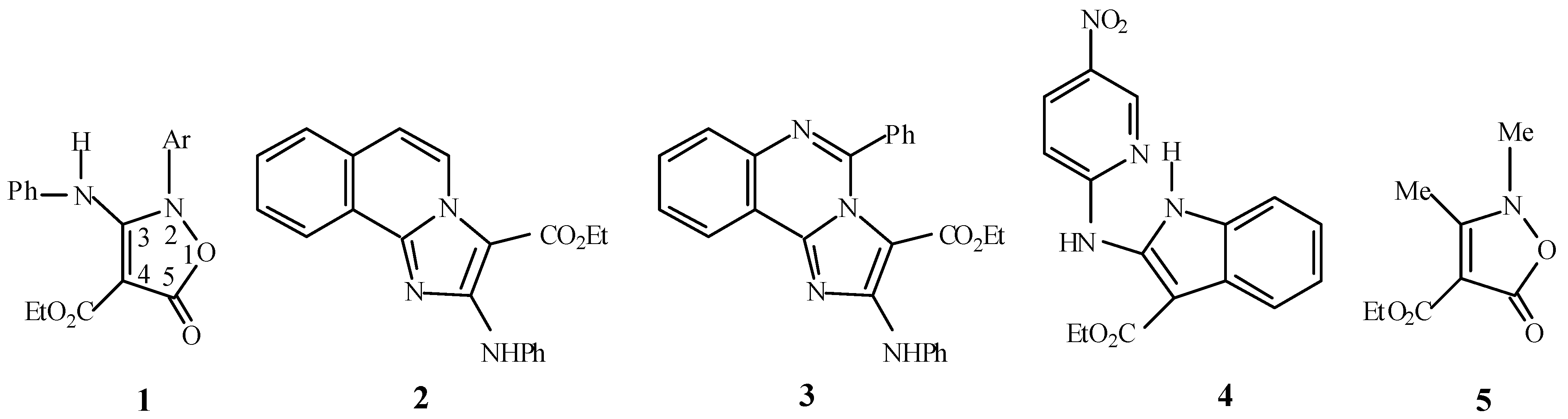
Experimental
General
Freshly distilled solvents were used throughout, and anhydrous solvents were dried according to Perrin and Amarego [
9].
1H-NMR and
13C-NMR spectra were recorded, in deuteriochloroform, unless otherwise stated, at 500 and 125 MHz respectively, with a Bruker DRX-500 Avance spectrometer. Tetramethylsilane was used as an internal standard and all signals due to amino protons were removed by exchange with D
2O. Infrared spectra were recorded on a Unicam Matsson 1000 Fourier-Transform Spectrometer. Mass spectra were recorded on a Varian Matt 311 spectrometer and relative abundance of fragments are quoted in parentheses after the m/z values. Melting points were determined on a Philip Harris C4954718 apparatus and are uncorrected. Micronalyses were preformed on a Carlo-Erba Analyzer 1104 at the University of Giessen, Germany.
