Introduction
Azides are considered very important compounds due to both their industrial as well as biological applications [
1]. Azide derivatives have been used in rubber vulcanization, polymer crosslinking, dyes, tire cored adhesives, foaming of plastics, pharmaceuticals, pesticides and herbicides [
1]. Many azide compounds show mutagenic activities [
2,
3,
4].
The chemistry of azides has thus attracted the attention of many chemists, since many of these compounds play an important role in organic chemistry [
5,
6,
7]. One of the more useful synthetic applications of azides is the preparation of 1,2,3-triazoles via 1,3-dipolar cycloaddition reactions of azides with substituted acetylene compounds [
8,
9,
10,
11,
12,
13].
The chemistry of 1,2,3-triazoles has also received much attention because of their wide range of applications. They have been used as fungicides, herbicides, light stabilizers, fluorescent whiteners, optical brightening agents, and corrosion retardants [
14,
15,
16]. Moreover 1,2,3-triazole derivatives show significant antimicrobial, cytostatic, virostatic, and anti-inflamatory activities [
17].
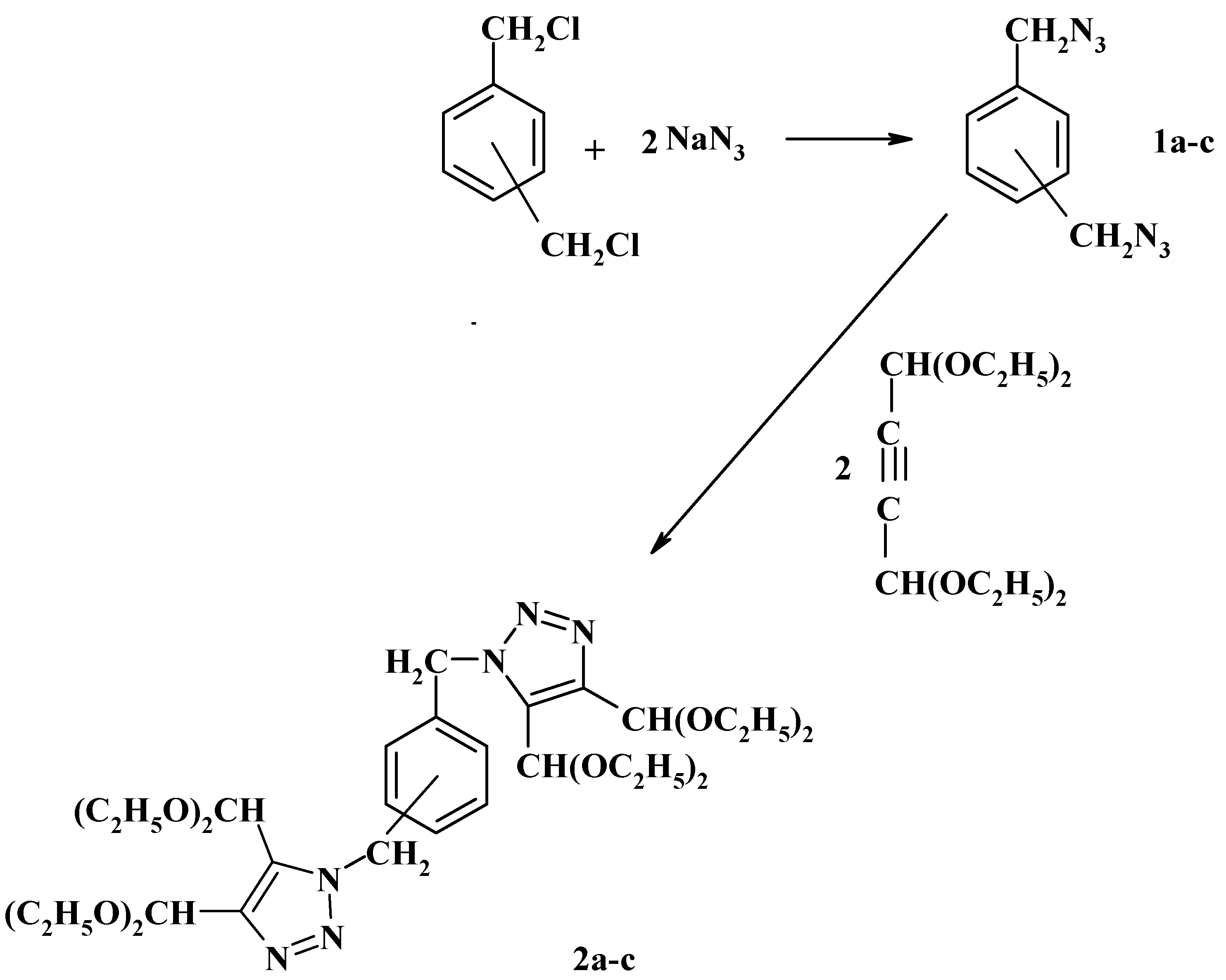
Over the last fifteen years we have contributed extensively to this field. Thus, in 1986 we reported the preparation of bis(azidomethyl)benzenes
1a-c and their reaction with acetylendicarboxaldehyde bisdiethylacetal to form the phenylenebis(methylene)bis(triazole-4,5-dicarboxaldehyde)tetrakis(ethyl acetals)
2a-c as shown in
Scheme 1 [
18].
