Highly Efficient, and Fast Solid State Deprotection of 1,3-Dithianes and 1,3-Dithiolanes using Mercury(II) Nitrate Trihydrate
Abstract
:Introduction
Results and Discussion
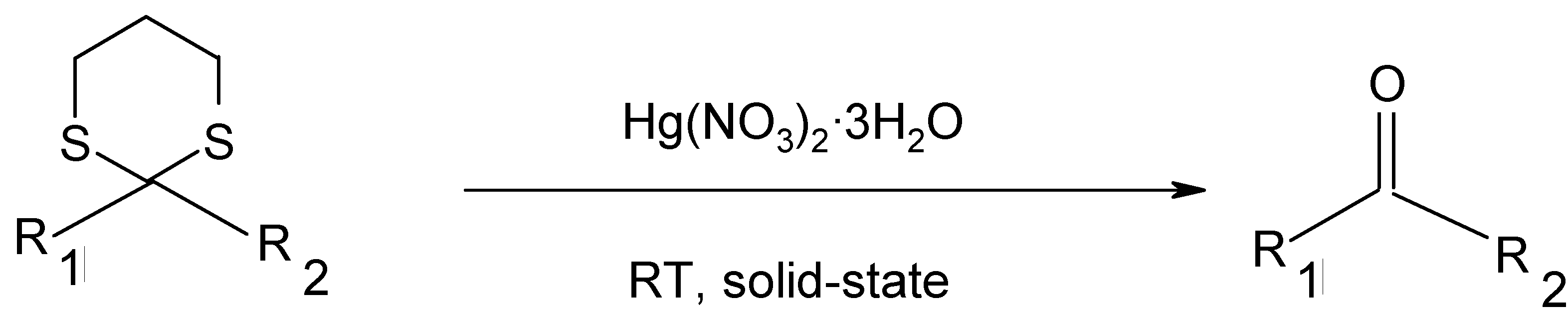
| Entry | R1 | R2 | Time/min | Yields/%a |
|---|---|---|---|---|
| 1 | 2-MeOC6H4 | H | 3 | 90 |
| 2 | 4-BrC6H4 | Me | 2 | 92 |
| 3 | 4-ClC6H4 | H | 2 | 90 |
| 4 | Ph | Ph | 3 | 90 |
| 5 | 4-PhC6H4 | Me | 4 | 88 |
| 6 | 4-BrC6H4 | CH2Br | 4 | 91 |
| 7 | 3-NO2C6H4 | H | 2 | 95 |
| 8 | 2-NO2C6H4 | H | 2 | 95 |
| 9 | 4-ClC6H4 | Ph | 3 | 92 |
| 10 | C6H13 | H | 1 | 96 |
Conclusions
Experimental
General
Typical procedure for solid phase deprotection of thioacetals with mercury(II) nitrate trihydrate: reaction of 2-(3-nitrophenyl)-1,3-dithiane.
References
- Greene, T. W.; Wuts, P. G. M. Protective Groups in Organic Synthesis; Wiley: New York, 1991. [Google Scholar] Komatsu, N.; Uda, M.; Suzuki, H. Synlett 1995, 984.
- Guanti, G.; Banfi, L.; Brusco, S.; Riva, R. Tetrahedron Lett. 1993, 34, 8549.
- Firouzabadi, H.; Iranpoor, N.; Karimi, B. Synthesis 1999, 58. Seebach, D.; Corey, E. J. J. Org. Chem. 1975, 40, 231. Groblel, B. T.; Seebach, D. Synthesis 1977, 357. Hatch, R. P.; Shringarpure, J.; Weinreb, S. M. J. Org. Chem. 1978, 43, 4172. Marshall, J. A.; Belletire, J. L. Tetrahedron Lett. 1971, 871.
- Lebouc, A.; Simonet, J.; Gelas, J.; Dehbi, A. Synthesis 1987, 320.
- Firouzabadi, H.; Iranpoor, N.; Zolfigol, M. A. Bull. Chem. Soc. Jpn. 1998, 77, 2169. Varma, R. S.; Saini, R. K. Tetrahedron Lett. 1997, 38, 2633. Meshram, H. M.; Reddy, G. S.; Yadav, J. S. Tetrahedron Lett. 1997, 38, 8891. Curini, M.; Marcotullio, M. C.; Pisani, E.; Rosati, 0. Synlett 1997, 769. Curini, M.; Ceccherelli, P.; Marcotullio, M. C.; Epifano, F.; Rosati, 0. Synlett 1996, 767. Komatsu, N.; Taniguchi, A.; Uda, M.; Suzuki, H. Chem. Commun. 1996, 1847. Schmittel, M.; Levis, M. Synlett 1996, 315. Haroutounian, S. A. Synthesis 1995, 39. Hirano, M.; Ukawa, K.; Yakabe, S.; Dark, J. H.; Morimoto, T. Synthesis 1997, 858. Lee, J. G.; Hwang, J. P. Chem. Lett. 1995, 507. Meshram, H. M.; Reddy, G. S.; Sumitra, G.; Yadav, J. S. Synth. Commun. 1999, 29, 1113.
- Firouzabadi, H.; Hazarkhani, H.; Karimi, B.; Niroumand, U.; Ghassamipour, S. Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), September 1-30, 2000; A0044. www.mdpi.org/ecsco-4.htm.
- Mohammadpoor-Baltork, I.; Nourozi, A. R. Synthesis 1999, 487. Meskens, F. A. J. Synthesis 1981, 501. Gros, P.; Perchec, P. L.; Senet, J. P. J. Chem. Res. (S) 1995, 196. Marcantoni, E.; Nobili, F. J. Org. Chem. 1997, 62, 4183.
