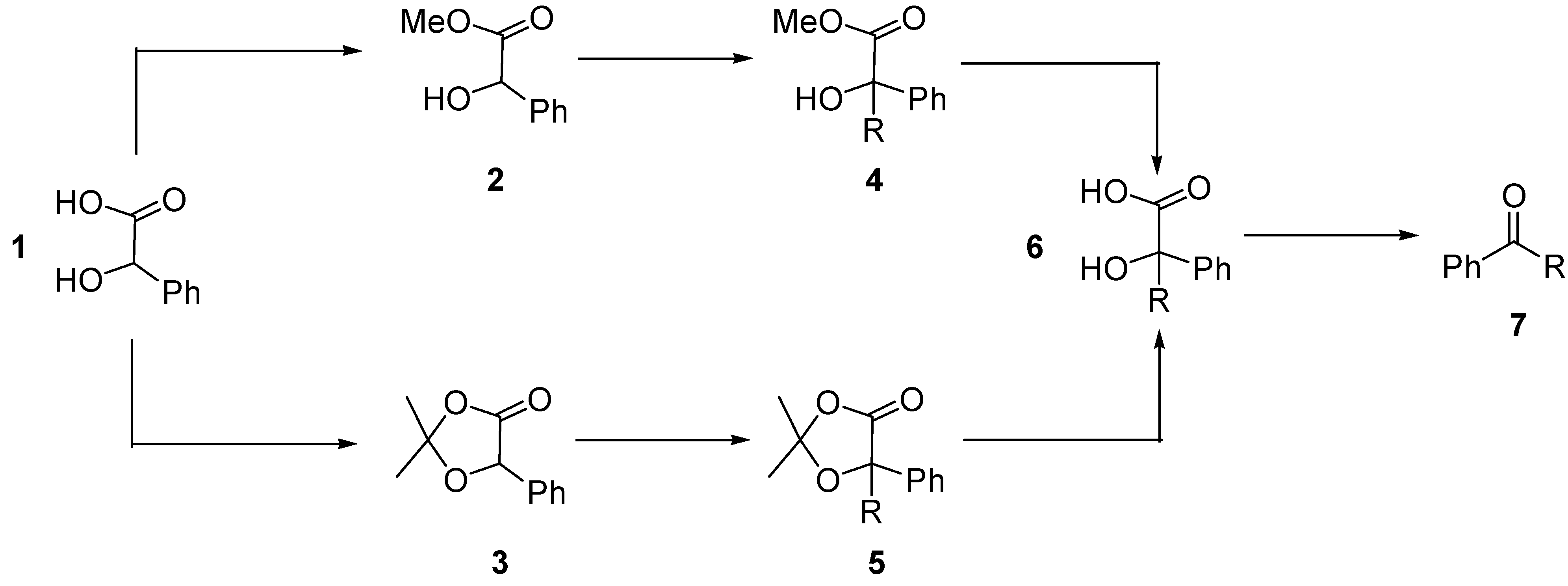
General procedure for alkylation of 2,2-dimethyl-5-phenyl-1,3-dioxolan-4-one (3)
A 1.6 M solution of n-BuLi in hexane (0.78 mL, 1.25 mmol) was added dropwise to a solution of diisopropylamine (0.175 mL, 1.25 mmol) in dry THF (0.8 mL) at 0 oC under argon. After 30 min, the solution was cooled to –78 oC and a solution of dioxolanone 3 (192 mg, 1 mmol) in THF (0.65 mL) was added. The resulting solution was stirred at –78 oC for 30 min and then the corresponding alkyl halide (1.25 mmol) in THF (0.39 mL) was added. The reaction mixture was stirred at this temperature for 2 hours and then at room temperature until the reaction was complete (2 hours). After this time, the reaction mixture was poured into aq. NH4Cl (30 mL), extracted with ether (3 x 30 mL), washed with brine and dried over MgSO4. After filtration and removal of the solvent under reduced pressure, the products 5 were obtained by column chromatography (elution with hexane-diethyl ether).