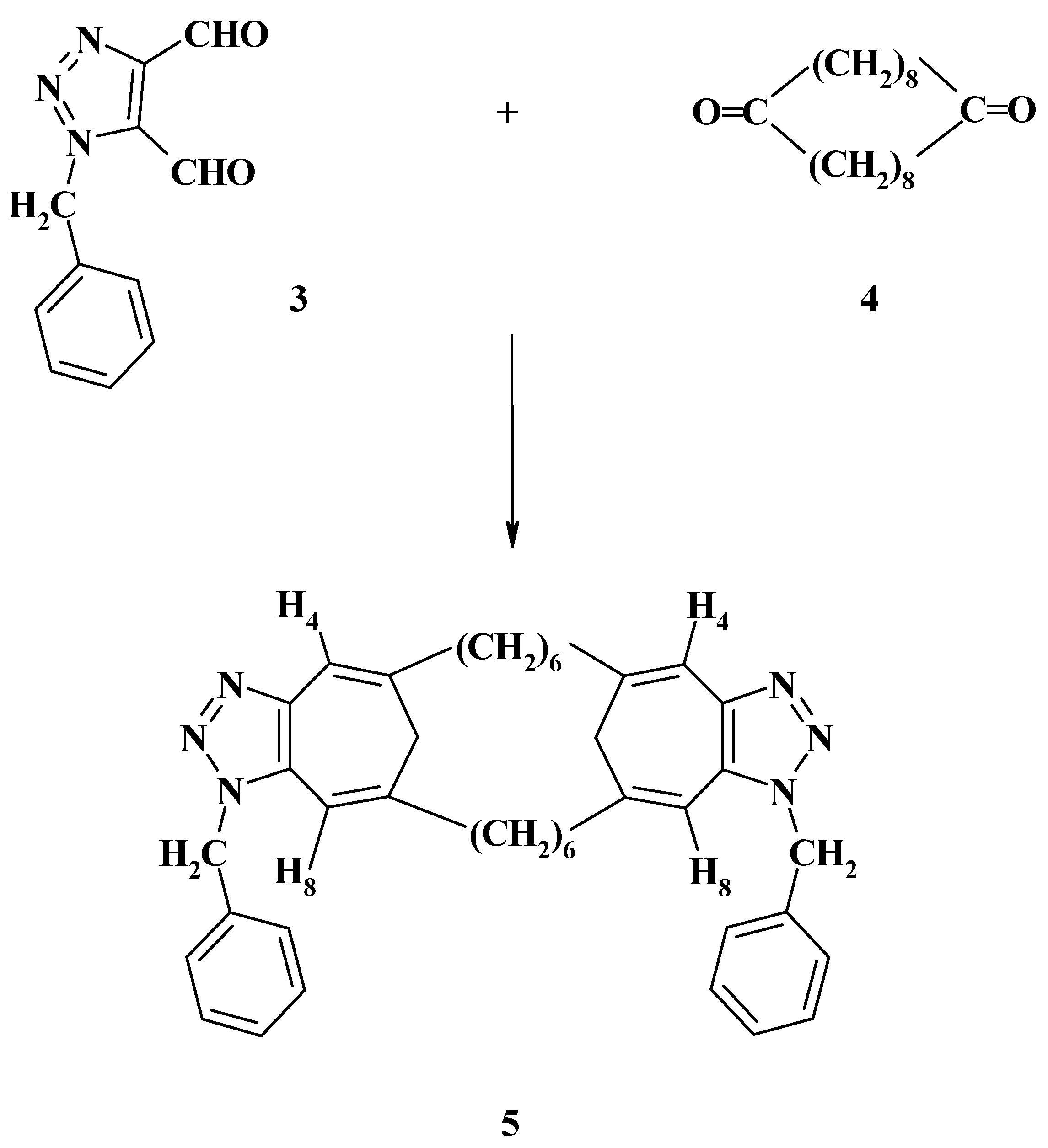
The same year we reported the synthesis of the novel ring system, 1,1΄-dibenzyl-5,5΄,7,7΄-bis(hexamethyleno)bis[6(2H)-cycloheptatriazolone] (
5) from the condensation reaction of 2 moles of 1-benzyl-
1H-triazole-4,5-dicarboxaldehyde (
3) with 1,10-cyclooctadecanedione (
4) as shown in
Scheme 2 [
19]. The aim of this synthesis was to study the effect of a polymethylene bridge on the aromaticity and planarity of a system containing two triazoletropone systems within the same molecule [
19].
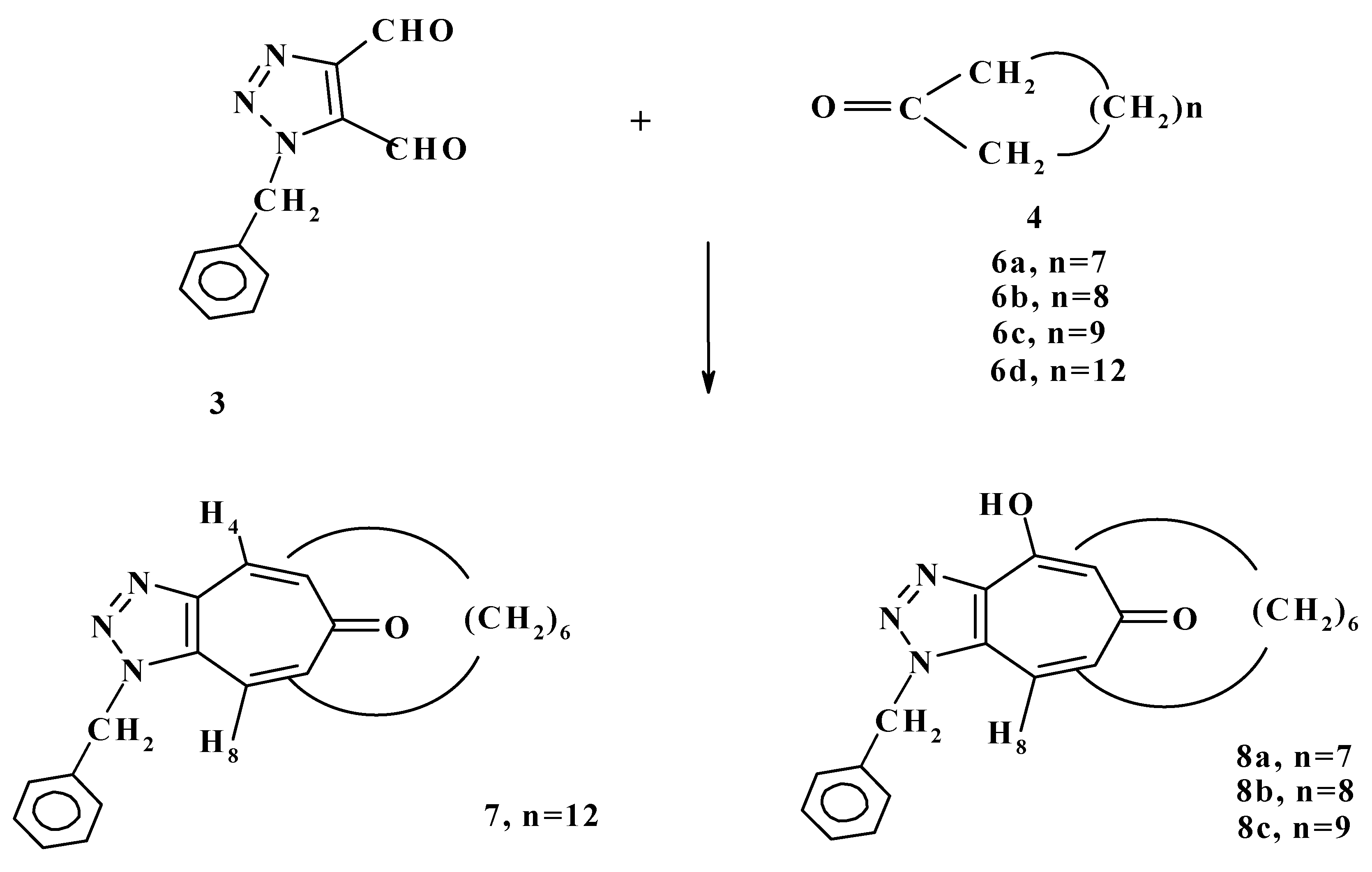
A year later we reported the condensation reaction of 1-benzyl-
1H-triazole-4,5-dicarboxaldehyde (
3) with cyclic ketones
6a-d to form 1-benzyl-5,7-polymethyleno-6-(2H)-cyclo-heptatriazolone (
7) or 4-hydroxy-1-benzyl-4,5-dihydro-5,7-polymethyleno-6-(2H)-cycloheptatriazol-ones (
8a-c) as shown in
Scheme 3 [
20].
