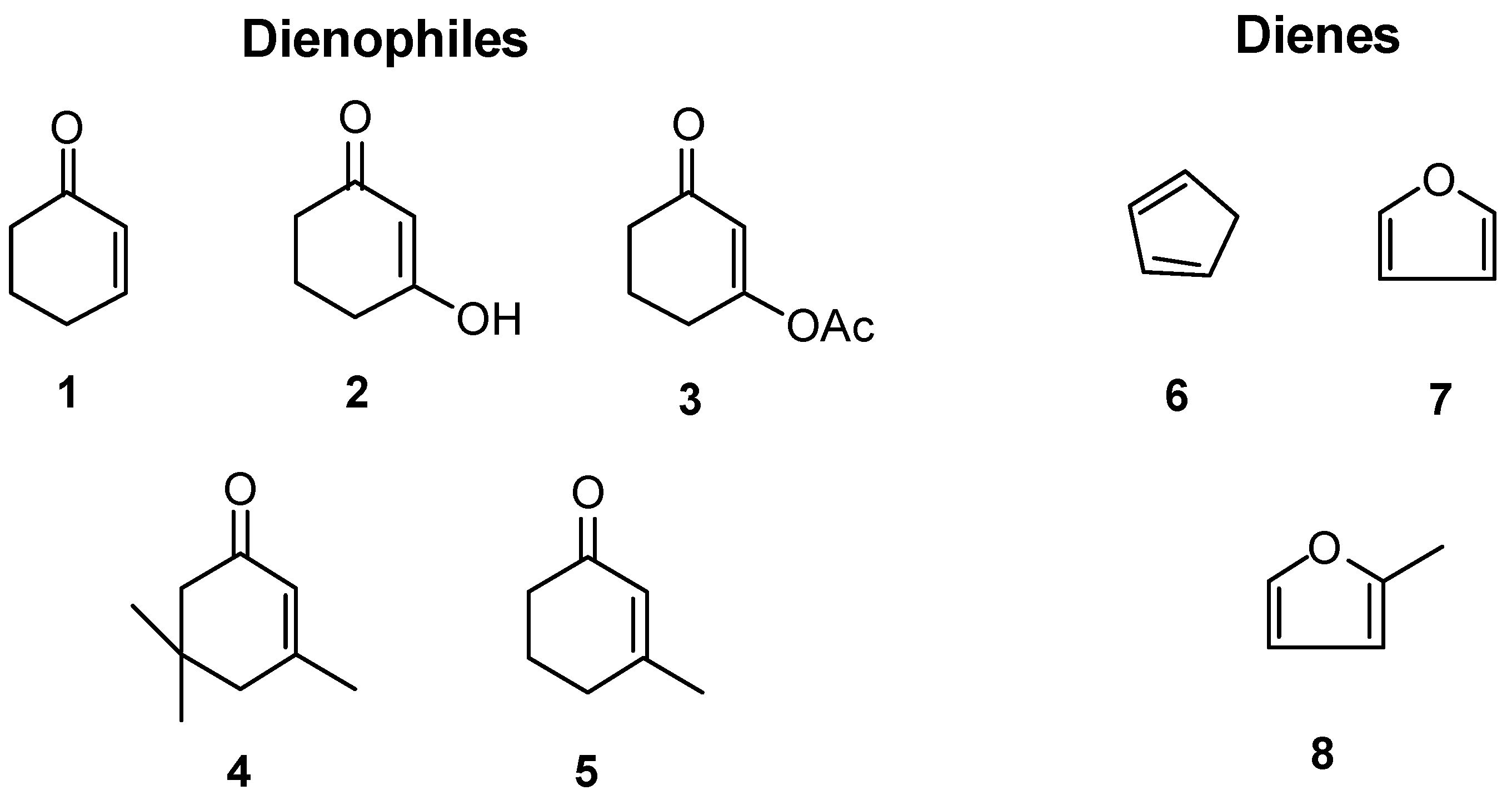
Spectral Data
Tricyclo[6.2.1.02,7]undec-9-en-3-one (9): 1H-NMR (300 MHz): δ 6.19 (dd, 1H, J1=5.7 and J2=2.9 Hz), 6.01 (dd, 1H, J1=5.7 and J2=2.9 Hz), 3.26 (br. s, 1H), 2.88 (br. s, 1H), 2.73 (dd, 1H, J1=10.2 and J2=3.6 Hz), 2.67 (m, 1H), 2.32 (dddd, 1H, J1=18.4, J2=6.0, J3=2.5 and J4=1.9 Hz), 2.03-1.92 (m, 1H), 1.93 (ddd, 1H, J1=18.4, J2=12.0 and J3=6.9 Hz), 1.85-1.62 (m, 2H), 1.45 (dt, 1H, J1=8.3 and J2=J3=1.9 Hz), 1.31 (dt, 1H, J1=8.3 and J2=J3=1.5 Hz), 0.77 (ddt, 1H, J1=J2=12.5, J3=10.6 and J4=3.5 Hz); 13C-NMR (75 MHz): δ 215.47 (C=O), 137.62 (CH), 134.91 (CH), 51.63 (CH), 48.32 (CH2), 46.52 (CH), 45.19 (CH), 41.38 (CH), 39.36 (CH2), 27.97 (CH2), 21.78 (CH2); IR (film) νmax: 3060, 2932, 2857, 1696, 1607, 1455, 1337, 1236, 1072 cm-1; MS m/z (rel. intensity) (%):162 [M+] (2), 121 (2), 97 (39), 91 (19), 79 (15), 66 (100), 43 (8), 41 (16).
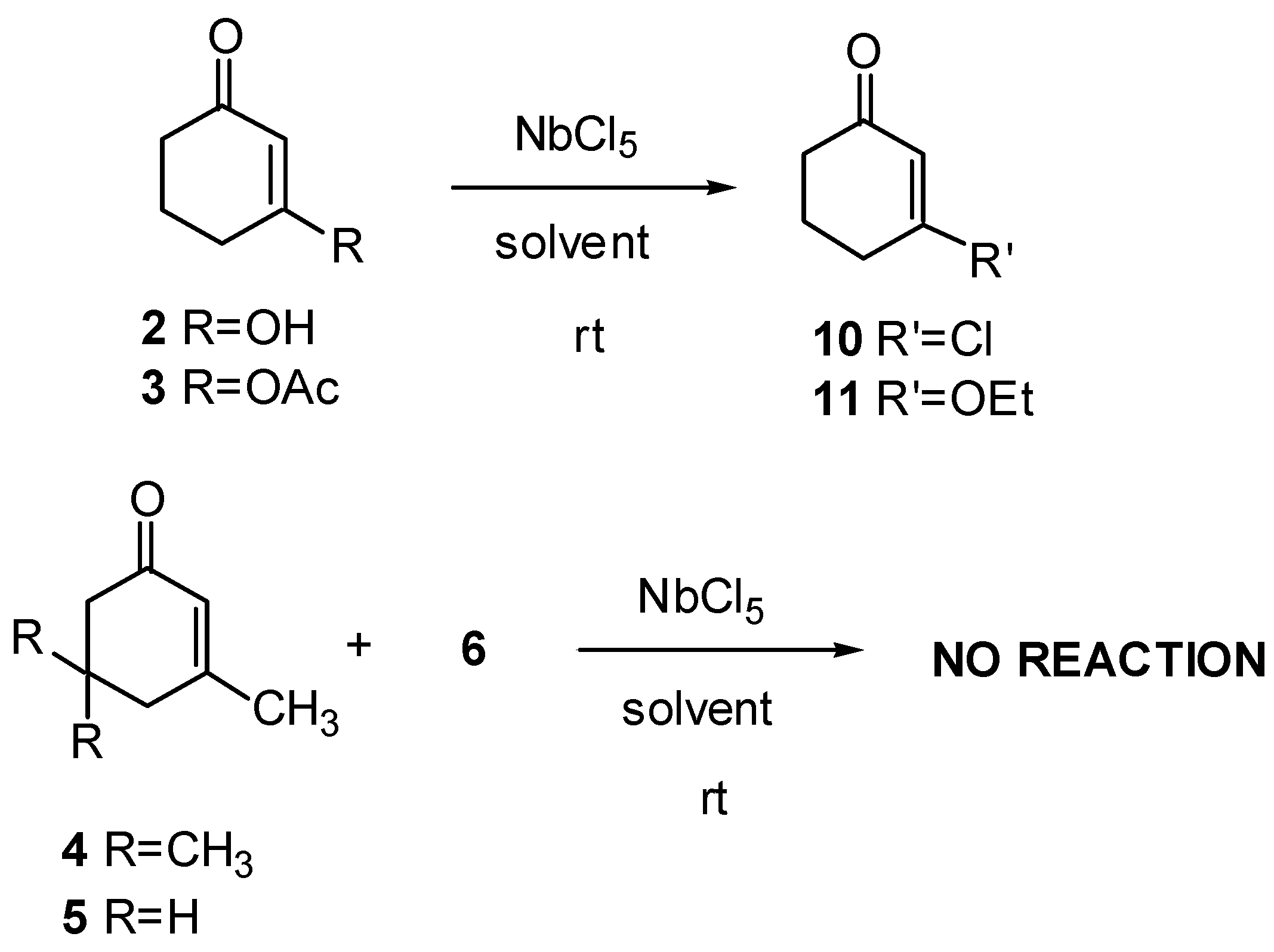
3-Chloro-2-cyclohexenone (10): 1H-NMR (300 MHz): δ 6.22 (t, 1H, J=1.5 Hz), 2.69 (td, 2H, J1=J2= 6.5 and J3=1.5 Hz), 2.40 (t, 2H, J=6.5 Hz), 2.09 (q, 2H, J=6.5 Hz); 13C-NMR (75 MHz): δ 196.85 (C=O), 158.56 (C), 128.44 (CH), 36.29 (CH2), 33.86 (CH2), 22.17 (CH2); IR (film) νmax: 3041, 2948, 2887, 1679, 1607, 1455, 1425, 1344, 1289, 1234, 1137, 1026 cm-1; MS m/z (rel. intensity) (%):132 [(M+2)+] (10), 130 (34), 104 (32), 102 (100), 67 (75), 65 (9), 39 (43), 28 (11).
3-Ethoxy-2-cyclohexenone (11): 1H-NMR (300 MHz): δ 5.34 (s, 1H), 3.91 (q, 2H, J=7.0 Hz), 2.41 (t, 2H, J=6.4 Hz), 2.33 (t, 2H, J=6.4 Hz), 1.98 (q, 2H, J=6.4 Hz), 1.37 (t, 3H, J=7.0 Hz), 13C-NMR (75 MHz): δ 199.69 (C=O), 177.92 (C), 102.67 (CH), 65.18 (CH2), 36.78 (CH2), 29.11 (CH2), 22.29 (CH2), 14.14 (CH3); IR (film) νmax: 3039, 2948, 2887, 1646, 1599, 1465, 1221, 1137, 1026 cm-1; MS m/z (rel. intensity) (%): 112 [(M-28)+] (28), 84 (81), 69 (23), 55 (35), 42 (100), 39 (37), 27 (37), 15 (9).
