Synthesis and Catalytic Activity of Two New Cyclic Tetraaza Ligands
Abstract
:Introduction
Results and Discussion
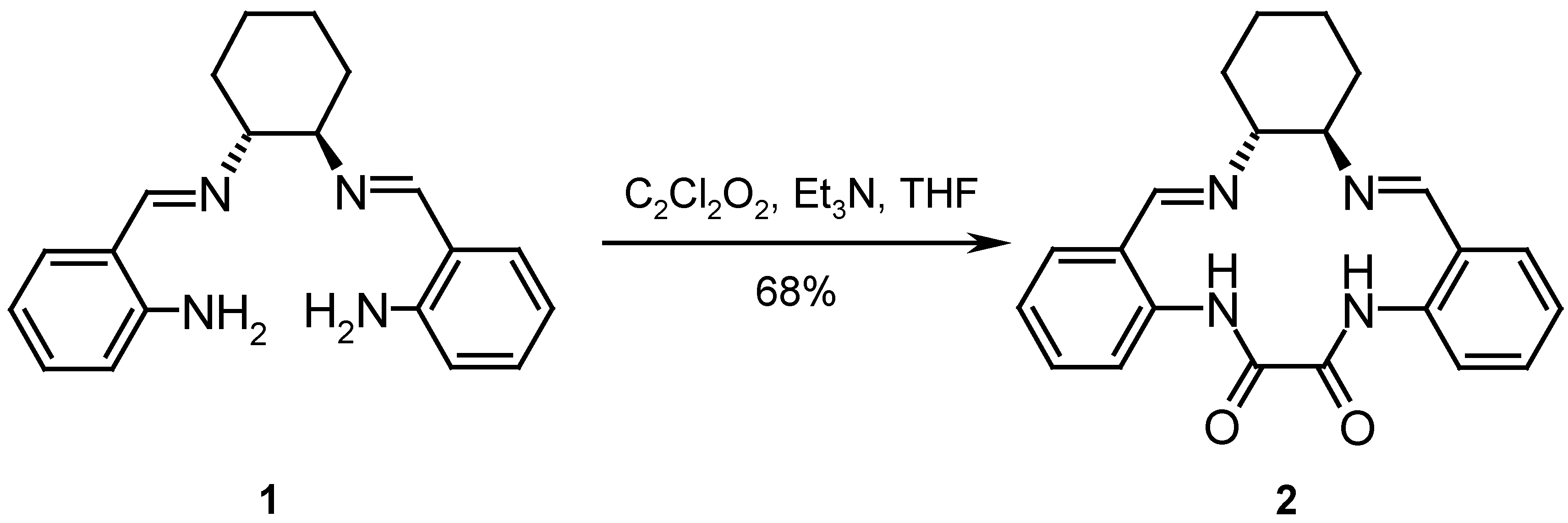
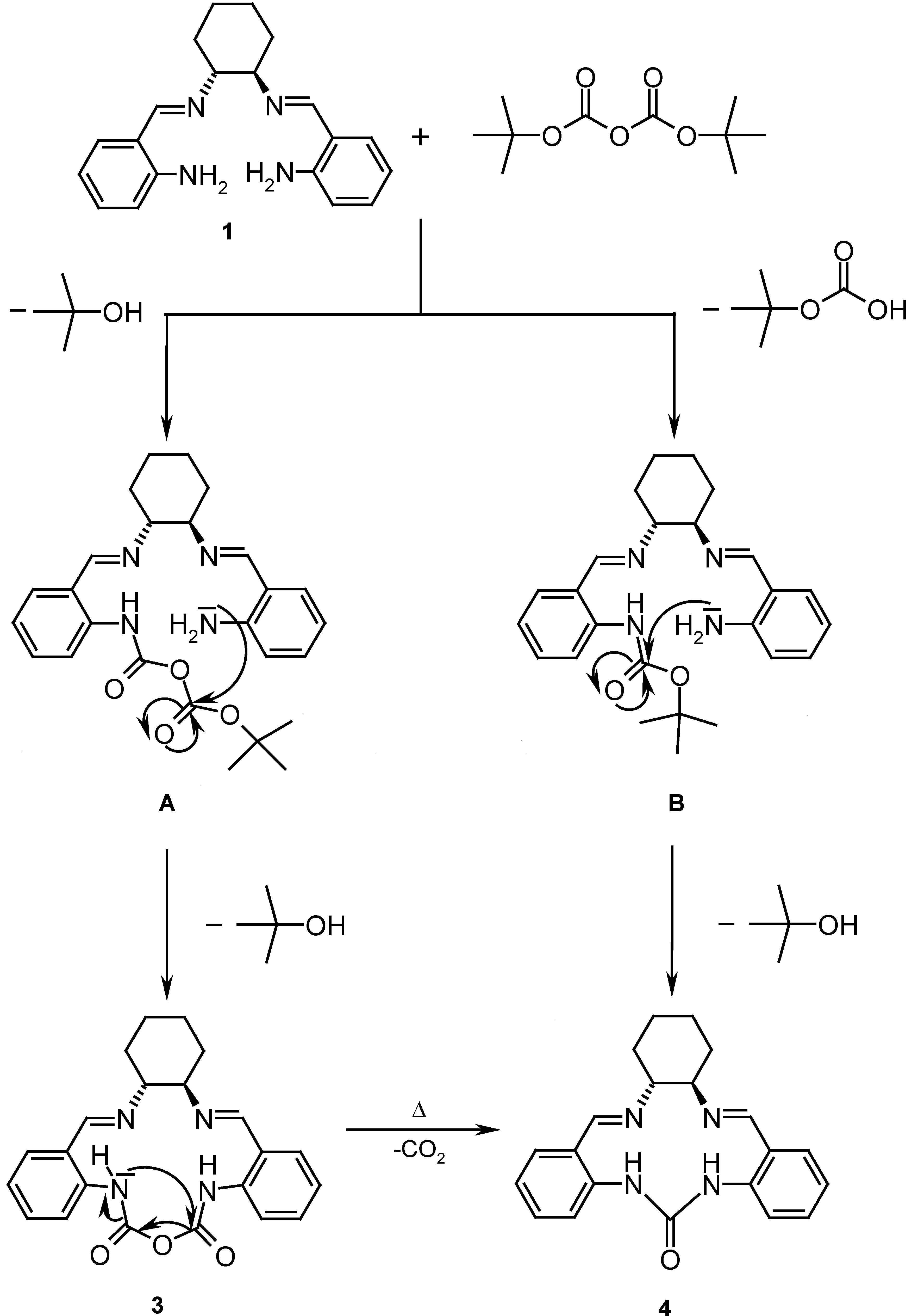