2,2,5-Trimethyl-5-phenyl-1,3-dioxolan-4-one (5a). An oil; 1H-NMR δ 7.54 (2H, dd, J = 8.1, 1.2 Hz), 7.4-7-2 (3H, m), 1.67 (3H, s), 1.61 (3H, s), 1.37 (3H, s); 13C-NMR δ 172.7 (s), 139.8 (s), 127.4 (d), 127.0 (d), 123.5 (d), 109.1 (s), 79.5 (s), 28.2 (q), 27.4 (q), 26.6 (q); MS (EI) m/z 206 (M+, 6.7), 191 (7.8), 162 (83.5), 121 (36.1), 104 (100), 77 (31.8); HRMS (EI) m/z required for C12H14O3 206.0943, found 206.0949.
5-Dodecyl-2,2-dimethyl-5-phenyl-1,3-dioxolan-4-one (5b). An oil; 1H-NMR δ 7.57 (2H, dd, J = 8.1, 1.2 Hz), 7.4-7.2 (3H, m), 1.89 (2H, m), 1.64 (3H, s), 1.36 (3H, s), 1.20 (20H, m), 0.83 (3H, t, J = 7.0 Hz); 13C-NMR δ 173.3 (s), 140.2 (s), 128.3 (d), 127.8 (d), 124.8 (d), 110.0 (s), 83.6 (s), 41.9 (t), 31.9 (t), 29.59 (t, two overlapped signals), 29.56 (t), 29.48 (t), 29.31 (t), 29.29 (t, two overlapped signals), 27.8 (q), 24.0 (t), 22.7 (t), 14.1 (q); MS (EI) m/z 360 (M+, 24.6), 316 (47.9), 275 (28.4), 191 (61.9), 163 (98.7), 105 (100), 77 (33.5); HRMS (EI) m/z required for C23H36O3 360.2664, found 360.2650.
2,2-Dimethyl-5-(3,7-dimethyl-6-octenyl)-5-phenyl-1,3-dioxolan-4-one (5c). An oil; 1H-NMR δ 7.56 (2H, dd, J = 8.1, 1.2 Hz), 7.4.7.2 (3H, m), 5.00 (1H, m), 2.0-1.8 (4H, m), 1.64 (3H, s), 1.61 (3H, s), 1.52 (3H, s), 1.36 (3H, s), 1.35-1.10 (5H, m), 0.79 (3H, t, J = 6.4 Hz); 13C-NMR δ 173.3 (s), 140.2 (s), 131.2 (s), 128.3 (d), 127.8 (d), 124.8 (d), 124.7 (d), 110.0 (s), 83.6 (s), 39.4 (t), 36.7 (t), 32.1 (d), 30.8 (t), 27.8 (q), 25.7 (q), 25.3 (t), 19.4 (q), 19.3 (q), 17.6 (q); MS (CI) m/z 331 (M++1, 0.5), 301 (8.1), 273 (100), 245 (15.9), 227 (32.0), 189 (17.7), 137 (13.1), 107 (8.7); HRMS (CI) m/z required for C21H31O3 331.2273, found 331.2263(M++1).
5-Benzyl-2,2-dimethyl-5-phenyl-1,3-dioxolan-4-one (5d). M.p. 47-49 oC (from CH2Cl2); 1H-NMR δ 7.63 (2H, dd, J = 8.1, 1.2 Hz), 7.35-7.22 (3H, m), 7.20-7.10 (5H, m), 3.31 (1H, d, J = 13.9 Hz), 2.98 (1H, d, J = 13.9 Hz), 1.27 (3H, s), 1.01 (3H, s); 13C-NMR δ 172.5 (s), 140.0 (s), 135.0 (s), 131.0 (d), 128.4 (d), 128.0 (d), 127.1 (d), 124.8 (d), 110.4 (s), 84.3 (s), 47.7 (t), 27.9 (q), 27.0 (q); MS (EI) m/z 282 (M+, 6.4), 191 (96.8), 178 (16.6), 163 (23.1), 105 (100), 77 (45.9); HRMS (EI) m/z required for C18H18O3 282.1256, found 282.1243.