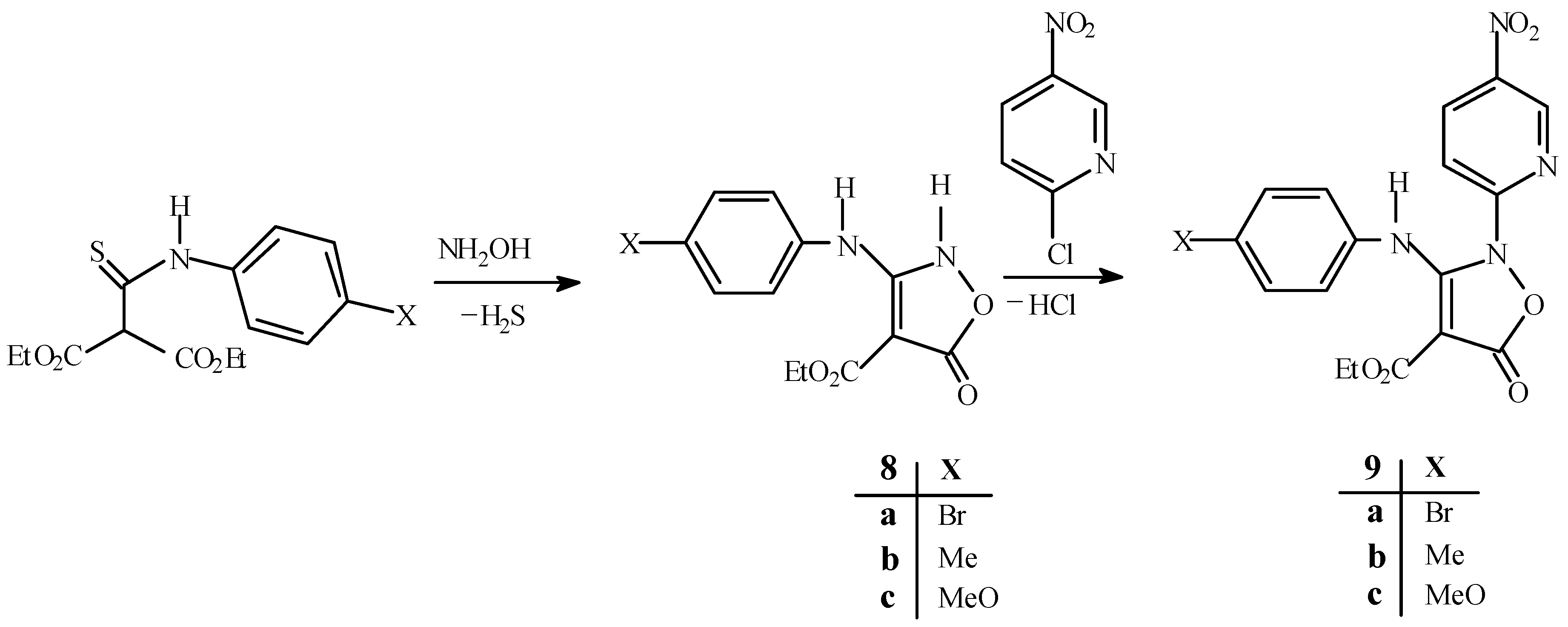
Diethyl (4-bromophenyl)thiocarbamoylmalonate (6a)
Sodium (2.9 g, 0.126 mol) was reacted with absolute ethanol (50 mL) and diethyl malonate (20g, 18.95 ml, 0.126 mol) was added at rt. The reaction mixture was stirred at room temperature for 15 min, 4‑bromophenyl isothiocyanate (26.96 g, 0.126 mol) was added and stirring was continued for further 2 h. The resulting precipitate was filtered off and washed with light petroleum to give the salt of 6a (39.91g, 80%) as yellow crystals, m.p.=163-164 °C. The salt was dissolved in water (30-40 mL) and neutralized by dropwise addition of dilute HCl. The mixture was stirred for 15 min and the precipitate was filtered to give the title compound (29.02g, 77%) as a pale yellow solid, m.p.= 52-53 °C; Rf (toluene): 0.27; 1H-NMR: δ= 1.35 (t, J=7.1Hz, 6H), 4.321 (q, J=7.1Hz, 2H), 4.326 (q, J=7.1Hz, 2H), 5.09 (s, 1H), 7.55 (d, J=8.6Hz, 2H), 7.73 (d, J=8.6Hz, 2H), 10.9 (bs, 1H, NH). 13C-NMR: δ= 14.34, 63.63, 67.68, 120.37, 125.13, 132.37, 137.89, 166.08, 188.05; IR: v= 3285, 1759, 1723, 1548, 1431, 1285, 1146, 1023, 831 cm -1.
The following thiocarbamates were made by the same procedure.
