Deprotection of Acetals and Ketals by Silica Sulfuric Acid and Wet SiO2
Abstract
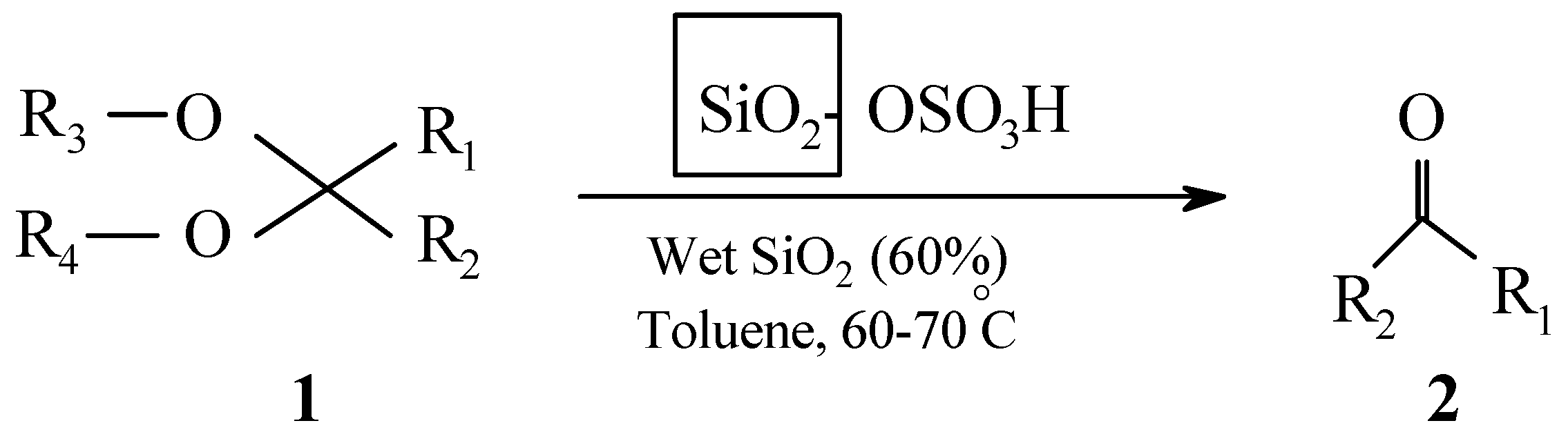
:Introduction

Results and Discussion
Conclusions
| Entry | Substrate | Substrate (mmole) / I (g) / II (g) | Time (min) | Yield (%) | Product |
|---|---|---|---|---|---|
| 1a |  | 0.2 / 0.12 / 0.2 | 60 | 95 |  |
| 1b |  | 0.35 / 0.1 / 0.1 | 60 | 94 |  |
| 1c |  | 0.26 / 0.25 / 0.27 | 60 | 98 |  |
| 1d |  | 0.25 / 0.2 / 0.2 | 60 | 93 |  |
| 1e |  | 0.25 / 0.2 / 0.2 | 75 | 94 |  |
| 1f |  | 0.3 / 0.11 / 0.15 | 60 | 97 |  |
| 1g |  | 0.25 / 0.3 / 0.3 | 60 | 98 |  |
| 1h |  | 0.2 / 0.3 / 0.3 | 60 | 97 |  |
| 1i |  | 0.25 / 0.3 / 0.3 | 75 | 95 |  |
| 1j |  | 0.25 / 0.4 / 0.4 | 60 | 98 |  |
| 1k |  | 0.25 / 0.3 / 0.3 | 60 | 96 |  |
| 1l |  | 0.25 / 0.3 / 0.3 | 60 | 98 |  |
| 1m |  | 0.25 / 0.3 / 0.4 | 60 | 97 |  |
Acknowledgments
Experimental
General
Preparation of silica sulfuric acid
Typical procedure for deacetalization of acetals (1) to the corresponding aldehydes (2): Preparation of 4-nitrobenzaldehyde (2g).