5-(p-Bromobenzyl)-2,2-dimethyl-5-phenyl-1,3-dioxolan-4-one (5e). M.p. 65-66 oC (from CH2Cl2); 1H-NMR δ 7.65 (2H, dd, J = 8.1, 1.2 Hz), 7.40-7.30 (5H, m), 7.05 (2H, d, J = 8.4 Hz), 3.29 (1H, d, J = 13.9 Hz), 3.02 (1H, d, J = 13.9 Hz), 1.34 (3H, s), 1.19 (3H, s); 13C-NMR δ 172.2 (s), 139.6 (s), 133.9 (s), 132.7 (d), 131.1 (d), 128.5 (d), 128.2 (d), 124.8 (d), 121.3 (s), 110.5 (s), 83.9 (s), 47.1 (t), 27.8 (q), 27.2 (q); MS (EI) m/z 362/360 (M+, 2.0/1.8), 277/275 (7.0/7.4), 191 (100), 178 (25.9), 163 (46.1), 105 (47.8), 77 (99.0); HRMS (EI) m/z required for C18H17O3Br 362.0341 / 360.0361, found 362.0382 / 360.0360.
5-Allyl-2,2-dimethyl-5-phenyl-1,3-dioxolan-4-one (5f). An oil; 1H-NMR δ 7.62 (2H, dd, J = 8.1, 1.2 Hz), 7.40-7.25 (3H, m), 5.72 (1H, m), 5.15 (1H, brd, J = 9.9 Hz), 5.13 (1H, brd, J = 17.4 Hz), 2.70 (2H, m), 1.66 (3H, s), 1.40 (3H, s); 13C-NMR δ 172.5 (s), 139.5 (s), 131.3 (d), 128.4 (d), 128.0 (d), 124.8 (d), 120.3 (t), 110.2 (s), 83.3 (s), 45.9 (t), 27.8 (q), 27.7 (q); MS (CI) m/z 233 (M++1, 0.8), 215 (2.3), 203 (5.0), 191 (4.5), 175 (100), 157 (7.9), 147 (12.0), 131(30.6) 129 (26.2), 105 (27.5); HRMS (CI) m/z required for C14H17O3 233.1178, found 233.1171 (M++1).
5-(2-Bromoallyl)-2,2-dimethyl-5-phenyl-1,3-dioxolan-4-one (5g). An oil; 1H-NMR δ 7.58 (2H, dd, J = 8.1, 1.2 Hz), 7.30-7.24 (3H, m), 5.61 (1H, s), 5.55 (1H, s), 3.14 (1H d, J = 15.1 Hz), 2.93 (1H, d, J = 15.1 Hz), 1.67 (3H, s), 1.36 (3H, s); 13C-NMR δ 172.0 (s), 139.0 (s), 128.5 (d), 128.4 (d), 125.2 (s), 124.9 (d), 122.9 (t), 110.9 (s), 83.1 (s), 51.6 (t), 27.9 (q), 27.6 (q); MS (CI) m/z 283/281 (M+- C2H5, 3.5/3.9), 255/253 (65.8/70.1), 237/235 (26.7/27.8), 227/225 (22.8/24.0), 191 (64.3), 173 (75.7), 129 (63.8), 105 (100); HRMS (CI) m/z required for C12H10O3Br 2872.9793 / 280.9813, found 282.9757 / 280.9824,.
2,2-Dimethyl-5-(methoxycarbonylmethyl)-5-phenyl-1,3-dioxolan-4-one (5h). M.p. 105-107 oC (from CH2Cl2); 1H-NMR δ 7.62 (2H, dd, J = 8.1, 1.2 Hz), 7.39-7.27 (3H, m), 3.69 (3H, s), 3.10 (1H, d, J = 16.8 Hz), 2.89 (1H, d, J = 16.8 Hz), 1.66 (3H, s), 1.35 (3H, s); 13C-NMR δ 171.9 (s), 169.0 (s), 138.9 (s), 128.6 (d), 128.4 (d), 124.7 (d), 110.8 (s), 80.6 (s), 51.9 (q), 45.5 (t), 27.8 (q), 26.7 (q); MS (EI) m/z 264 (M+, 4.4), 220 (31.8), 205 (8.3), 179 (25.6), 133 (21.5), 105 (100), 77 (49.5); HRMS (EI) m/z required for C14H16O5 264.0998, found 264.1005.