Diethyl (4-methylphenyl)thiocarbamoylmalonate (
6b). Pale yellow solid (90%); m.p.= 54 °C (lit [
10], 55-56 °C); R
f (toluene): 0.37;
1H-NMR: δ=1.35 (t, J=7.15Hz, 6H), 2.38 (s, 3H), 4.321 (q, J=7.15Hz, 2H), 4.325 (q, J=7.15Hz, 2H), 5.11 (s, 1H), 7.23 (d, J=8.3Hz, 2H), 7.66 (d, J=8.3Hz, 2H), 10.77 (bs, 1H, NH);
13C-NMR: δ= 14.35, 21.57, 63.47, 67.62, 123.64, 129.86, 136.41, 137.43, 166.16, 187.68; IR
v= 3284,1760, 1723, 1515, 1430, 1315, 1223, 1148, 1020, 831 cm
-1.
Diethyl (4-methoxyphenyl)thiocarbamoylmalonate (
6c). Pale yellow solid (87%); m.p.= 57-58 °C (lit [
10], 58.5-59.5°C); R
f (toluene): 0.74;
1H-NMR: δ= 1.34 (t, J=7.15Hz, 6H), 3.83(s, 3H), 4.312 (q, J=7.15 Hz, 2H), 4.316, (q, J=7.15 Hz, 2H), 5.10 (s, 1H), 6.94 (d, J=8.9Hz, 2H), 7.67 (d, J=8.9Hz, 2H), 10.7 (bs, 1H, NH);
13C-NMR: δ= 14.33, 55.87, 63.46, 67.38, 114.39, 125.29, 131.94, 158.62, 166.18, 187.55; IR
v=3284, 1760, 1723, 1515, 1306,1254, 1151, 1030, 842 cm
-1.
Ethyl 3-(4-bromophenyl)amino-5-oxo-2,5-dihydroisoxazole-4-carboxylate (8a).
To a solution of hydroxylamine hydrochloride (7.06 g, 102 mmol) in water (30 mL), sodium bicarbonate (10.17 g, 102 mmol) was added slowly. Ethanol (80 mL) was added and the resulting potassium chloride was filtered off. Diethyl (4-bromophenyl)thiocarbamoylmalonate (6a, 12.71g, 34 mmol) was added to the filtrate and the mixture was stirred at room temperature for 24 h. The reaction mixture was acidified with dilute HCl and the white precipitate was collected and recrystallized from acetone to give the title product (8.78 g, 79%) as colourless needles, m.p.= 200 º C (dec.); 1H-NMR (d6-DMSO): δ= 1.25 (t, J=7.1Hz, 3H), 4.21 (q, J=7.1 Hz, 2H), 7.37(d, J=8.4Hz, 2H), 7.57 (d, J=8.4Hz, 2H), 8.30 (bs, 1H, NH), 9.39 (bs, 1H, NH); 13C-NMR (d6-DMSO): δ= 15.31, 59.96, 74.69, 118.02, 125.08, 132.94, 137.10, 163.53, 164.74, 167.39; IR v= 3250, 2950, 2740, 1723, 1696, 1666, 1607, 1563, 1456, 1398, 1316, 1183, 1018, 818 cm-1.
The following compounds were made by the same procedure:
Ethyl 3-(4-methylphenyl)amino-5-oxo-2,5-dihydroisoxazole-4-carboxylate (8b). Refluxing for 24h gave colourless crystals (85%), m.p.= 164-166 °C (dec.); 1H-NMR (d6-DMSO + CDCl3): δ= 0.95 (t, J=7Hz, 3H), 1.94 (s, 3H), 3.91(q, J=7Hz, 2H), 6.78 (d, J=9.2Hz, 2H), 6.79(bs, 1H, NH), 6.80(d, J=9.2Hz, 2H), 8.85 (bs, 1H, NH); 13C-NMR (D6-DMSO+ CDCl3): δ= 14.52, 20.85, 60.08, 74.69, 121.53, 130.13, 133.29, 135.64, 163.59, 165.51, 166.74; IR: v= 3669, 2979, 2746, 1705, 1669, 1615, 1331, 1208, 1115, 1023, 800 cm-1.
