Silane Reduction of 5-Hydroxy-6-methyl-pyridine-3,4-dicarboxylic Acid Diethyl Ester: Synthesis of Vitamin B6
Abstract
:Introduction
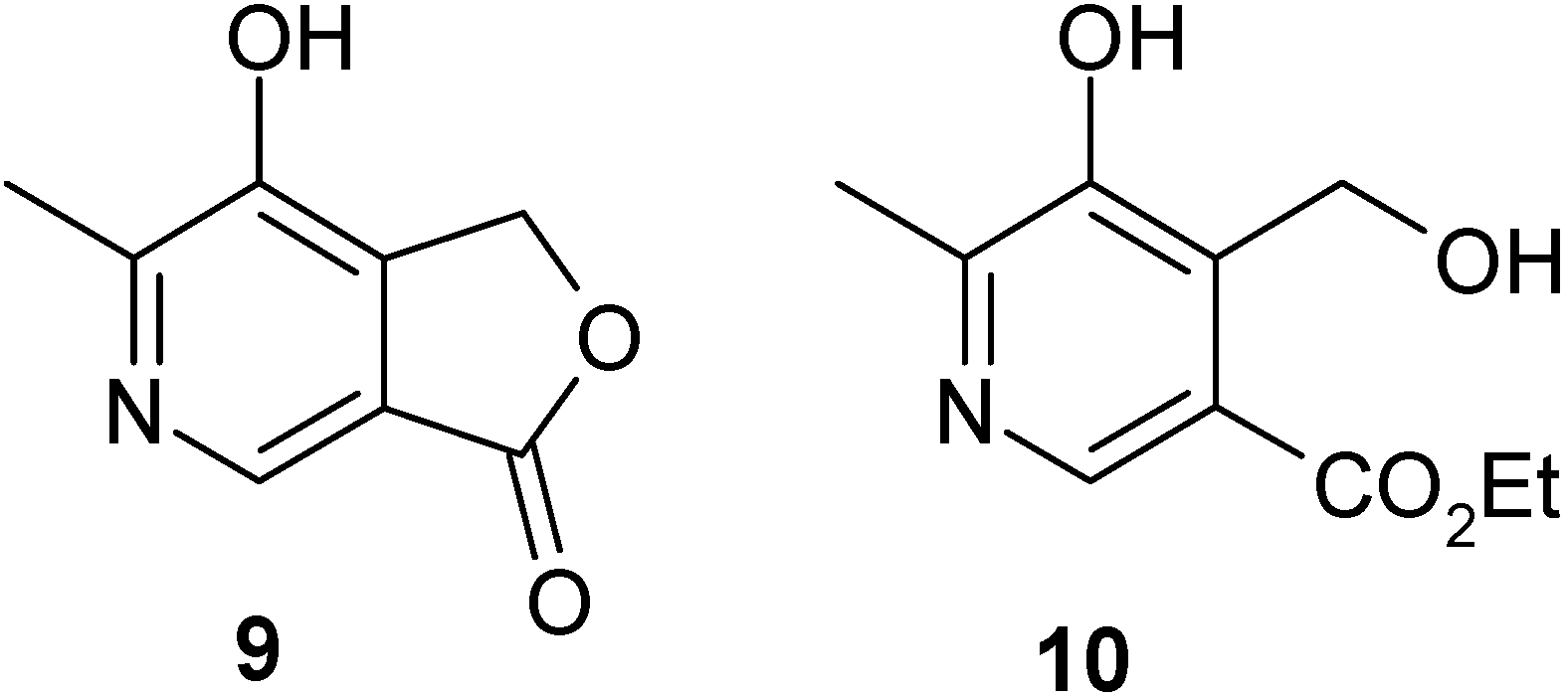
Results and Discussion
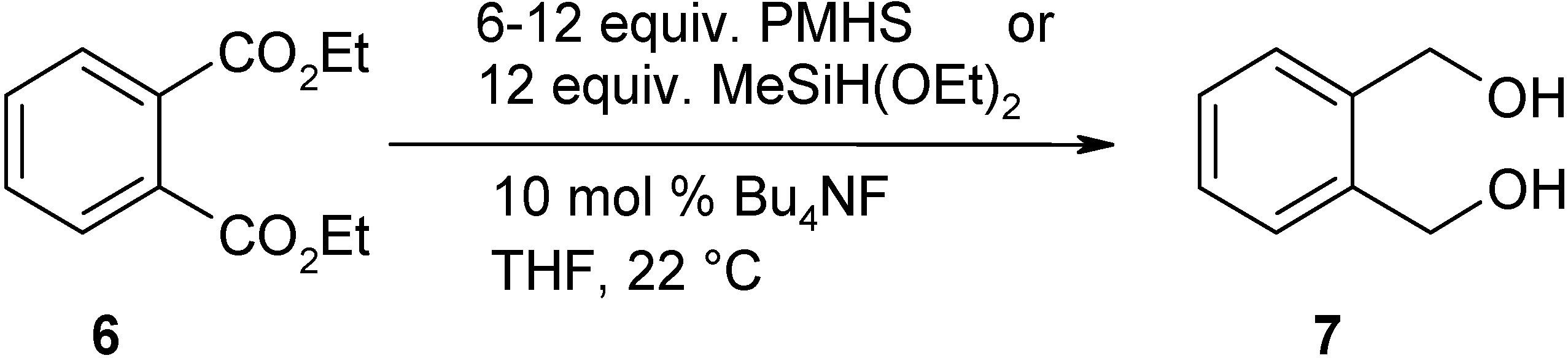
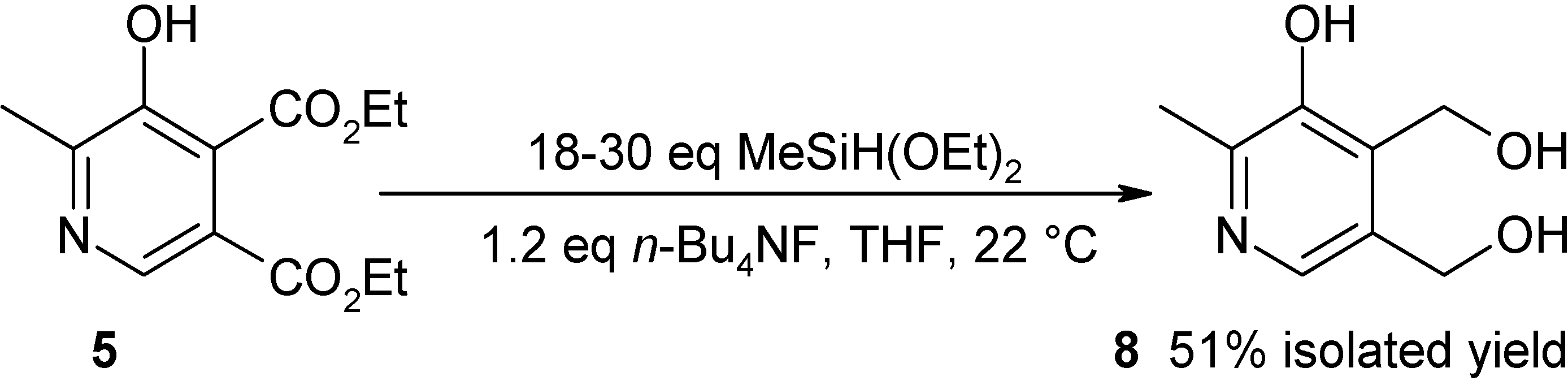
| Conditions (silane reagent-catalyst, solvent, temperature, time) | Exp. | Overall yielda [LC yield] % | Purity % |
|---|---|---|---|
| 8 equiv. PMHS-10 mol% Bu4NF in THF, 22 °C, 24 h | 1 | 0 | |
| 7.5 equiv. MeSiH(OEt)2-10 mol% Bu4NF in THF, 22 °C, 24 h | 2 | 0b | |
| 12 equiv. Si(OEt)4-30 equiv. PMHS, 1.2 equiv. Bu4NF in THF, 22 °C, 24 h | 3 | 38 | 5.1 |