Catalysis
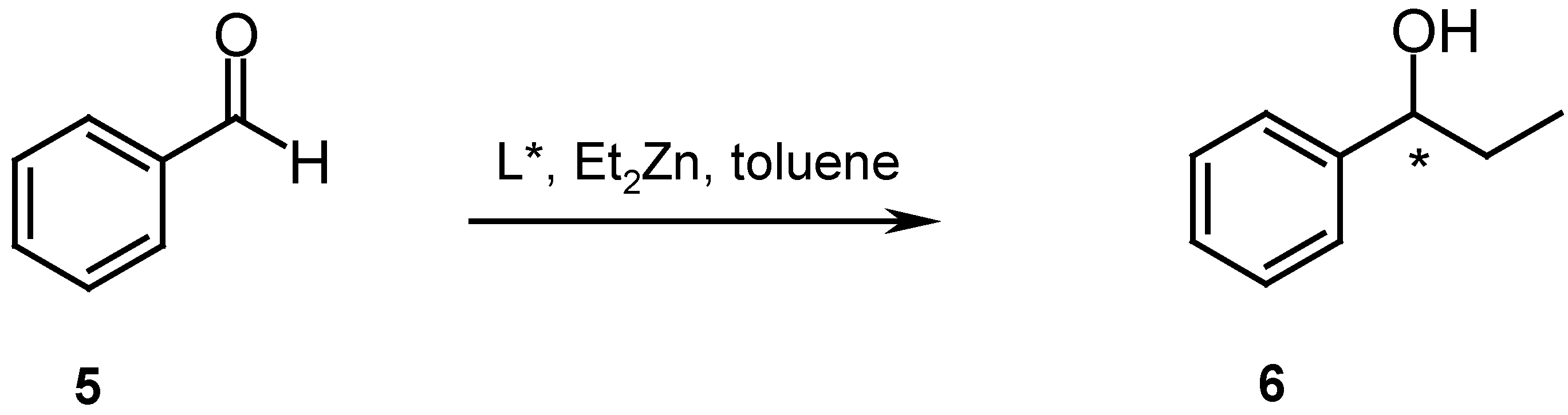
Experimental
General
Synthesis of the ligands
References and Notes
- Fache, F.; Schulz, E.; Tommasino, M.; Lemaire, M. Chem. Rev. 2000, 100, 2159–2231.