Ethyl 3-(4-methoxyphenyl)amino-5-oxo-2,5-dihydroisoxazole-4-carboxylate (8c). Refluxing for 24 h gave the desired product (7.75g, 80%) which was recrystallized from ethanol/acetone (1:1) as a white solid, m.p.= 206-207 °C (dec.); 1H-NMR (d6-DMSO+CDCl3): δ= 0.95 (t, J= 7Hz, 3H), 3.35 (s, 3H), 3.83 (q, J=7Hz, 2H), 6.38 (d, J=8.5Hz, 2H), 6.94 (d, J=8.5Hz, 2H), 6.96 (bs,1H, NH), 7.70 (bs, 1H, NH); 13C-NMR(d6-DMSO+CDCl3): δ= 15.64, 55.55, 58.37, 73.5, 114.6, 118.6, 135.73, 153.7, 165.73, 168.14, 174.81; IR: v=3407, 1708, 1615, 1554, 1248, 1077, 792 cm-1.
Ethyl 3-(4-bromophenyl)amino-2-(5-nitropyrid-2-yl)-5-oxo-2,5-dihydroisoxazole-4-carboxylate (9a).
A mixture of 2-chloro-5-nitropyridine (48.5 mg, 0.30 mmol) and ethyl 3-(4-bromophenyl) amino-5-oxo-2,5-dihydroisoxazole-4-carboxylate (8a, 100 mg, 0.30 mmol) was heated neat under nitrogen in an oil bath at 130 °C for 2 h. The residue was recrystallized from ethanol to give the desired isoxazolone as yellow crystals (112 mg, 82%), m.p.= 218 °C; Rf (CH2Cl2): 0.28; Analysis: found C, 46.10, H, 2.82, N, 12.45%; C17H13BrN4O6 requires C, 45.43, H, 2.89, N, 12.47%; 1H-NMR (D6-DMSO+CDCl3): δ= 0.99 (t, J=7Hz, 3H), 3.94 (q, J=7Hz, 2H), 6.87 (d, J=8.5Hz, 2H), 7.18 (d, J=8.5Hz, 2H), 7.73 (d, J=9.1Hz,1H), 8.40 (dd, J1=9.1Hz, J2=2.3Hz, 1H), 8.75 (d, J=2.3Hz, 1H), 10.29 (bs, 1H, NH); 13C‑NMR (d6-DMSO+CDCl3): δ= 14.41, 60.99, 79.05, 114.63, 119.46, 124.11, 132.46, 135.08, 137.21, 141.66, 143.79, 153.92, 158.31, 161.38, 165.88; IR v=3140, 2965, 1773, 1683, 1591, 1531, 1324, 1188, 1114, 1010, 961, 832 cm-1; MS m/z: 450 (M+, 27%), 448(M+, 30%), 406(74), 404(77), 279(100), 251(20), 184(35), 182(36), 157(29), 155(29), 102(22), 72(23), 44(59).
The following compounds were made by the same procedure.
Ethyl 3-(4-methylphenyl)amino-2-(5-nitropyrid-2-yl)-5-oxo-2,5-dihydroisoxazole-4-carboxylate (9b). Yellow needles (85%), m.p.= 156-158 °C, after recrystalization from ethanol; Rf (CH2Cl2) 0. 24; Analysis: found C, 56.25, H, 3.93, N, 24.74%; C18H16N4O6 requires C, 56.25, H, 4.16, N, 24.58%; 1H-NMR : δ= 1.29 (t, J=7.05Hz, 3H), 2.30 (s, 3H), 4.26 (q, J=7.05Hz, 2H), 7.04 (d, J=8.5Hz,2H),7.07(d, J=8.5Hz, 2H), 7.07 (d, J= 9.0Hz, 1H), 8.55 (dd, J1=9.0Hz, J2=2.5Hz, 1H), 8.91 (d, J=2.5Hz, 1H), 10.33 (s, 1H, NH); 13C-NMR: δ= 14.66, 21.35, 61.34, 79.05, 115.42, 122.40, 130.29, 134.74, 135.36, 136.83, 141.89, 143.92, 154.28, 160.88, 163.62, 164.19; IR v= 3177, 1762, 1700, 1600, 1515, 1338, 1208, 1123, 976, 838 cm-1; MS m/z 384 (M+, 13%), 340 (100), 294 (57), 269 (16), 248 (40), 230 (16), 220 (16), 158 (39), 144 (13), 118 (21), 117 (20), 107 (16), 91 (67), 78 (16), 65 (20), 44 (33).