| 30 equiv. MeSiH(OEt)2-1.2 equiv. Bu4NF in THF, 22 °C, 24 h | 4 | 51 | 9.2 |
| 8 equiv. MeSiH(OEt)2-10 mol% Bu4NF in THF, reflux, 2 h | 5 | 0b | |
| 8 equiv. MeSiH(OEt)2-10 mol% Bu4NF in THF, reflux, 96 h | 6 | [21]b | |
| 16 equiv. PMHS-8 equiv. Si(OEt)4-10 mol% Bu4NF in DMF, 150 °C, 24 h | 7 | [11]b,c | |
| 8 equiv. Si(OEt)4-8 equiv. PMHS-10 mol% Bu4NF, 160 °C, 24 h | 8 | [9]d | |
| 16 equiv. MeSiH(OEt)2-1.2 equiv. Bu4NF in THF, 22 °C, 24 h | 9 | [60]b | |
| 10 equiv. MeSiH(OEt)2-10 mol% Bu4NF, no solvent, 100 °C, 21 h* | 10 | 51 [60]b | 55.8e |
| 15 equiv. MeSiH(OEt)2-10 mol% Bu4NF, no solvent, 100 °C, 21 h | 11 | 42 [60]b | 65.2f |
| 15 equiv. MeSiH(OEt)2-20 mol% Bu4NF, no solvent, 100 °C, 26 h | 12 | 46 [65]b | 64.8f |