In 1988, we described the interaction of synthetic organic azides with highly purified camel gluathione S-transferase in an attempt to elucidate the role of these enzymes in the detoxification of organic azides [
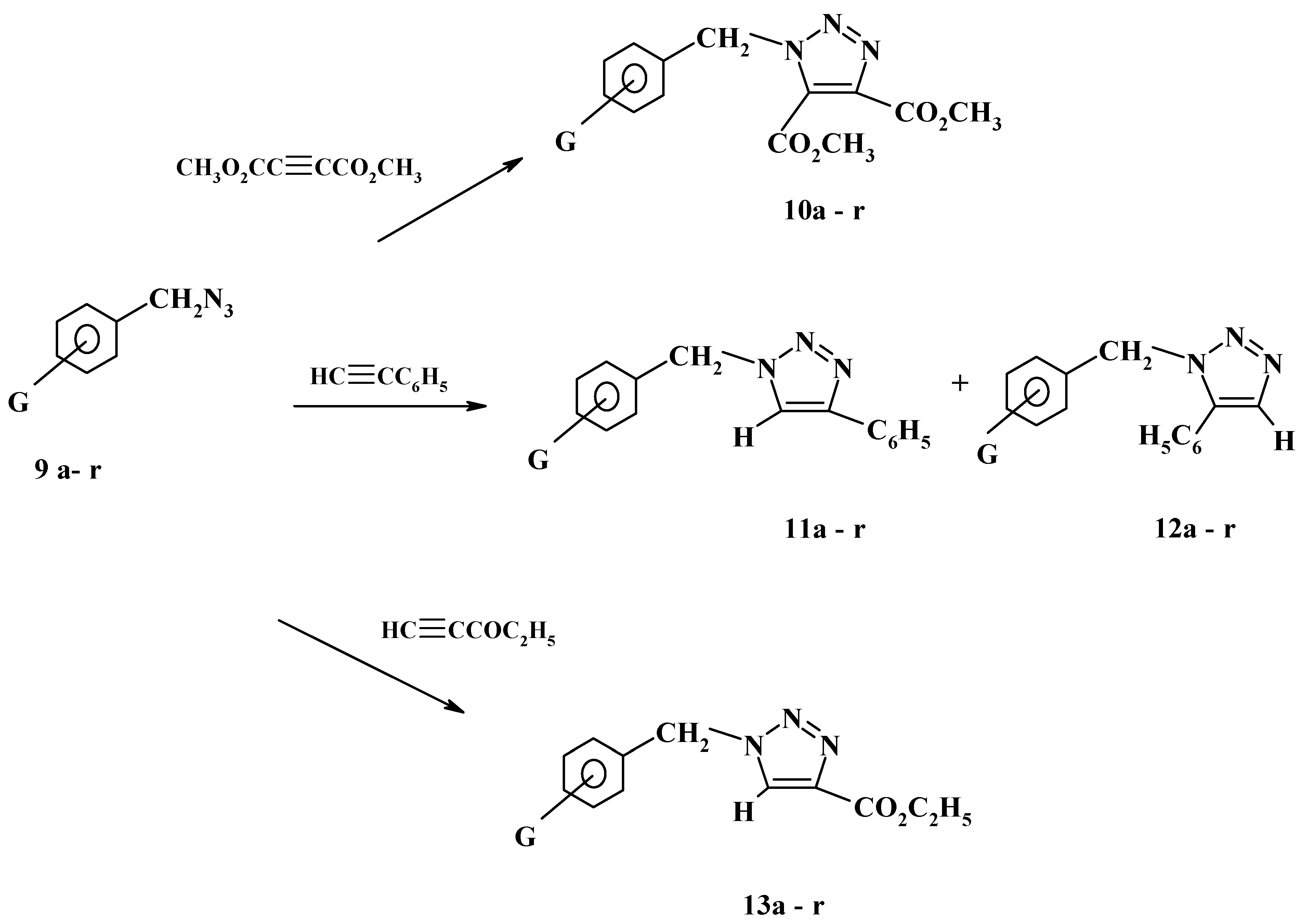
21]. A year later our group described the reaction of substituted benzyl azides with some acetylenic esters and with phenylacetylene [
22]. Thus, substituted benzyl azides
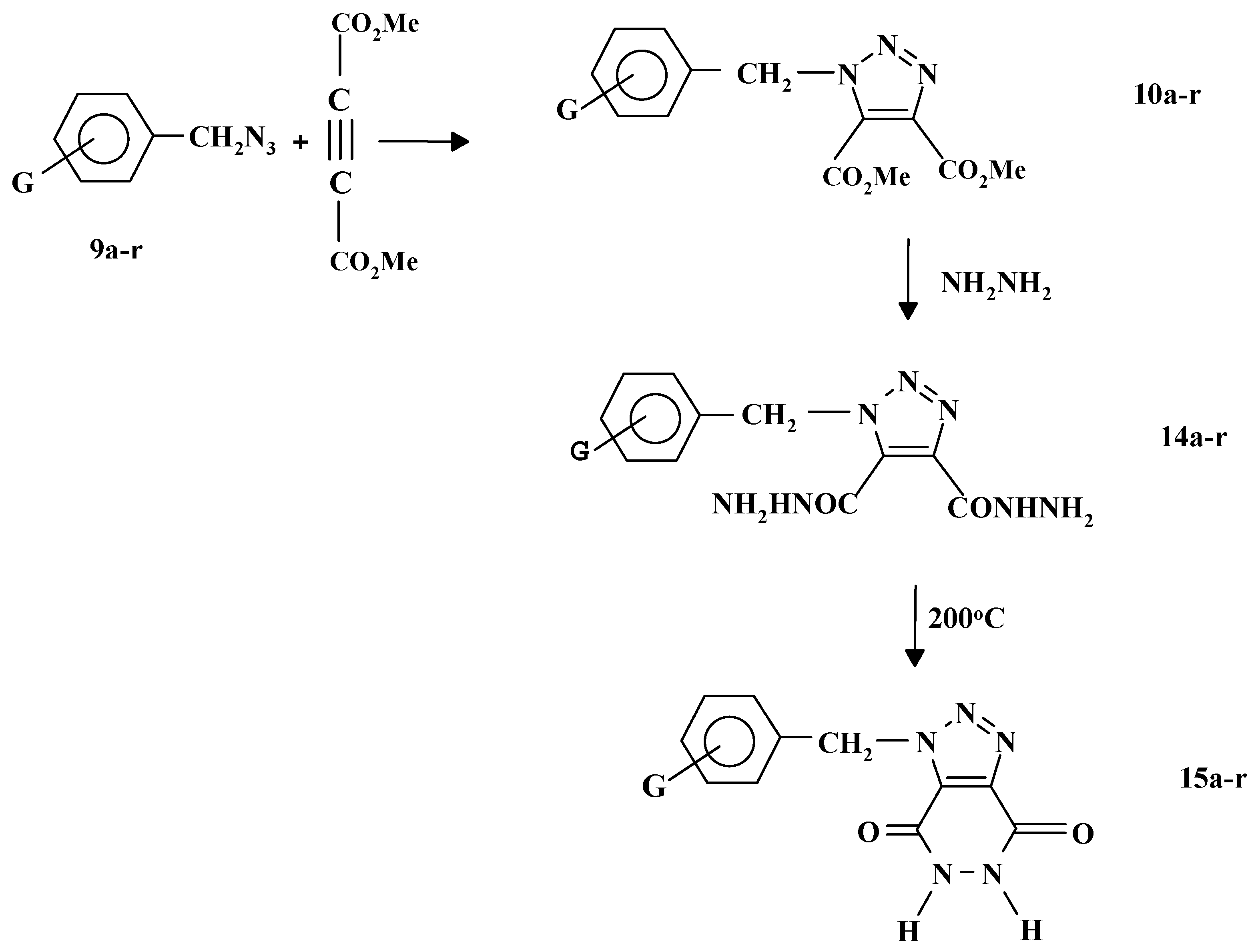
9a-r were reacted with dimethyl acetylenedicarboxylate in boiling ethanol to give the corresponding triazoles
10a-r in more than 80% yield as shown in
Scheme 4.
On the other hand, the reactions of azides
9a-r with phenylacetylene afforded, as expected and revealed by thin layer chromatography, a mixture of two isomeric products in over 85% overall yield. The isomeric triazoles
11a-r,
12a-r are shown in
Scheme 4. The product mixtures were separated by preparative thin layer chromatography using silica gel and a toluene-ethyl acetate mixture as eluent. The relative ratios of the two isomeric products was found to be in the range of 10:90 to 40:60. Surprisingly, the isomers separated in each reaction gave identical IR and
1H-NMR spectral data and melting points. This might be due to the similarity of the chemical environment of the protons in either position 4 or 5 in the triazole ring. The less hindered triazoles
11a-r are believed to be the major products on the basis of steric considerations. This assignment is quite compatible with the reported results of Tsypin and Mihelcic and their co-workers on the addition of heterocyclic and aliphatic azides to phenylacetylene [
23,
24]. Unlike the reactions with phenylacetylene, the reactions of azides
9a-r with ethyl propiolate were found to be completely regiospecific. Thin layer chromatography using different solvent systems confirmed the presence of a single product in each reaction. These products are believed to be triazoles
13a-r as depicted in
Scheme 4.