General procedure for hydrolysis of α-alkylated dioxolanones 5
The α-alkylated dioxolanones
5 (0.3 mmol) were treated with 5% ethanolic KOH (0.75 mL, 0.6 mmol) at room temperature until complete reaction of the starting material (as indicated by TLC). The solution was poured into ice and acidified with 1M HCl to pH ~ 2. The aqueous mixture was extracted with EtOAc (3 x 20 mL), the organic layers were washed with brine until neutral, dried, filtered and concentrated under reduced pressure to give the α–alkylated mandelic acids
6 in almost quantitative yield. For characterisation of compounds
6a, 6b,
6d and
6f-6h see ref [
1].
2-Hydroxy-5,9-dimethyl-2-phenyl-8-decenoic acid (6c). M.p. 78-80 oC (from ethyl acetate); 1H-NMR δ 7.61 (2H, d, J = 7.0 Hz), 7.40-7.20 (3H, m), 5.06 (1H, m), 2.3-1.8 (4H, m), 1.67 (3H, s), 1.57 (3H, s), 1.45-1.10 (5H, m), 0.87 (3H, t, J = 6.2 Hz); 13C-NMR δ 180.6 (s), 141.0 (s), 131.1 (s), 128.3 (d), 128.0 (d), 125.5 (d), 124.7 (d), 78.4 (s), 37.1 (t), 36.7 (t), 32.3 (d), 30.3 (t), 25.7 (q), 25.4 (t), 19.4 (q), 17.6 (q); MS (EI) m/z 290 (M+, 0.8), 272 (49.4), 245 (22.2), 133 (22.3); 110 (77.5), 105 (100), 69 (64.3); HRMS (EI) m/z required for C18H26O3 290.1882, found 290.1872.
3-(p-Bromophenyl)-2-hydroxy-2-phenylpropanoic acid (6e). M.p. 210-212 oC (from CH2Cl2); 1H-NMR (DMSO-d6) δ 7.62 (2H, d, J = 7.2 Hz), 7.40-7.25 (5H, m), 7.16 (2H, d, J = 7.2 Hz) 3.44 (1H, d, J = 13.6 Hz), 3.19 (1H, d, J = 13.6 Hz); 13C-NMR (DMSO-d6) δ 175.0 (s), 142.4 (s), 136.2 (s), 132.7 (d), 130.1 (d), 127.7 (d), 127.1 (d), 125.6 (d), 119.4 (s), 77.8 (s), 44.2 (t); MS (EI) m/z 304/302 (M+- H2O, 4.0/3.8), 178 (9.3), 171 (12.3), 169 (13.0), 149 (16.5), 105 (100), 77 (28.8); HRMS (EI) m/z required for C15H11BrO2 303.9922 / 301.9942, found 303.9919 / 301.9937.