| 15 equiv. MeSiH(OEt)2-10 mol% Bu4NF in dioxane,100 °C, 24 h* | 13 | 54 | 75.9 |
| 15 equiv. MeSiH(OEt)2,10 mol% CsF, 20 mol% 18-crown-6 ether, dioxane, 100 °C, 24 h | 14 | [52]b |
Conclusions
Acknowledgements
Experimental
General
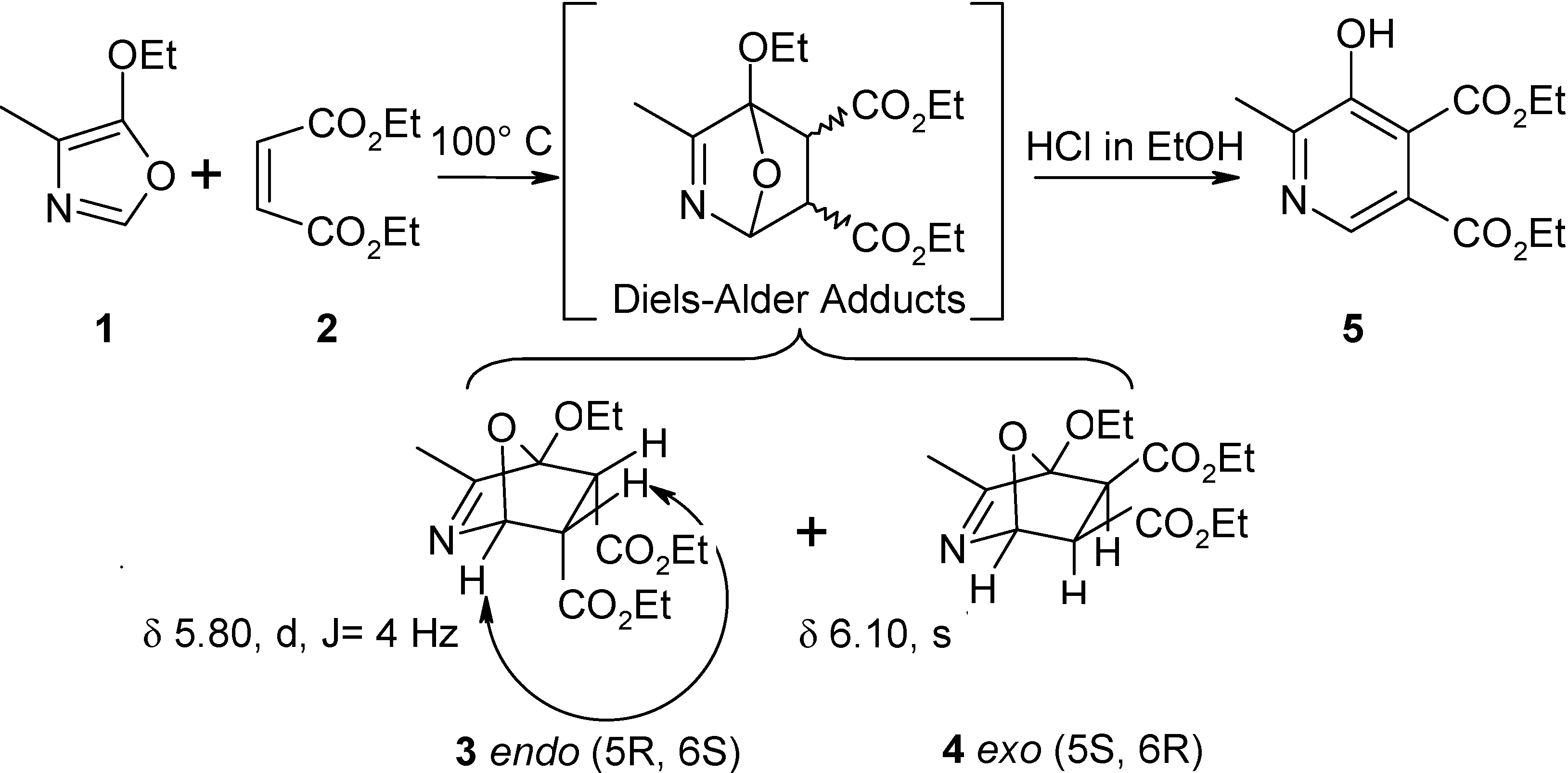
Isolation of Diels-Alder adducts endo (5R,6S)-4-ethoxy-3-methyl-7-oxa-2-aza-bicyclo[2.2.1]hept-2-ene-5,6-dicarbocyclic acid diethyl ester (3) and exo (5S,6R)-4-ethoxy-3-methyl-7-oxa-2-aza-bicyclo[2.2.1]hept-2-ene-5,6-dicarbocyclic acid diethyl ester (4).
Spectral and Analytical Data
Hydrosilylation/reduction of hydroxypyridine diester (5) to vitamin B6
References and Notes
- Coffen, D.L. Vitamins (B6). In Kirk-Othmer: Encyclopedia of Chemical Technology, 3rd Edition ed; John Wiley & Sons, Inc.: New York, 1984; vol. 24, pp. 94–107. [Google Scholar]
- Pauling, H.; Weimann, B.J. Vitamin B6. In Ullmann’s Encyclopedia of Industrial Chemistry; VCH Verlagsgesellschaft mbH: Weinheim, Germany, 1996; vol. A27, pp. 530–540. [Google Scholar]
- Kondrat’eva, G.Y. Khim. Nauk Prom. 1957, 2, 666.
- Kondrat’eva, G.Y. Izv. Akad. Nauk SSSR, Ser. Khim. 1959, 484.
- Kondrat’eva, G.Y. Dokl. Akad. Nauk SSSR 1961, 141, 628, 861, [Proc. Acad. Sci. USSR, Chem. Sect. 1961, 1169, 1221].
- Huang, C.H.; Kondrat’eva, G.Y. Izv. Akad. Nauk SSSR, Otd. Khim. Nauk 1962, 525.