Ethyl 3-(4-methoxyphenyl)amino-2-(5-nitropyrid-2-yl)-5-oxo-2,5-dihydroisoxazole-4-carboxylate (9c).
Yellow needles (80%), m.p.= 186-188 °C; Rf (CH2Cl2): 0.68; Analysis: found C, 54.10, H, 3.78, N, 14.11%; C18H16N4O7 requires C, 54.00, H, 4.00, N, 14.00%; 1H-NMR: δ= 1.30 (t, J=7Hz, 3H), 3.77 (s, 3H), 4.26 (q, J=7Hz, 2H), 6.79 (d, J=8.7Hz, 2H), 7.10 (d, J=8.7Hz, 2H), 7.52 ( d, J=9.0Hz,1H), 8.54(dd, J1=9 Hz, J2=2.1Hz, 1H), 8.93 (bd, J=2.1, 1H), 10.26 (s,1H, NH); 13C-NMR: δ= 14.72, 55.89, 61.36, 78.87, 114.85, 115.59, 124.34, 130.74, 134.68, 141.93, 143.95, 154.33, 158.40, 161.38, 163.69, 164.32; IR v=3823, 1785, 1700, 1592, 1345, 1207, 1115, 1030, 838 cm-1; MS m/z: 400 (M+, 10%), 356 (100), 310 (49), 295 (43), 264 (21), 249 (13), 221 (14), 193 (12), 194 (10), 174 (21), 146 (10), 134 (34), 133 (22), 123 (17), 92 (16), 77 (29), 44 (37).
Butyl 3-(4-methylphenyl)amino-2-(5-nitropyrid-2-yl)-5-oxo-2,5-dihydroisoxazole-4-carboxylate (10).
Ethyl 3-(4-methylphenyl)amino-5-oxo-2,5-dihydroisoxazole-4-carboxylate (100 mg, 0.30 mmol), and 2-chloro-5-nitropyridine (48.5 mg, 0.30 mmol) were refluxed in 1-butanol (5 mL), for 12 hours. On cooling, the product was collected and washed with cold ethanol to give the title compound as bright pale needles (126 mg, 80 %), m.p.=176 °C; Rf (CH2Cl2): 0.26; Analysis: found C, 57.41, H, 4.62, N, 13.66%; C20H20N4O6 requires C, 58.25, H, 4.85, N, 13.59%; 1H-NMR: δ= 0.96 (t, J=7.5Hz, 3H), 1.42 (sx, J=7.5Hz, 2H), 1.65 (qn, J=7.1Hz, 2H), 2.30 (s, 3H), 4.20 (t, J=6.7Hz, 2H), 7.04 (d, J=8.3Hz, 2H), 7.07 (d, J=8.3Hz, 2H), 7.54 ( d, 9.1 Hz, 1H), 8.55 (dd, J1=9.1 Hz, J2=1.8 Hz, 1H), 8.91 (bd, J=1.8 Hz, 1H), 10.32 (s,1H, NH); 13C-NMR: δ= 14.12, 19.43, 21.33, 30.99, 65.07, 79.03, 115.39, 122.39, 130.27, 134.7, 135.35, 136.74, 141.86, 143.92, 154.28, 160.74, 163.52, 164.15; IR v= 3446, 2831, 1792, 1700, 1600, 1515, 1345, 1123, 846 cm-1; MS m/z : 412 (M+, 7%), 368 (100), 340(13) ,338(18) ,312(12) ,294(65) ,269 (22), 248 (32), 220 (15), 158 (24), 144 (12), 118 (20), 107 (18), 91 (44), 78 (10), 77 (12), 57 (17), 44 (38).