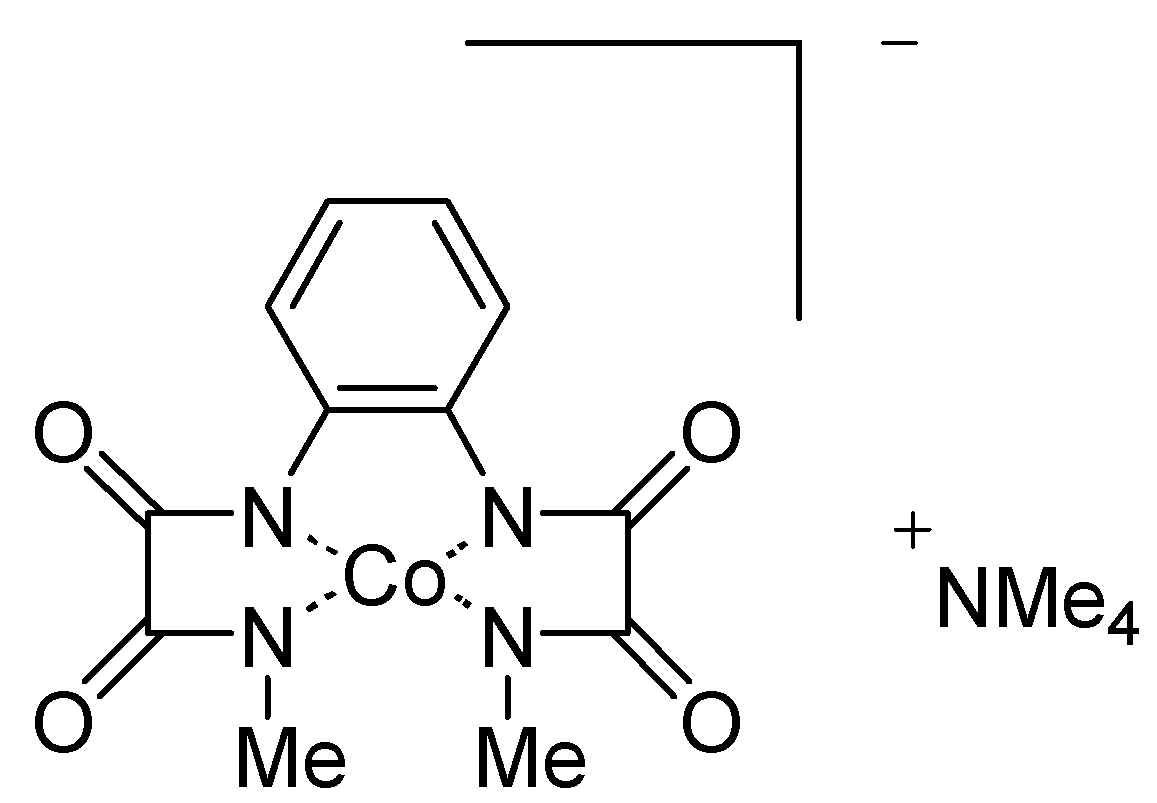
General procedure for catalytic aerobic decarboxylation of α–hydroxyacids 6
Co (III)-Me
2opba complex (3.3 mg, 7.7 x 10
-3 mmol) and pivalaldehyde (46 μL, 0.39 mmol) were added to a stirred solution of alkylated α-hydroxyacids
6 (0.13 mmol) in acetonitrile (0.5 mL) under a dioxygen atmosphere. The mixture was stirred at the indicated temperature until consumption of the starting α-hydroxyacid, as indicated by TLC. The reaction products
7 were purified by flash chromatography. For characterisation of compounds
7a, 7b,
7d and
7f-7h see ref [
1].
3,7-Dimethyl-6-octenyl phenyl ketone (7c). An oil; 1H-NMR δ 7.94 (2H, dd, J = 8.1 and 1.2 Hz), 7.53 (1H, tt, J = 8.1 and 1.2 Hz) 7.43 (2H, td, J = 8.1 and 1.2 Hz), 5.08 (1H, m), 2.94 (2H, m), 1.98 (2H, m), 1.75 (2H, m), 1.66 (3H, s), 1.58 (3H, s), 1.50 (2H, m), 1.35 (1H, m), 0.92 (3H, d, J = 6.2 Hz); 13C-NMR δ 200.8 (s), 137.0 (s), 132.8 (d), 131.2 (s), 128.5 (d), 128.0 (d), 124.7 (d), 36.9 (t), 36.3 (t), 32.2 (d), 31.3 (t), 25.7 (q), 25.5 (t), 19.4 (q), 17.6 (q); MS (EI) m/z 244 (M+, 58.8), 201 (6.9), 173 (18.5), 133 (78.9); 122 (77.5), 120 (71.4); 105 (100), 77 (46.6); HRMS (EI) m/z required for C17H24O 244.1827, found 244.1829.
p-Bromobenzyl phenyl ketone (7e). M.p. 136-138 oC (from CH2Cl2); 1H-NMR δ 8.00 (2H, dd, J = 8.1, 1.2 Hz), 7.65-7.40 (5H, m), 7.15 (2H, d, J = 8.4 Hz), 4.24 (2H, s); 13C-NMR δ 197.2 (s), 136.6 (s), 135.3 (s), 133.6 (d), 132.0 (d), 131.5 (d), 129.0 (d), 128.8 (d), 121.2 (s), 45.0 (t); MS (EI) m/z 276/274 (M+, 9.3/9.8), 185/183 (15.8/15.0), 171 (9.7), 165 (5.0), 157 (5.8), 105 (73.8), 90 (22.4), 77 (100); HRMS (EI) m/z required for C14H11OBr 275.9973 / 273.9993, found 275.9963 / 273.9982.