- Harris, E.E.; Firestone, R.A.; Pfister, K.; Boettcher, R.R.; Cross, F.J.; Currie, R.B.; Monaco, M.; Peterson, E.R.; Reuter, W. J. Org. Chem. 1962, 27, 2705.
- Firestone, R.A.; Harris, E.E.; Reuter, W. Tetrahedron 1967, 23, 943.
- Pfister III, K.; Harris, E.; Firestone, R.A.; Merck & Co. US 3,227,722, 4 Jan. 1966.
- Naito, T.; Yoshikawa, T. Chem. Pharm. Bull. 1966, 14, 921. [CrossRef]
- Doktorova, N.D.; Ionova, L.V.; Karpeisky, M.Y.; Padyukova, N.S.; Turchin, K.F.; Florentiev, V.L. Tetrahedron 1969, 25, 3527.
- Itov, Z.I.; Gunar, V.I. Chem. Pharm. J. (Engl. Transl.) 1988, 2, 151.
- Kreher, R. Houben-Weyl Methoden der Organischen Chemie; Georg Thieme Verlag: Stuttgart-New York, 1992; Band E7b Hetearene II Teil 2, Chap 4.2.1.3.4; pp. 532–539. [Google Scholar]
- Karpeiskii, M.Y.; Florent’ev, V.L. Russ. Chem. Rev. 1969, 38, 540, and references therein.
- Turchi, I.J.; Dewar, M.J.S. Chem. Rev. 1975, 75, 389, and references therein.
- Boger, D.A. Chem. Rev. 1986, 86, 781.
- Jones, R.G.; Kornfeld, E.C. J. Am. Chem. Soc. 1951, 73, 107.
- Lutz, A.H.; F. Hoffmann La Roche & Co. CH 408914, Switzerland, 1966.
- Merck & Co., Inc. GB 1,116,118 [US 3,410,862] 6 June 1968.
- Jones, R.G.E.; Lilly & Co. US 2,744,114, 1 May 1952.
- Okawara, R.; Sakiyama, M. Bull. Chem. Soc. Jpn. 1956, 29, 236.
- Koerner, G.Th.; Goldschmidt, A.G. DE 1,162,365, 5 May 1962.
- Sagami Chemical Research Center. GB 1,401,948, 28 Feb. 1974.
- Nagai, Y.; Ojima, I.; Inaba, S.; Sagami Chemical Research Center. US 3,910,980, 7 Oct. 1975.
- Kötzsch, H.J.; Seiler, C.D.; Vahlensieck, H.J.; Dynamit Nobel, A.G. US 4,228,092, 14 Oct. 1980.
- Boyer, J.; Corriu, R.J.P.; Perz, R.; Reye, C. Tetrahedron 1981, 37, 2165.
- Chuit, C.; Corriu, R.J.P.; Perz, R.; Reye, C. Synthesis 1981, 981.
- Berk, S.C.; Buchwald, S.L. J. Org. Chem. 1992, 57, 3751. [CrossRef]
- Berk, S.C.; Buchwald, S.L. J. Org. Chem. 1993, 58, 3221. [CrossRef]
- Buchwald, S.L.; Gutierrez, A.; Berk, S.C.; Kreutzer, K.A.; Massachusetts Institute of Technology. US 5,220,020, 15 Jun. 1993.
- Breeden, S.W.; Lawrence, N.J. Synlett 1994, 833.
- Mimoun, H.; Firmenich. WO 96/12694, 2 May 1996.
- Mimoun, H.; Firmenich. WO 96/28497, 19 Sept. 1996.
- Mimoun, H.; Firmenich. WO 99/50211, 7 Oct. 1999.
- Mimoun, H. J. Org. Chem. 1999, 64, 2582.
- Mimoun, H.; de Saint Laumer, J.Y.; Giannini, L.; Scopelliti, R.; Floriani, C. J. Am. Chem. Soc. 1999, 121, 6158. [CrossRef]
- Kohra, S.; Hayashida, H.; Tominaga, Y.; Hosomi, A. Tetrahedron Lett. 1988, 29, 89.