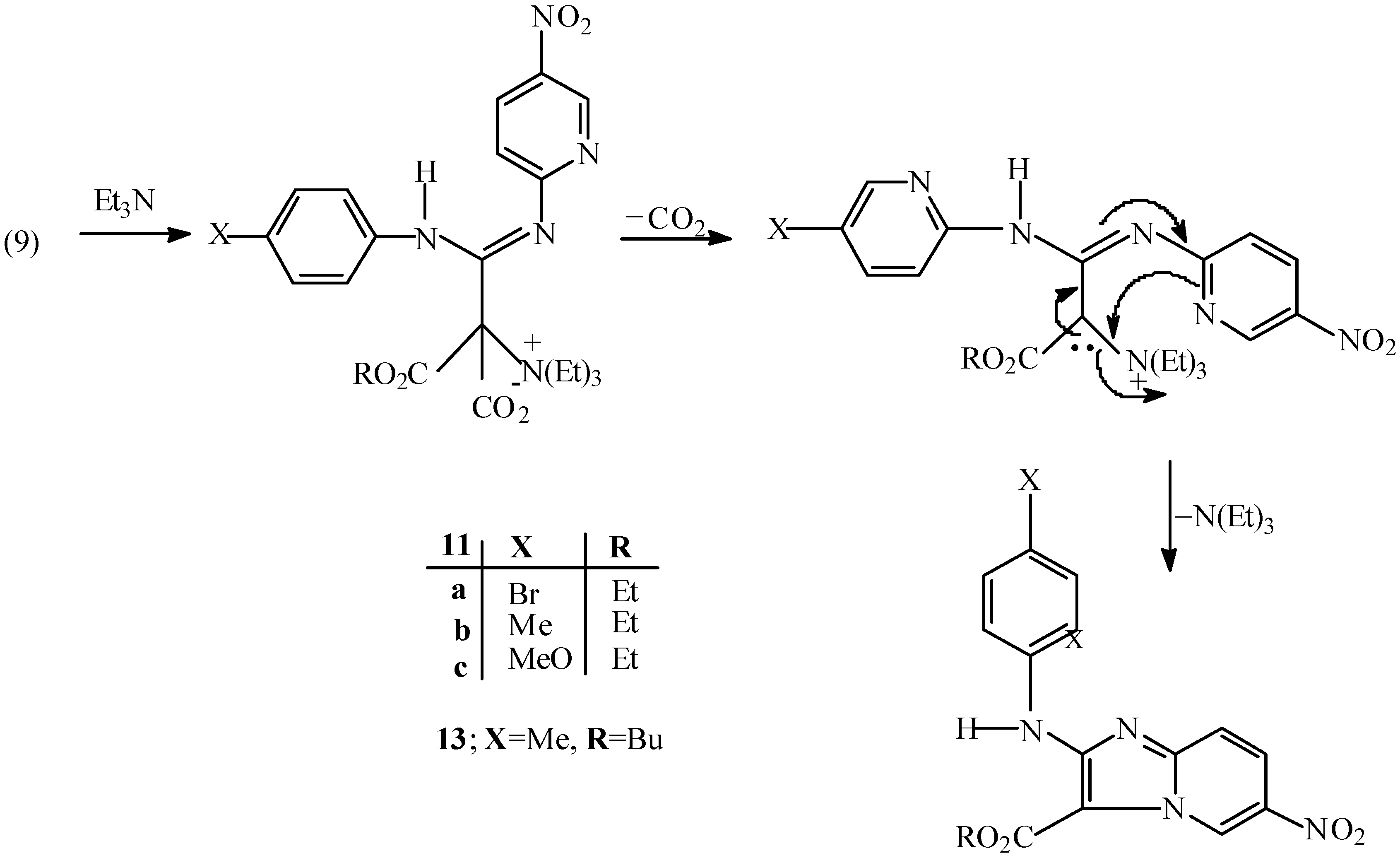
Ethyl 2-(4-bromophenyl)amino-6-nitroimidazo[1,2-a] pyridine-3-carboxylate (11a).