Reactions of
10a-r with excess hydrazine hydrated under reflux conditions gave the corresponding bis (hydrazinocarbonyl) derivatives
14a-r. Heating compounds
14a-r at 200 °C for about 4-5 hrs was found to be a convenient method for the synthesis of 1-substituted benzyl-1H-1,2,3-triazolo [4,5-d] pyridazidine-4,7-diones
15a-r as shown in
Scheme 5 [
25].
Scheme 5.
| Compound | G |
|---|
| a | H |
| b | 4-CH3 |
| c | 3-CH3 |
| d | 2-CH3 |
| e | 4-OCH3 |
| f | 3-OCH3 |
| g | 4-NO2 |
| h | 3-NO2 |
| i | 2-NO2 |
| j | 2-Cl |
| k | 3-Cl |
| l | 4-Cl |
| m | 4-Br |
| n | 3-Br |
| o | 2-Br |
| p | 2-F |
| q | 3-F |
| r | 4-F |
In 1991, we reported the reactions of bis(azidomethyl) benzenes
1a-c with several substituted acetylenes that formed the corresponding bistriazoles [
26]. Thus 1,2-, 1,3- and 1,4-bis (azidomethyl) benzenes (
1a-c) underwent cycloaddition reactions with dimethyl-, diethyl- and di-
tert-butyl acetylenedicarboxylate, respectively, in methanol or ethanol at reflux temperature to form the corresponding tetramethyl, tetraethyl and tetra-
tert-butyl 1,1΄-(phenylenedimethylene) bis-1
H-1,2,3-triazole-4,5-dicarboxylates
16a-c,
17a-c and
18a-c, respectively, in quantitative yields as shown in
Scheme 6. The completion of the reaction was monitored by the disappearance of the azide IR absorption in the range 2170-2220 cm
-1.
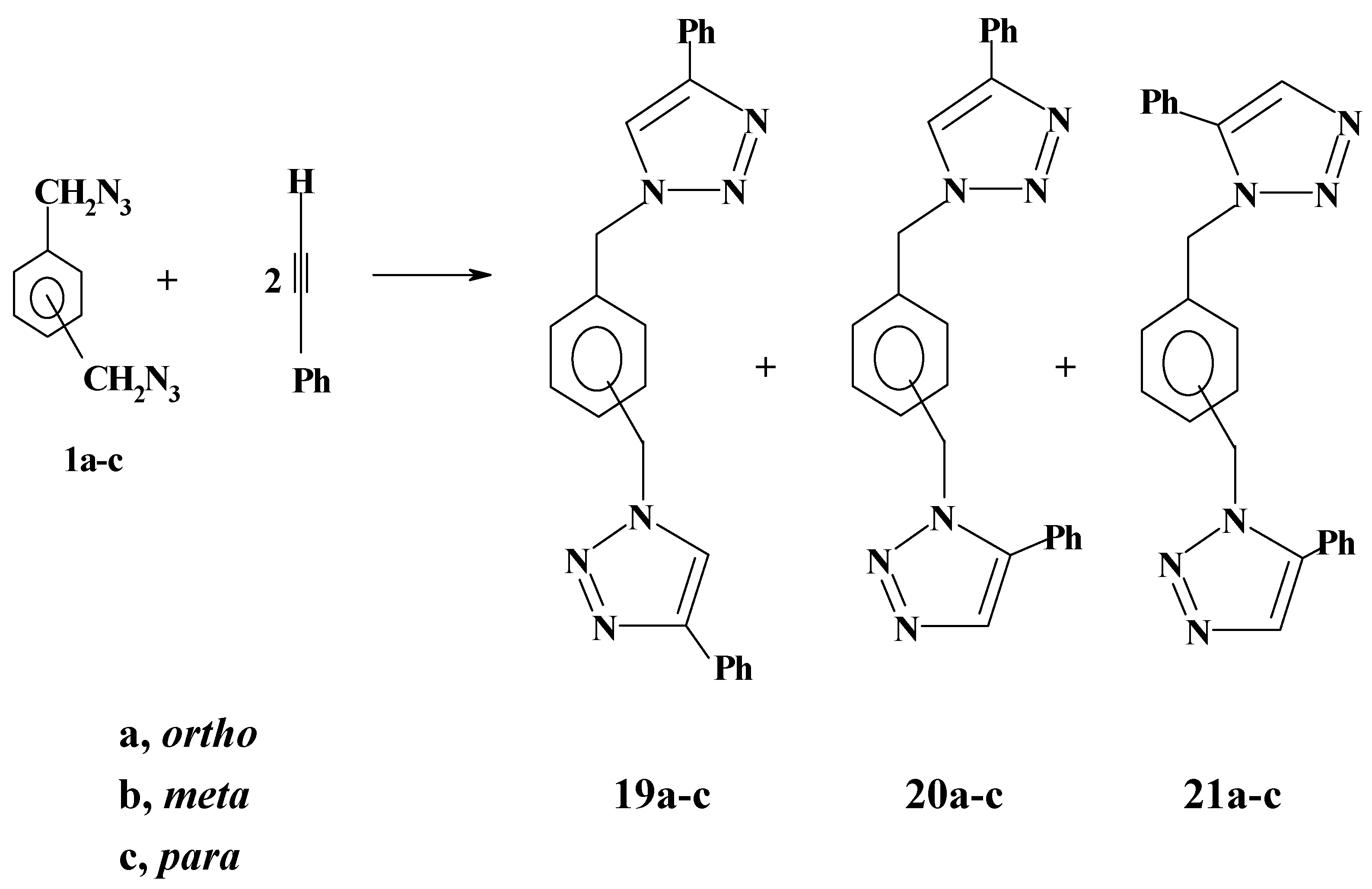
Likewise, bis-azides
1a-c reacted with phenylacetylene to give a mixture of two isomeric products as revealed by thin-layer chromatography, although three isomeric products are theoretically possible as shown in
Scheme 7.