- Balogh, M.; Comelis, A.; Laszlo, P. Tetrahedron Lett. 1984, 25, 3313.
- Curini, M.; Marcotullio, M. C.; Pisani, E.; Rosati, 0. Synlett 1997, 769.
- Mehta, G.; Uma, R. Tetrahedron Lett. 1996, 37, 1897.
- Komatsu, N.; Taniguchi, A.; Uda, M.; Suzuki, H. Chem. Commun. 1996, 1847.
- Schmittel, M.; Levis, M. Syn!ett 1996, 315.
- Tanermura, K.; Dohya, H.; Imamura, M.; Suzuki, T.; Horaguchi, T. J. Chem. Soc. Perkin Trans. 1 1996, 453.
- Haroutounian, S. A. Synthesis 1995, 39.
- Kamata, M.; Yukiko, M.; Tamagawa, Y.; Kato, M.; Hasegawa, E. Tetrahedron 1994, 50, 12821.
- Kiselyvo, SA. S.; Strekowski, L. Tetrahedron 1993, 49, 2151.
- Epiing, G.; Wang, Q. Tetrahedron Lett. 1992, 33, 5909.
- Kamata, M.; Otogawa, H.; Hasegawa, E. Tetrahedron Lett. 1991, 32, 7421.
- Saigo, K.; Hashimoto, Y.; Kihara, N. Chem. Lett. 1990, 831.
- Stork, G.; Zhao, K. Tetrahedron Lett. 1989, 30, 287.
- Cossy, J. Synthesis 1987, 1113.
- El-Wassimy, M. T. M.; Jorgensen, K. A.; Lawesson, S. 0. J. Chem. Soc. Perkin Trans. 1 1983, 2201.
- Olah, G.; Mehrotra, A. K.; Narang, S. C. Synthesis 1982, 151.
- Prato, M.; Quintily, U.; Scorrano, G.; Sturaro, A. Synthesis 1982, 679.
- Olah, A. G.; Narang, S. C.; Mehrotra, A. K. Synthesis 1982, 965.
- Ikehira, H.; Tanimoto, S.; Oida, T.; Okano, M. Synthesis 1982, 1087.
- Degani, I.; Fochi, R.; Regondi, V. Synthesis 1981, 51.
- Cussans, N. J.; Ley, S. V. J. Chem. Soc. Perkin Trans. 1 1980, 1654.
- Carins, J.; Logan, R. T. J.C. S. Chem. Comm. 1980, 886.
- Fuji, K.; Ichikawa, K.; Fujita, E. Tetrahedron Lett. 1978, 3561.
- Tamura, Y.; Sumoto, K.; Fujii, S.; Satoh, H.; Ikeda, M. Synthesis 1973, 312.
- Oishi, T.; Kamemoto, K.; Ban, Y. Tetrahedron Lett. 1972, 1085.
- Fetizon, M.; Jurion, M. J. C. S. Chem. Comm. 1972, 382.
- Chang, H-L. W. Tetrahedron Lett. 1972, 1989.
- Ho, T. L.; Ho, H. C.; Wong, C. M. J. C. S. Chem. Comm. 1972, 791. Ho, T. L. Synthesis 1973, 347. Vedjes, E.; Fuchs, P.L. J. Org. Chem. 1971, 36, 366.
- Huurdeman, W. F. J.; Wynberg, H. Tetrahedron Lett. 1971, 3449.
- Heaton, P. R.; Midgley, J. M.; Whalley, W. B. Chem. Comm. 1971, 750.
- Corey, E. J.; Erickson, B. W. J. Org. Chem. 1971, 36, 3553.
- Narasaka, K.; Sakashita, T.; Mukaiyama, T. Bull. Chem. Soc. Jpn. 1972, 3724.
- Hajipour, A.R.; Mallakpour, S.E.; Mohammadpoor, I.; Abedi, H. Molecules 2002, 7, 674.
- Habibi, M.H.; Mallouk, T. E. J. Fluorine Chem. 1991, 53, 53. Habibi, M.H.; Mallouk, T. E. J. Fluorine Chem. 1991, 51, 291. Habibi, M.H.; Farhadi, S. J. Chem. Res. (S) 1998, 776. Habibi, M.H.; Farhadi, S. Asian Chem. Lett. 1998, 2, 111. Habibi, M.H.; Farhadi, S. Tetrahedron Lett. 1999, 40, 2821.
- Dictionary of Organic Compounds, 6th ed.; Chapman and Hall: London, 1982.
- Sample Availability: Available from the authors.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Habibi, M.H.; Tangestaninejad, S.; Montazerozohori, M.; Mohamadpoor-Baltork, I. Highly Efficient, and Fast Solid State Deprotection of 1,3-Dithianes and 1,3-Dithiolanes using Mercury(II) Nitrate Trihydrate. Molecules 2003, 8, 663-669. https://doi.org/10.3390/80900663
Habibi MH, Tangestaninejad S, Montazerozohori M, Mohamadpoor-Baltork I. Highly Efficient, and Fast Solid State Deprotection of 1,3-Dithianes and 1,3-Dithiolanes using Mercury(II) Nitrate Trihydrate. Molecules. 2003; 8(9):663-669. https://doi.org/10.3390/80900663
Chicago/Turabian StyleHabibi, Mohammad H., Shahram Tangestaninejad, Morteza Montazerozohori, and Iraj Mohamadpoor-Baltork. 2003. "Highly Efficient, and Fast Solid State Deprotection of 1,3-Dithianes and 1,3-Dithiolanes using Mercury(II) Nitrate Trihydrate" Molecules 8, no. 9: 663-669. https://doi.org/10.3390/80900663