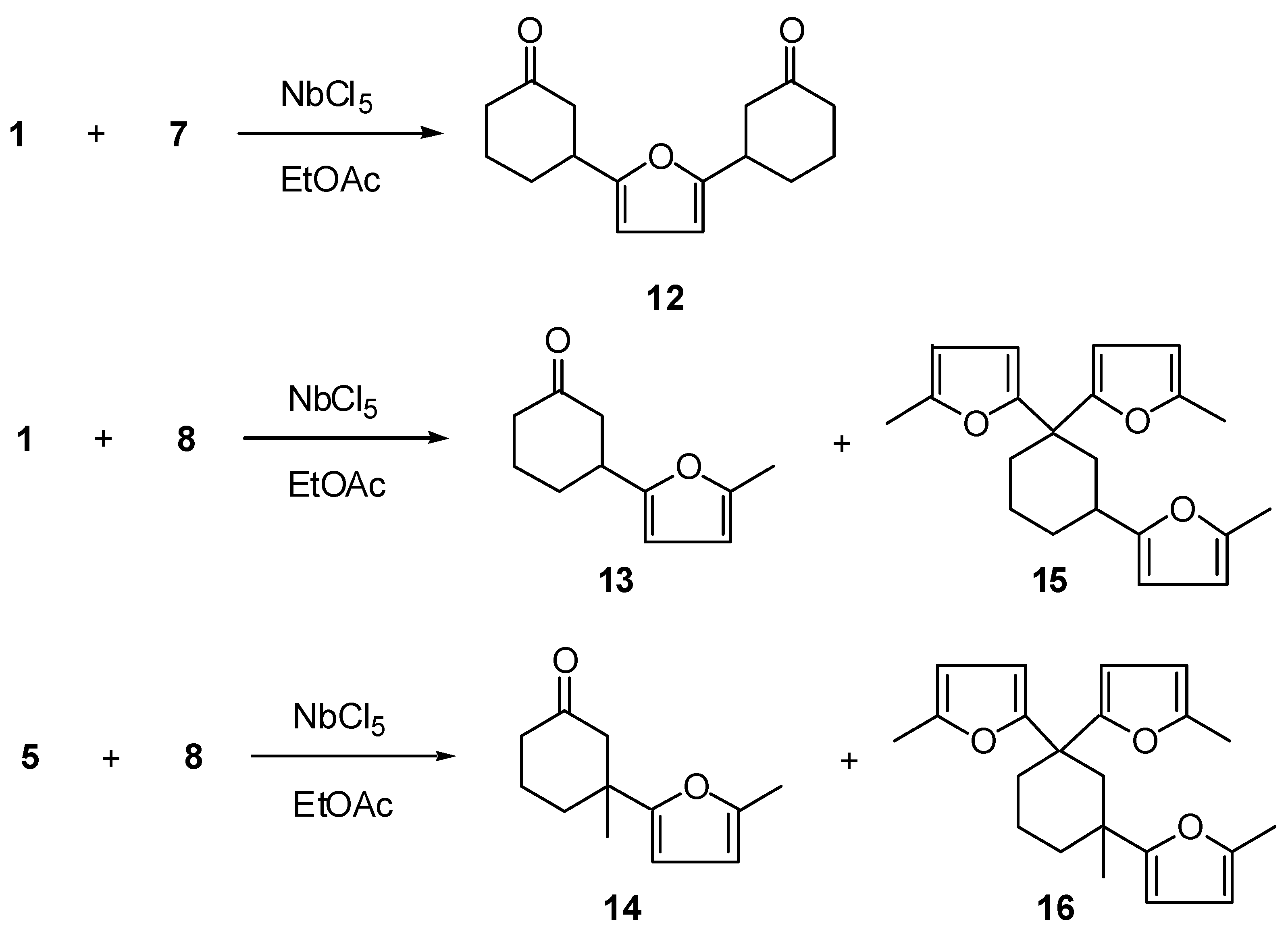
3-[5-(3-Oxo-cyclohexyl)-furan-2-yl]-cyclohexanone (12): 1H-NMR (300 MHz): δ 5.91 (s, 2H), 3.15 (ddt, 2H, J1=10.5, J2= 8.6 and J3=J4= 4.8 Hz), 2.66 (ddt, 2H, J1=14.2, J2=4.8 and J3=J4=0.9 Hz), 2.49 (ddd, 2H; J1=14.2, J2=10.5 and J3=0.9 Hz), 2,35 (m, 4H), 2.10 (m, 4H), 1.82 (m, 4H); 13C-NMR (75 MHz): δ 210.11 (C=O), 155.81 (C), 104.80 (CH), 45.51 (CH2), 41.16 (CH2), 37.49 (CH), 29.77 (CH2), 24.26 (CH3); IR (film) νmax: 3041, 3037, 2948, 2872, 1709, 1599, 1557, 1455, 1421, 1221, 1175, 1099, 1018 cm-1; MS m/z (rel. intensity) (%): 260 [M+] (2), 203 (3), 55 (3), 42 (4), 41 (3), 32 (20), 28 (100), 27 (2).