1H-NMR spectroscopy did not differentiate between the two isomeric bistriazoles. Thus, the four benzylic protons in the product mixture appeared as a sharp singlet in the range 5.52-5.70 ppm. This is quite consistent with our previously reported results on the reactions of monoazides with phenylacetylene [
22]. Although the two products were not separated, it is believed that the major products are the less sterically hindered 1,1΄- (phenylendimethylene) bis-[4-phenyl-
1H-1,2,3-triazoles]
19a-c while the minor products should be the isomers
20a-c on the basis of steric and statistical considerations. The isomeric bistriazoles
21a-c are excluded on the same basis. This regiochemical assignment is compatible with the literature reports, one of the recent reports being published by Fouli and co-workers [
27].
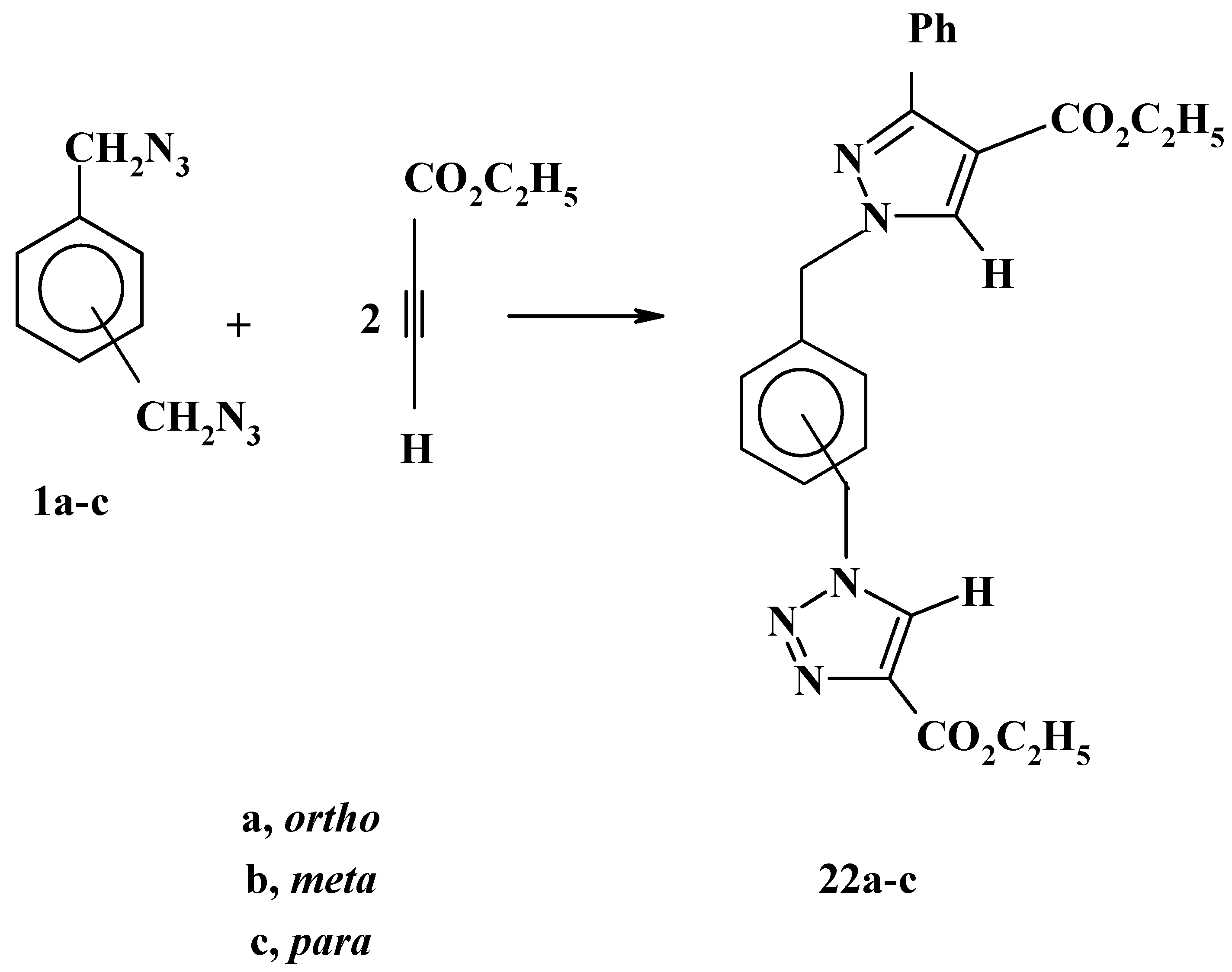
In great contrast with the latter reactions, the reactions of bis-azides
1a-c with ethyl propiolate, have displayed complete regiospecificity under similar conditions. Only one product was obtained in a high yield, again, this result is consistent with our previous findings [
22]. In addition, on the basis of steric and electronic factors, the products are assigned structures
22a-c as shown in
Scheme 8. The remarkable difference in the reactivities of the three bis-azides
1a-c is worthy of comment. The trend is quite clear. The 1,4-isomer
1c is the most reactive whereas the 1,2-isomer
1a is the least reactive. indeed, the decisive factor is the steric hindrance which is lowest in the 1,4-bis-azide
1c and highest in the 1,2-isomer
1a [
26].