The isoxazolone 9a (100 mg, 0.23 mmol) and triethylamine (0.2 mL) were refluxed in ethanol (10 mL) for 1 h. The mixture was left to cool to room temperature and the desired compound was collected as pale cream needles (75.76 mg, 84 %), m.p.= 195 °C; Rf (CH2Cl2): 0.72; 1H-NMR: δ= 1.57 (t, J=7.1Hz, 3H), 4.59(q, J=7.1Hz, 2H), 7.48 (d, J=8.7Hz, 2H), 7.56 (d, J=9.7Hz, 1H), 7.65 (bd, J=8.7Hz, 2H), 8.19(dd, J1=9.7Hz, J2=1.3 Hz, collapsed to doublet, J=8.7Hz, by irradiation at δ=9.87, 1H), 8.97 (bs, 1H, NH), 9.87 (bd, J=1.3Hz, 1H); 1H-NMR (d6-DMSO): δ= 1.44 (t, J=7Hz, 3H), 4.46 (q, J=7Hz, 2H), 7.49 (d, J=8.5Hz), 7.68 (d, J=9.7Hz, 1H), 7.74 (d, J=8.5Hz , 2H), 8.19 (dd, J1=9.7Hz, J2=1.6Hz, 1H), 8.87 (bd, J=1.6, 1H), 9.88 (s,1H, NH); 13C-NMR (d6-DMSO): δ= 15.25, 61.57, 99.72, 114.62, 114.97, 121.76, 123.93, 127.61, 132.38, 137.66, 140.01, 147.17, 155.28, 160.87; IR v=3285, 2955, 1643, 1611, 1555, 1475, 1331, 1294, 1201, 1102, 1079, 1002, 820 cm-1; MS m/z : 406 (M+, 62%), 404 (M+, 64%), 360 (5), 358 (6), 279 (100), 251 (16), 233 (14), 205 (12), 184 (11), 182 (12), 157 (12), 155 (12), 102 (14), 78 (13), 77 (11).
Ethyl 2-(4-methylphenyl)amino-6-nitroimidazo[1,2-a]pyridine-3-carboxylate (11b).
The above procedure using 9b (100 mg, 0.26 mmol) and triethylamine (0.2 mL) gave the desired imidazole as pale cream needles (66.4 mg, 75%), m.p.=187-188 °C; Rf (CH2Cl2): 0.66; Analysis: found C, 60.03, H, 4.63, N, 16.68% calc; for C17H16N4O4 required: C, 60.00, H, 4.70, N, 16.47%; 1H-NMR: δ=1.27 (t, J=6.9Hz, 3H, CH3), 2.36 (s, 3H, Me), 4.57 (q, J=6.9Hz, 2H, CH2), 7.19 (d, J=8.2Hz, 2H, Ar), 7.51 (d, J=9.7Hz, 1H, Ar), 7.59 (bd, J=8.2Hz, 2H, Ar), 8.15 (bd, J=6.7Hz, 1H, Ar), 8.85 (bs, 1H, NH), 9.84 (bs, 1H, Ar); 13C-NMR: δ= 15.03, 21.22, 61.44, 98.99, 114.30, 119.33, 122.80, 127.26, 130.07, 132.93, 137.11, 147.34, 157.76, 161,15; IR v=3455, 1662, 1608, 1555, 1308, 1208, 1015, 822 cm-1; MS m/z: 340 (M+, 100%), 294(48), 248(27), 220(9), 144(10), 118(13), 91(20), 78(6), 65(6).
Butyl 2-(4-methylphenyl)amino-6-nitroimidazo [1,2-a] pyridine-3-carboxylate (13).