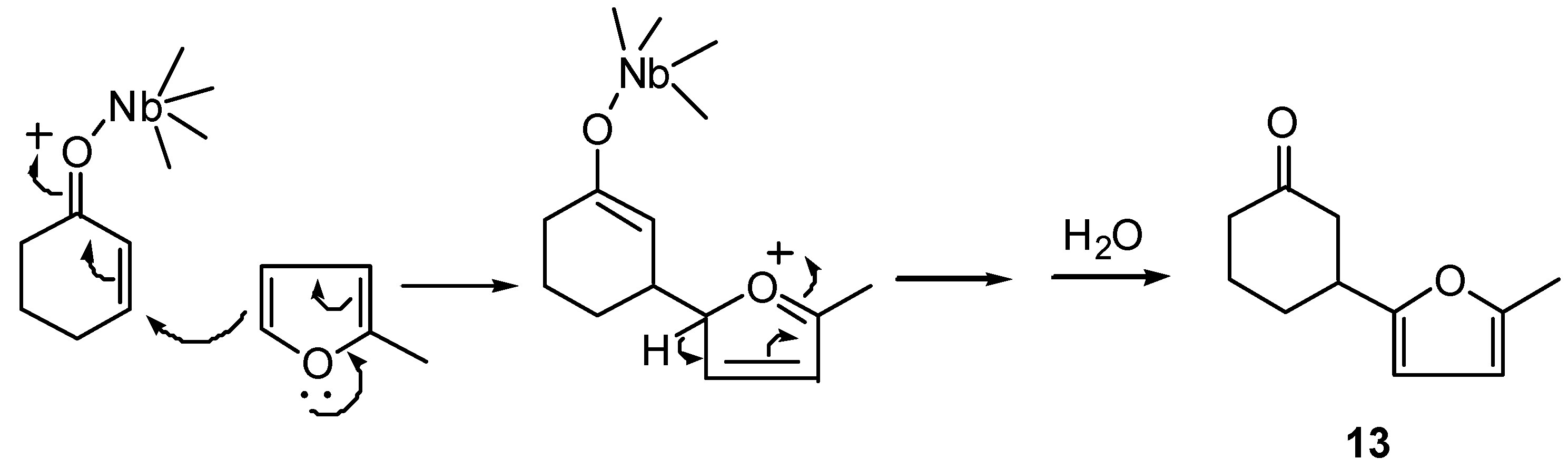
(±)-3-(5-Methyl-furan-2-yl)-cyclohexanone (13): 1H-NMR (400 MHz): δ 5.88 (d, 1H, J=3.3 Hz), 5.85 (d, 1H, J=3.3 Hz), 3.12 (tt, 1H, J1=J2=10.0 and J3=J4=4.5 Hz,), 2.66 (ddt, 1H, J1 = 14.5, J2 = 4.5 and J3=J4=1.6 Hz), 2.51 (ddd, 1H, J1=14.5, J2=10,0 and J3=1.3 Hz), 2.37 (m, 2H), 2.25 (s, 3H), 2.15 (m, 1H), 2.01 (m, 1H), 1.82 (m, 2H); 13C-NMR (100 MHz): δ 210.41 (C=O), 155.38 (C), 150.39 (C), 105.80 (CH), 105.05 (CH), 45.75 (CH2), 41.27 (CH2), 37.65 (CH), 30.04 (CH2), 24.41 (CH2), 13.47 (CH3); IR (film) νmax: 3115, 2948, 2872, 1713, 1616, 1569, 1446, 1319, 1221, 1022 cm-1; MS m/z (rel. intensity) (%): 178 [M+] (6), 121 (15), 108 (10), 77 (4), 55 (6), 42 (13), 32 (28), 28 (100).
(±)-3-Methyl-3-(5-methyl-furan-2-yl)-cyclohexanone (14): 1H-NMR (400 MHz): δ 5.86 (d, 1H, J =3.0 Hz), 5.81 (d, 1H, J=3.0 Hz), 2.71 (dt, 1H, J1=14.0 and J2=J3=1.5 Hz), 2.31 (m, 3H), 2.23 (s, 3H), 2.20 (m, 1H), 1.86 (m, 1H), 1.73 (m, 1H), 1.64 (m, 1H), 1.30 (m, 3H); 13C-NMR (100 MHz): δ 210.79 (C=O), 157.89 (C), 150.79 (C), 105.68 (CH), 105.64 (CH), 51.88 (CH2), 40.71 (CH2), 40.44 (C), 35.91 (CH2), 27.18 (CH3), 22.06 (CH2), 13.47 (CH3); IR (film) νmax: 3115, 2948, 2857, 1709, 1607, 1557, 1455, 1349, 1221, 1115, 1022 cm-1; MS m/z (rel. intensity) (%): 192 [M+] (4), 149 (4), 135 (10), 122 (8), 98 (3), 77 (4), 55 (35), 28 (100).
