineau, A.; Auge, J.; Queneau, Y. Water-promoted organic reactions. Synthesis 1994, 741–60. [Google Scholar]; (c) Katritzky, A. R.; Nichols, D. A.; Siskin, M.; Murugan, R.; Balasubramanian, M. Reactions in high-temperature aqueous media. Chem. Rev. 2001, 101, 837–92. [Google Scholar]; (d) Savage, P.E. Organic Chemical Reactions in Supercritical Water. Chem. Rev. 1999, 99, 603–21s. [Google Scholar]. For examples from our lab see: (e) An, J.; Bagnell, L.; Cablewski, T.; Strauss, C.R.; Trainor, R. W. Applications of High-Temperature Aqueous Media for Synthetic Organic Reactions. J. Org. Chem. 1997, 62, 2505–11. [Google Scholar]; For reviews on solvent-free reactions see: (f) Tanaka, K.; Toda, F. Solvent-Free Organic Synthesis. Chem. Rev. 2000, 100, 1025–74. [Google Scholar]; (g) Loupy, A.; Petit, A.; Hamelin, J.; Texier-Boullet, F.; Jacquault, P.; Mathe, D. New solvent-free organic synthesis using focused microwaves. Synthesis 1998, 1213–34. [Google Scholar]; For examples from our lab see: (h) Cave, G. W. V.; Raston, C. L.; Scott, J. L. Recent advances in solventless organic reactions: towards benign synthesis with remarkable versatility. Chem. Commun. 2001, 2159–69. [Google Scholar]; (i) Kreher, U.; Strauss, C. R.; Walther, D. 5th International Electronic Conference on Synthetic Organic Chemistry ECSOC-5); 2001. http://www.mdpi.net/ecsoc-5.; For reviews on applications of supercritical carbon dioxide as solvent in chemical synthesis see: (j) Oakes, R. S; Clifford, A. A.; Rayner, C. M. The use of supercritical fluids in synthetic organic chemistry. J. Chem. Soc., Perkin Trans. 1 2001, 917–41. [Google Scholar]; (k) Baiker, A. Supercritical Fluids in Heterogeneous Catalysis. Chem. Rev. 1999, 99, 453–73. [Google Scholar].
- For reviews on ionic liquids and their applications see: (a) Wasserscheid, P.; Keim, W. Ionic liquids - new "solutions" for transition metal catalysis. Angew. Chem Int. Ed. 2000, 39, 3772–89. [Google Scholar] [CrossRef]; (b) Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–83. [Google Scholar].
- Fadeev, A. G.; Meagher, M. M. Opportunities for ionic liquids in recovery of biofuels. Chem. Commun. 2001, 295–6. [Google Scholar] [CrossRef]
- Bates, E. D.; Mayton, R. D.; Ntai, I.; Davis, J. H. CO2 Capture by a Task-Specific Ionic Liquid. J. Am. Chem. Soc. 2002, 124, 926–7. [Google Scholar] [CrossRef] [PubMed]
- Schroth, W.; Andersch, J.; Schändler, H.-D.; Spitzner, R. The dimethylamine-carbon dioxide complex DIMCARB and its preparative use. Chemiker-Z. 1989, 113, 261–71. [Google Scholar]
- Wilkes, J. S. A short history of ionic liquids—from molten salts to neoteric solvents. Green Chem. 2002, 4, 73. [Google Scholar] [CrossRef]
- Schroth, W.; Schädler, H.-D.; Andersch, J. Structure and aggregation of dimethylammonium dimethylcarbamate (DIMCARB) and analogous dialkylammonium dialkylcarbamates. Z. Chem. 1989, 29, 129–35. [Google Scholar] [CrossRef]
- Hess, U.; Dunkel, S.; Reck, G. Synthesis of cyano-substituted di- and tetrahydropyridines in DIMCARB (dimethylamine CO2 adduct). J. Prakt. Chem. 1997, 339, 414–19. [Google Scholar]
- Kreher, U.; Raston, C. L.; Strauss, C. R.; Nichols, P. N,N-Dimethylammonium N',N'- dimethylcarbamate. Acta Cryst. 2002, E58, 948–9. [Google Scholar]
- Schroth, W.; Schädler, H.-D.; Andersch, J. Z. Chem., Dimethylammonium dimethylcarbamate (DIMCARB) as solvent and extractant. 1989, 29, 56–7. [Google Scholar]
- CRC Handbook of Chemistry and PhysiscsWeast, R. C.; Astle, M. J. (Eds.) CRC Press, Inc.: Boca Raton, Florida, 1982, 63rd ed.; table B73-166.
- Maschmeier, C.-P.; Krahnstöver, J.; Matschiner, H.; Hess, U. Electrochemical study of a dimethylamine-carbon dioxide addition compound - a new electrolyte. Electrochimica Acta 1990, 35, 769–70. [Google Scholar] [CrossRef]
- Radeglia, R.; Andersch, J.; Schroth, W. The dynamic behavior of the dimethylamine-carbon dioxide complex (DIMCARB). Z. Naturforsch.B: Chem. Sci. 1989, 44, 181–6. [Google Scholar]
- Results and Discussion are primarily taken from and can be viewed in this journal article: Kreher, U. P.; Rosamilia, A. E.; Raston, C. L.; Scott, J. L.; Strauss, C. R. Direct Preparation of Monoarylidene Derivatives of Aldehydes and Enolizable Ketones with DIMCARB. Org. Lett. 2003, 5, 3107–10. [Google Scholar] [CrossRef] [PubMed]. Presented in preliminary form as an oral presentation, Rosamilia, A. E. A Distillable Ionic Liquid and its Application in Organic Synthesis, at the Royal Australian Chemical Institute’s 27th Annual Synthesis Symposium. Melbourne, 6–12–2002..
- Zheng, M.; Wang, L.; Shao, J.; Zhong, Q. A facile synthesis of α,α’-bis(substituted benzylidene)cycloalkanones catalysed by bis(p-ethoxyphenyl)telluroxide(BMPTO) under microwave irradiation. Synth. Commun. 1997, 27, 351. [Google Scholar]
2004 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kreher, U.P.; Rosamilia, A.E.; Raston, C.L.; Scott, J.L.; Strauss, C.R. Self-associated, “Distillable” Ionic Media. Molecules 2004, 9, 387-393. https://doi.org/10.3390/90600387
Kreher UP, Rosamilia AE, Raston CL, Scott JL, Strauss CR. Self-associated, “Distillable” Ionic Media. Molecules. 2004; 9(6):387-393. https://doi.org/10.3390/90600387
Chicago/Turabian StyleKreher, Ulf P., Anthony E. Rosamilia, Colin L. Raston, Janet L. Scott, and Christopher R. Strauss. 2004. "Self-associated, “Distillable” Ionic Media" Molecules 9, no. 6: 387-393. https://doi.org/10.3390/90600387
APA StyleKreher, U. P., Rosamilia, A. E., Raston, C. L., Scott, J. L., & Strauss, C. R. (2004). Self-associated, “Distillable” Ionic Media. Molecules, 9(6), 387-393. https://doi.org/10.3390/90600387