- Alcón, M. J.; Iglesias, M.; Sánchez, F.; Viani, I. J. Organomet. Chem. 2001, 634, 25–33.
- Uhlemann, E.; Plath, M. Z. Chem. 1969, 9, 234–235.
- Trost, B. J. Am. Chem. Soc. 1997, 119, 7879–7880.
- Skog, K. Tetrahedron Lett. 1992, 33, 1751–1754.
- Lindauer, D.; Atzrodt, J.; Beckert, R.; Görls, H. Liebigs Ann. 1995, 199–201.
- Knölker, H.-J.; Braxmeier, T.; Schlechtingen, G. Synlett 1996, 502–504.
- Christie, C. C.; Kirby, G. W.; McGuigan, H.; Mackinnon, J.W. J. Chem. Soc. Perkin Trans. 1 1985, 2469–2473.Ye, Y.; Aulinger, K.; Arnold, N.; Spahl, W.; Steglich, W. Tetrahedron Lett. 1997, 38, 8013–8016.Wallace, E. G. U.S. Patent 4117153, 1978. 1978-09-26.Nilsson, J. L. G.; Sievertsson, H.; Dahlbom, R. Acta Chem. Scand. 1968, 22, 683–685.Sievertsson, H.; Nilsson, J. L. G. Acta Chem. Scand. 1970, 24, 939–945.Bogentoft, C.; Sievertsson, H. Acta Chem. Scand. 1972, 26, 4172–4174.
- A sample of 7.041 mg of 3 was heated under N2 from 25 to 300 °C at a rate of 10 °C/min. The reaction set in at 259.1 °C and the sample lost 0.818 mg in weight, which corresponds to the loss of CO2 (theoretically 0.794 mg).
- The preparative synthesis of 4 thus never succeeded. Possible alternate ways of obtaining this compound could be to react the tetraaza ligand 1 with urea while heating (Davis, T. L.; Underwood, H. W., Jr. J. Am. Chem. Soc. 1922, 44, 2595–2604.), with phosgene in benzene (Jones, L. W.; Root, F. B. J. Am. Chem. Soc. 1926, 48, 181–195.), with diphosgene in dioxane (Cordier, D.; Coulet, P. R. J. Chem. Soc. Perkin Trans. 2 1994, 4, 891–894.) or with carbon dioxide in the presence of a strong base or with methyl chloroformate and Et3N in CH2Cl2 (Naito, R.; Takeuchi, M.; Morihira, K.; Hayakawa, M.; Ikeda, K.; Shibanuma, T.; Isomura, Y. Chem. Pharm. Bull. 1998, 43, 1286–1294.).
- While with ligand 2 the racemic alcohol was obtained, ligand 3 gave the R-isomer in 6 % ee.
- a)Collective of authors. In Organikum, 18. Edition ed; Deutscher Verlag der Wissenschaften: Berlin, 1990; pp. 638–659.b)Tietze, L. F.; Eicher, T. Reaktionen und Synthesen in organisch-chemischen Grundpraktikum, 2nd Edition ed; Georg Thieme Verlag: Stuttgart, 1991; pp. 547–551. [Google Scholar]
- Oriyama, T. Chem. Lett. 1984, 12, 2071–2074.
- Sample availability: Samples of compounds 1, 2 and 3 are available from MDPI.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Fonseca, M.H.; Hjelmgaard, T.; König, B. Synthesis and Catalytic Activity of Two New Cyclic Tetraaza Ligands. Molecules 2003, 8, 453-458. https://doi.org/10.3390/80500453
Fonseca MH, Hjelmgaard T, König B. Synthesis and Catalytic Activity of Two New Cyclic Tetraaza Ligands. Molecules. 2003; 8(5):453-458. https://doi.org/10.3390/80500453
Chicago/Turabian StyleFonseca, María Hechavarría, Thomas Hjelmgaard, and Burkhard König. 2003. "Synthesis and Catalytic Activity of Two New Cyclic Tetraaza Ligands" Molecules 8, no. 5: 453-458. https://doi.org/10.3390/80500453