(±)-2-[3,3-Bis-(5-methyl-(2-furan-2-yl)-cyclohexyl]-5-methyl-furan (15): 1H-NMR (400 MHz): δ 6.11 (d, 1H; J=3.0 Hz), 5.90 (d, 1H, J=3.0 Hz), 5.84 (d, 1H, J=3.3 Hz), 5.81 (d, 1H, J=3.3 Hz), 5.76 (d, 1H, J=3.0 Hz), 5.64 (d, 1H, J=3.0 Hz), 2.81 (tt, 1H, J1=J2=12.0 and J3=J4=3.3 Hz), 2.74 (m, 1H), 2.48 (m, 1H), 2.23 (s, 3H), 2.21 (s, 3H), 2.80 (s, 3H), 1.95 (m, 1H), 1.92 (t, 1H, J=12.0 Hz), 1.84 (dd, 1H, J1=12.0 and J2=3.5 Hz), 1.73 (m, 1H), 1.55 (ddt, 1H, J1=25.0, J2=14.0 and J3=J4=3.5 Hz), 1.37 (dd, 1H, J1=25.0, J2=12.5 Hz), 13C-NMR (100 MHz): δ 159.88 (C), 159.02 (C), 155.06 (C), 151.07 (C), 150.70 (C), 150.43 (C), 108.06 (CH), 106.60 (CH), 106.23 (CH), 106.14 (CH), 104.66 (CH), 103.96 (CH), 42.40 (C), 39.12 (CH2), 33.85 (CH2), 33.81 (CH), 31.69 (CH2), 22.70 (CH2), 14.10 (CH3), 14.04 (CH3), 13.97 (CH3); IR (film) νmax: 3115, 2948, 2872, 1612, 1557, 1450, 1378, 1217, 1026 cm-1; MS m/z (rel. intensity) (%): 242 [(M-82)+] (8), 199 (7), 188 (4), 108 (5), 95 (5), 61 (4), 43 (53), 28 (100).
(±)-2-[1,3-Bis(5-methyl-(2-furan-2-yl))-3-methyl-cyclohexyl]-5-methyl-furan (16): 1H-NMR (400 MHz): δ 5.91 (d, 1H; J=3.0 Hz), 5.87 (d, 1H, J=3.0 Hz), 5.75 (s, 2H), 5.72 (d, 1H, J=3.0 Hz), 5.62 (d, 1H, J=3.0 Hz), 2.47 (d, 1H, J=13.5 Hz), 2.42 (d, 1H, J=13.5), 2.25 (s, 3H), 2.23 (s, 3H), 2.23 (m, 1H), 2.17 (s, 3H), 2.02 (m, 1H), 1.83 (m, 3H), 1.59 (m, 1H), 0.96 (s, 3H); 13C-NMR (100 MHz): δ 162.25 (C), 158.26 (C), 156.66 (C), 150.09 (C), 150.00 (C), 149.53 (C), 106.08 (CH), 105.63 (CH), 105.51 (CH), 105.35 (CH), 104.78 (CH), 102.64 (CH), 42.50 (CH2), 40.54 (C), 36.00 (CH2), 35.63 (C), 33.45 (CH2), 25.32 (CH3), 19.07 (CH2), 13.63 (CH3), 13.57 (CH3), 13.55 (CH3); IR (film) νmax: 3100, 2948, 2842, 1612, 1561, 1450, 1319, 1221, 1022 cm-1; MS m/z (rel. intensity) (%): 338 [(M)+] (2), 188 (5), 175 (5), 135 (6), 122 (6), 95 (4), 91 (4), 28 (100).