Synthesis and Reactivity of New 1-Pentafluorophenyl-1Hpyrrole Derivatives
Abstract
:Introduction
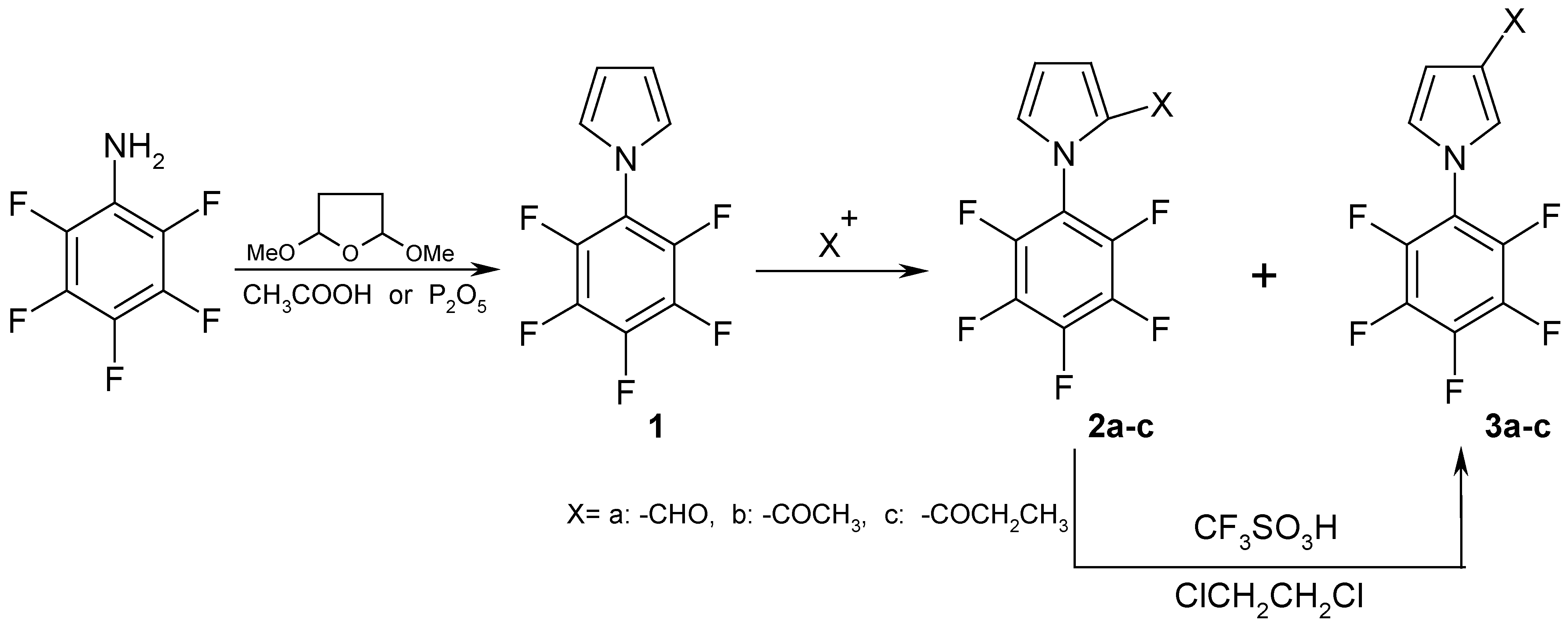
Results and Discussion
Conclusions
Experimental
General
Synthesis of 1-Pentafluorophenyl-1H-Pyrrole (1).
Synthesis of 1-Pentafluorophenyl-1H-Pyrrole-2-Carbaldehyde (2a).
Synthesis of Compounds 2b,c.
Synthesis of Compounds 3a-c.
References
- Müllen, K.; Wegner, G. Electronic Materials: The Oligomer Approach; Wiley-VCH: New York, 1998. [Google Scholar]
- Shastri, V. R.; Pishko, M. V. Electrical and Optical Polymer Systems; Wnek, G. E., Trantolo, D. J., Cooper, T. M., Gresser, J. D., Eds.; Marcel Dekker: New York, 1998; p. 1031. [Google Scholar] Buchanan, J. G.; Sable, H.Z. Selective Organic Transformations; Thyagarajan, B. S., Ed.; Wiley-Interscience: New York, 1972; Vol. 2, pp. 1–95. [Google Scholar]
- Coates, G. W.; Dunn, A. R.; Henling, I. M.; Ziller, J. W.; Lobkovsky, E. B.; Grubbs, R. H. Phenyl-Perfluorophenyl Stacking Intercations: Topochemical [2+2] Photodimerization and Photopolymerization of Olefinic Compounds. J. Am. Chem. Soc. 1998, 120, 3641–3649. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Li, Z.-T. Pentafluorophenylation of Aromatics with Pentafluorophenyl Perfluoro- and Polyfluoroalkanesulfonates. A Photoinduced Electron Transfer Cation Diradical Coupling Process. J. Org. Chem. 1993, 58, 2599–2604. [Google Scholar] [CrossRef]
- Henrie, R. N., II; Yeager, W. H. Reaction of Diazole Anions with Hexafluorobenzene: an Unexpectedly Facile Entry Into Hexa(Diazol-1-yl)-Benzenes. Heterocycles 1993, 35, 415–425. [Google Scholar]
- Biemans, H. A. M.; Ahang, C.; Smith, P.; Koojiman, H.; Smeets, W. J. J.; Spek, A. L.; Meijer, E. W. Hexapyrrolylbenzene and Octapyrrolylnaphtalene. J. Org. Chem. 1996, 61, 9012–9015. [Google Scholar] [CrossRef]
- Clauson-Kaas, N.; Tyle, Z. Preparation of Cis and Trans 2,5-Dimethoxy-2-(acetamidomethyl)-2,5-dihydrofuran, of Cis and Trans 2,5-Dimethoxy-2-(acetamidomethyl)-tetrahydrofuran and of 1-Phenyl-2-(acetamidomethyl)pyrrole. Acta Chem. Scand. 1952, 6, 667–670. [Google Scholar] Elming, N.; Clauson-Kaas, N. The preparation Pyrroles from Furans. Acta Chem. Scand. 1952, 6, 867–874. [Google Scholar]
- Fang, Y.; Leysen, D.; Ottenheijm, H. C. J. A Facile Synthesis of N-Substituted Pyrroles. Synth. Commun. 1995, 25, 1857–1861. [Google Scholar] [CrossRef]
- Dellemagne, P.; Rault, S.; Fabis, F.; Dumoulin, H.; Robba, M. A Convenient Rearrangement of 1-Phenylpyrrole-2-Carboxaldehydes into Their 3-Isomers Synth. Commun. 1994, 24, 1855–1857. [Google Scholar]
- Sample Availability: Available from the authors.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Hrnčariková, K.; Végh, D. Synthesis and Reactivity of New 1-Pentafluorophenyl-1Hpyrrole Derivatives. Molecules 2003, 8, 536-540. https://doi.org/10.3390/80700536
Hrnčariková K, Végh D. Synthesis and Reactivity of New 1-Pentafluorophenyl-1Hpyrrole Derivatives. Molecules. 2003; 8(7):536-540. https://doi.org/10.3390/80700536
Chicago/Turabian StyleHrnčariková, Katarína, and Daniel Végh. 2003. "Synthesis and Reactivity of New 1-Pentafluorophenyl-1Hpyrrole Derivatives" Molecules 8, no. 7: 536-540. https://doi.org/10.3390/80700536