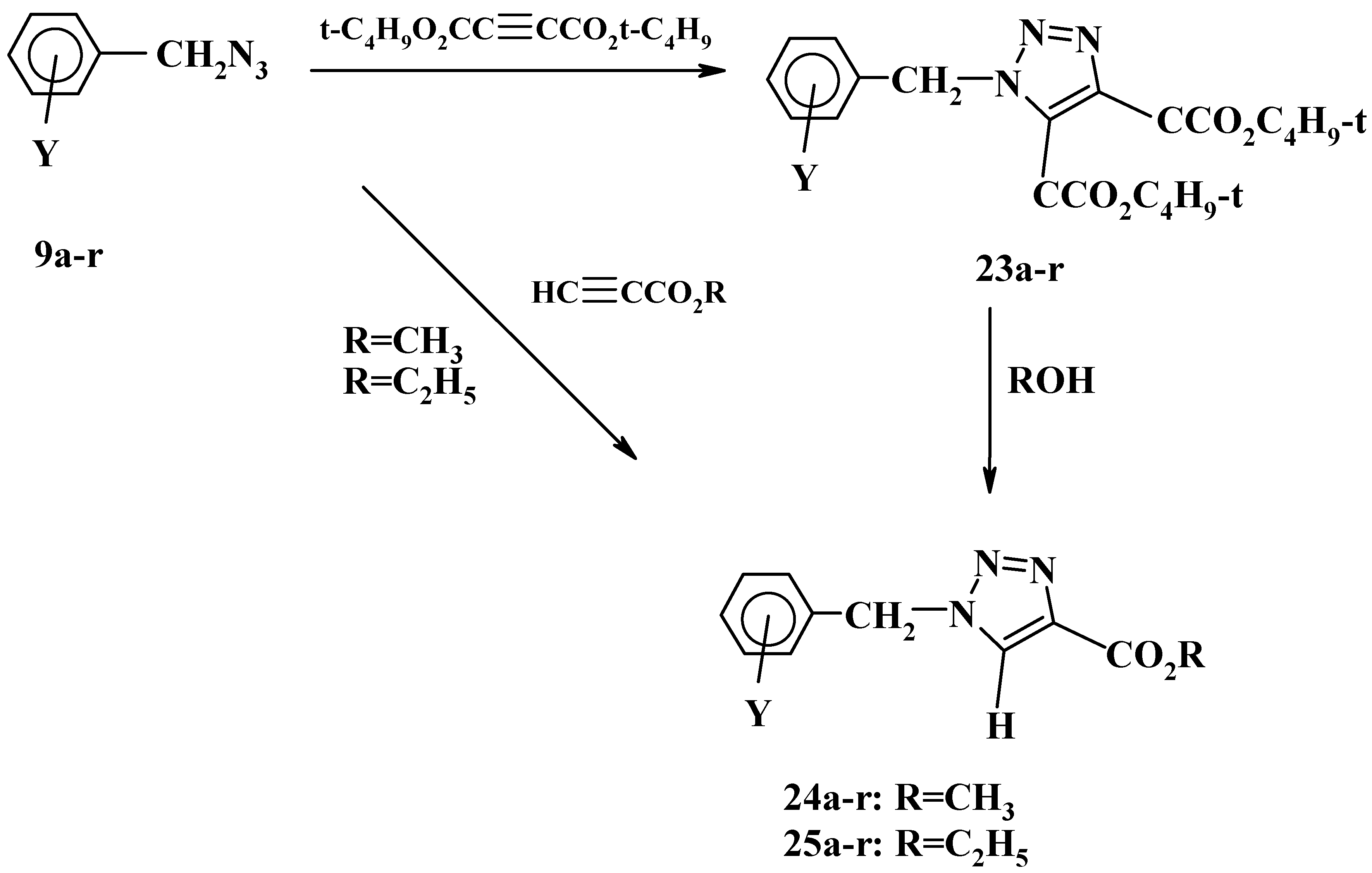
Moreover, we have studied the effect of solvent and reaction time on the products of the 1,3-dipolar cycloaddition of substituted benzyl azides with di-
tert-butyl acetylenedicarboxylate [
28]. Substituted benzyl azides
9a-r underwent cycloaddition reactions with di-
tert-butyl acetylenedicarboxylate, in refluxing methanol or ethanol for 3-20 hrs producing di-
tert-butyl 1-(substituted benzyl)-1
H-1,2,3-triazole-4,5-dicarboxylates
23a-r in 73-94% yield, as shown in
Scheme 9.
The reactions of azides
9a-r with di-
tert-butyl acetylenedicarboxylate, however, was found to be sensitive to the nature of the solvent being used and the type of the substituents on the phenyl ring. Thus prolonged reflux (2-3 days) of azides
9e-r in protic solvents such as methanol or ethanol resulted in the formation of the triazole monoesters, methyl 1-(substituted benzyl)-
1H-1,2,3-triazole-4-carboxylates
24e-r and ethyl 1-(substituted benzyl)-
1H- 1,2,3-triazole-4-carboxylates
25e-r, respectively. Unexpectedly, the reaction of azides
9a-d with di-
tert-butyl acetylenedicarboxylate did not show the same trend under the same conditions since the triazole diesters
23a-d were the only products obtained [
28].