The above procedure using 10b (100 mg, 0.26 mmol) and triethylamine (0.2 mL) gave the desired imidazole as pale cream needles, (72.35mg, 81%), m.p.= 156 °C; Rf (CH2Cl2): 0.63; Analysis: found C, 61.33, H, 5.20, N, 15.15%; C19H20N4O4 requires C, 61.95, H, 5.43, N, 15.21%; 1H-NMR: δ= 1.08 (t, J= 7.4Hz, 3H), 1.59 (sx, J=7.3Hz, 2H), 1.91 (qn, J= 7.2Hz, 2H), 2.36 (s, 3H), 4.52 (t, J=6.5Hz, 2H), 7.20 (d, J=8.0Hz, 2H), 7.52 (d, J=9.7Hz, 1H), 7.60 (bd, J=8.0Hz, 2H), 8.15 (bd, J=9.3Hz, 1H), 8.85 (bs, 1H, NH), 9.86 (bs, 1H); 13C-NMR: δ= 14.17, 19.02, 21.20 ,31.30, 65.19, 99.05, 114.30, 119.23, 122.66, 127.27, 130.07, 132.89, 137.13, 147.31, 157.56, 160.72; IR v=3328, 2961, 1669, 1608, 1572, 1315, 1208, 1085, 815 cm-1; MS m/z: 368 (M+, 100%), 340 (17), 294 (67), 248 (32), 220 (16), 144 (13), 118 (17), 91 (26), 78 (9), 42 (17).
Ethyl 2-(4-methoxyphenyl)amino-6-nitroimidazo[1,2-a]pyridine-3-carboxylate (11c) and ethyl 5-methoxy-2-(5-nitropyrid-2-ylamino)indole-3-carboxylate (12).
The isoxazolone 9c (100 mg, 0.25 mmol) and triethylamine (0.2 mL) were refluxed in ethanol (10 mL) for 3 h. On cooling to room temperature, the precipitate was filtered to give an orange solid (71.20 mg), shown (NMR and TLC) to be a mixture of two compounds, which were separated by silicagel p.l.c eluting three times with dichloromethane. The first band was separated and washed with n-hexane to give 12 as orange needles (17.80 mg, 20%), m.p.= 210-213 °C; Rf (CH2Cl2): 0.90; 1H-NMR: δ=1.52 (t, J=7.1Hz, 3H), 3.92 (s, 3H), 4.47 (q, J=7.1Hz, 2H), 6.86 (dd, J1=8.7Hz,J2=2.5Hz,1H), 6.92(d, J=9.1Hz, 1H), 7.32 (d, J=8.7Hz, 1H), 7.45 (bs, 1H), 8.42 (dd, J1=9.1Hz, J2=2.6Hz,1H), 9.26 (d, J=2.6Hz, 1H), 10.72 (bs, 1H, NH), 11.45 (s,1H, NH); 13C-NMR: δ= 15.02, 56.13, 50.43, 89.99, 103.86, 111.00, 111.69, 112.14, 125.67, 126.89, 133.76, 138.65, 145.26, 145.59, 156.41, 156.58; IR v= 3345, 1642, 1615, 1500, 1331, 1215, 1117, 1035, 838 cm-1; MS m/z: 356 (M+, 70%), 310 (100), 295 (27), 264 (32), 249 (13), 221 (29), 193 (12), 194 (10), 150 (13), 78 (6), 77 (5), 40 (5). The second band was separated and washed with n-hexane to give 11c as a red solid (53.4 mg, 59%), m.p.= 160-161 °C; Rf (CH2Cl2): 0.79; Analysis: found C, 57.88, H, 4.50, N, 16.12%; C17H16N4O5 requires C, 57.30, H, 4.49, N, 15.73%; 1H-NMR: δ= 1.56 (t, J=7.0Hz, 3H), 3.85 (s, 3H), 4.58 (q, J=7.0Hz, 2H), 6.95 (d, J=8.9Hz, 2H), 7.50 (d, J=9.7Hz, 1H) 7.62 (bd, J=8.9Hz, 2H), 8.16 (dd, J1=9.7Hz, J2=1.9Hz, 1H), 8.78 (bs, 1H, NH), 9.88 (bs, 1H); 13C-NMR: δ= 15.05, 55.97, 61.40, 98.76, 114.19, 114.82, 121.31, 122.86, 127.24, 132.85, 137.15, 147.48, 156.16, 160.86; IR v=3130, 1685, 1615, 1515, 1315, 1222, 1199, 1092, 824 cm-1; MS m/z : 356 (M+, 100%), 310 (34), 295 (43), 264 (12), 249 (12), 221 (10), 194 (8), 193 (11), 134 (16), 92 (8), 90 (8), 78 (8).