- Hosomi, A.; Chisso Corporation. JP 63,010,738, 18 Jan. 1988.
- Hojo, M.; Murakami, C.; Fuji, A.; Hosomi, A. Tetrahedron Lett. 1999, 40, 911.
- Corriu, R.J.P.; Guerin, C.; Henner, B.; Wang, Q. Organometallics 1991, 10, 2297.
- Corriu, R.J.P.; Guerin, C.; Henner, B.; Wang, Q. Inorg. Chim. Acta 1992, 198-200, 705.
- Deneux, M.; Akhrem, I.C.; Avetissian, D.V.; Myssoff, E.I.; Volp’in, M.E. Bull. Soc. Chim. Fr. 1973, 2638.
- Akhrem, I.S.; M. Dene, M.; Volp’in, M.E. Izv. Akad. Nauk SSSR Ser. Khim. (Engl. Transl.) 1973, 897.
- Boyer, J.; Corriu, R.J.P.; Poirier, M.; Reye, C. Synthesis 1981, 558.
- Boyer, J.; Corriu, R.J.P.; Perz, R.; Reye, C. Tetrahedron 1981, 17, 2165.
- Chuit, C.; Corriu, R.J.P.; Perz, R.; Reye, C. Synthesis 1982, 981.
- Fujit, M.; T. Hiyama, T. J. Org. Chem. 1988, 53, 5405.
- Furin, G.G.; Vyazankina, O.A.; Gostevsky, B.A.; Vyazankin, N.S. Tetrahedron 1988, 44, 2675.
- Goldberg, Y.; Abele, E.; Shymanska, M.; Lukevics, E. J. Organomet. Chem. 1991, 410, 127. [CrossRef]
- Drew, M.D.; Lawrence, N.J.; Fontaine, D.; Sehkri, L. Synlett 1997, 989.
- Drew, M.D.; Lawrence, N.J. Tetrahedron Lett. 1997, 38, 5857.
- Kobayashi, Y.; Takahisa, E.; Nakano, M.; Watani, K. Tetrahedron 1997, 53, 1627.
- Kobayashi, Y.; Shin-Etsu Chemical Industry Co. JP 10,087,530, 7 Apr. 1998.
- Lawrence, N.J.; Drew, M.D.; Bushell, S.M. J. Chem. Soc., Perkin Trans. 1 1999, 3381.
- Abele, E.; Lukevics, E. Main Group Met. Chem. 2001, 24, 315.
- Asakawa, H.; Fukushima, Y.; Imamiya, E.; Kawamatsu, Y. Chem. Pharm. Bull. 1979, 27, 522.
- Goldberg, Y.; Abele, E.; Shymanska, M.; Lukevics, E. J. Organomet. Chem. 1991, 410, 127. [CrossRef]
- Samples Availability: Starting materials, intermediates and final products are all known compounds. Some are available from the author upon request.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Dumond, Y.R.; Gum, A.G. Silane Reduction of 5-Hydroxy-6-methyl-pyridine-3,4-dicarboxylic Acid Diethyl Ester: Synthesis of Vitamin B6. Molecules 2003, 8, 873-881. https://doi.org/10.3390/81200873
Dumond YR, Gum AG. Silane Reduction of 5-Hydroxy-6-methyl-pyridine-3,4-dicarboxylic Acid Diethyl Ester: Synthesis of Vitamin B6. Molecules. 2003; 8(12):873-881. https://doi.org/10.3390/81200873
Chicago/Turabian StyleDumond, Yves René, and Andrew G. Gum. 2003. "Silane Reduction of 5-Hydroxy-6-methyl-pyridine-3,4-dicarboxylic Acid Diethyl Ester: Synthesis of Vitamin B6" Molecules 8, no. 12: 873-881. https://doi.org/10.3390/81200873