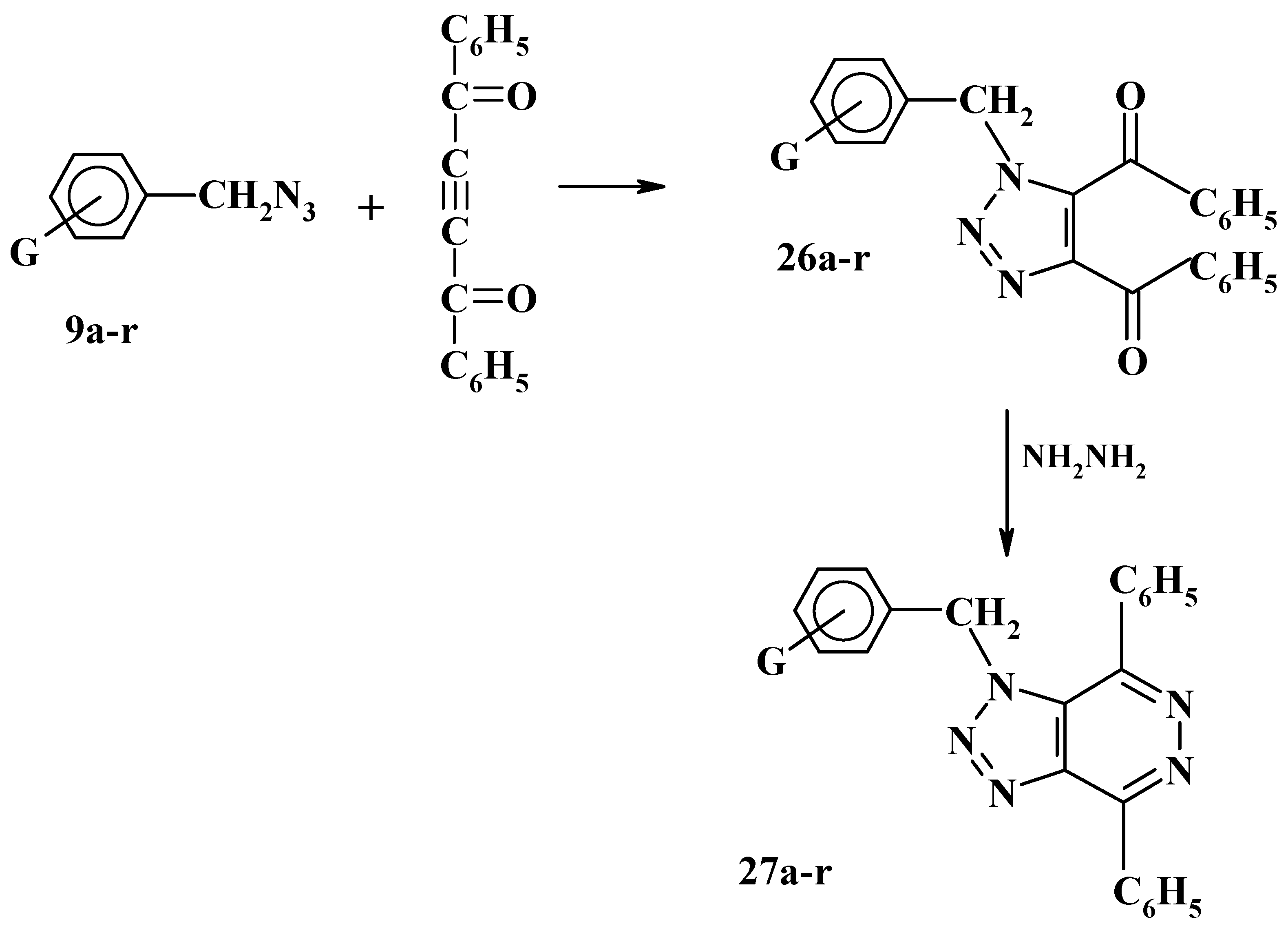
Recently, we have investigated the 1,3-dipolar cycloaddition reactions of substituted benzyl azides
9a-r and bis (azdiomethyl) benzenes
1a-c with dibenzoylacetylene to afford the corresponding 1H-1,2,3-triazole derivatives
26a-r and
28a-c respectively. Reaction of these triazoles
26a-r and
28a-c with hydrazine hydrated in ethanolic solution was found to produce in high yield the corresponding triazolopyridazine and bis (triazolopyridazine) derivatives
27a-r and
29a-c, respectively, as shown in
Scheme 10 and
Scheme 11 [
29]. The inhibition effect of some of these 1-substituted benzyl-4,5-dibenzoyl-1,2,3-triazoles
26a-r on the corrosion activity of mild steel in acid media has been investigated recently and it was found that these triazoles can be used as corrosion inhibitors [
16,
30,
31].
In the last few years, we have studied the cycloaddition reactions of substituted benzyl azides
9a-r with trans-1,2-dibenzoylethylene. This study presented another good example of the drastic difference in the final products due to the difference in thermal stability of theadducts resulting from the cycloaddition process, namely triazoles and triazolines [
32,
33,
34]. Thus, when azides
9a-r reacted with trans-1,2-dibenzoylethylene in boiling ethanol, the enamines
31i-k,n-q and enolimines
32a-h,l,m,r were formed unexpectedly. The formation of enamines
31 and enolimines
32 might be explained by the formation of the corresponding thermally unstable triazoline
30a-r, followed by ring cleavage and loss of a nitrogen molecule to afford the final products as keto-enol tautomers
31 and
32 as shown in
Scheme 12 [
32,
33,
34]. In 1999, we succeeded in the preparation of a series of 2-[1-benzyl-1,2,3-triazolo-4]-5-aryl-1,3,4-oxadiazole compounds
35 from ethyl 1-substituted benzyl-1,2,3-triazole-4-carboxylate
13 as shown in
Scheme 13 [
35].
The same year, we synthesized a series of 4,5-bis [5-aryl-1,3,4-oxadiazol-2-yl]-1-substituted benzyl-1,2,3-triazole compounds
38 from the reactions of dimethyl-1-substituted benzyl-1,2,3-triazole-4,5-dicarboxylate (
10) with hydrazine, followed with addition of aroyl chlorides in aqueous THF in the presence of K
2CO
3 which resulted in dehydration and cyclization as shown in
Scheme 14 [
36].