References
- Pourjavadi, A.; Mirjalili, B.F. J. Chem. Res. 1999, 562.
- Firouzabadi, H.; Iranpour, N.; Zolfigol, M.A. Bull. Chem. Soc. Jap. 1998, 71, 2169. [CrossRef]
- Green, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis; John Wiley and Sons, Inc.: New York, 1991. [Google Scholar]
- Sen, S.E.; Roach, S.L.; Boggs, J.K.; Ewing, G.J.; Magrath, J. J. Org. Chem. 1997, 62, 6684. [CrossRef]
- Butler, D.N.; Halton, B.; Warrener, R.N. Aus. J. Chem. 2000, 53, 561.
- Hashemi, M.M.; Kalantari, F. Synth. Commun 2000, 30, 1857.
- Sterzycki, R. Synthesis 1979, 724.
- Gautier, E.G.L.; Graham, A.E.; Mckillop, A.; Standen, S.P.; Taylor, R.J.K. Tetrahedron Lett. 1997, 38, 1881.
- Ates, A.; Gautier, A.; Leroy, B.; Plancher, J.M.; Quesnel, Y.; Marko, I.E. Tetrahedron Lett. 1999, 40, 1799.
- Riego, J.M.; Sedin, Z.; Zaldivar, J.M.; Marziano, N.C.; Tortato, C. Tetrahedron Lett. 1996, 37, 513.
- Turro, N.J. Tetrahedron 1987, 43, 1589.
- Zolfigol, M.A. Synth. Commun. 2000, 30, 1593.Zolfigol, M.A.; Bagherzadeh, M.; Chehardoli, G.; Mallakpour, S.E. ibid. 2001, 31, 1149.Zolfigol, M.A.; Bagherzadeh, M.; Ghorbani Choghamarani, A.; Keypour, H.; Salehzadeh, S. ibid. 2001, 31, 1661.Zolfigol, M.A.; Sadeghi, M.M.; Mohammadpoor-Baltork, I.; Ghorbani Choghamarani, A.; Taqian-nasab, A. Asian J. Chem. 2001, 13, 887.Zolfigol, M.A.; Bagherzadeh, M.; Madrakian, E.; Ghaemi, E.; Taqian-nasab, A. J. Chem. Res. 2001, 140.Zolfigol, M.A.; Ghaemi, E.; Madrakian, E. Molecules 2001, 6, 614.Zolfigol, M.A.; Ghaemi, E.; Madrakian, E.; Kiany-Borazjani, M. Synth. Commun. 2000, 30, 2057.Zolfigol, M.A.; Madrakian, E.; Ghaemi, E. Indian J. Chem. 2000, 39B, 308.Zolfigol, M.A.; Kiany-Borazjani, M.; Sadeghi, M.M.; Memarian, H.R.; Mohammadpoor-Baltork, I. Synth. Commun. 2000, 30, 2945.Zolfigol, M.A.; Kiany-Borazjani, M.; Mallakpour, S.E.; Nassr-Isfahani, H. ibid. 2000, 30, 2573.Zolfigol, M.A.; Kiany-Borazjani, M.; Sadeghi, M.M.; Mohammadpoor-Baltork, I.; Memarian, H.R. ibid. 2000, 30, 3919.Zolfigol, M.A.; Kiany-Borazjani, M.; Sadeghi, M.M.; Memarian, H.R.; Mohammadpoor-Baltork, I. J. Chem. Res. 2000, 197.Salehi, P.; Khodaie, M.; Zolfigol, M.A.; Keyvan, A. Synth. Commun. 2001, 31, 1947.
- Olah, G.A; Molhotra, R.; Narang, S.C. J. Org. Chem. 1978, 43, 4628. [CrossRef]
- Zolfigol, M.A. Tetrahedron 2001, 57, 9509.
- Sample Availability: Samples of substrates 1c, 1g-1l and the 3-nitro and 4-cyano analogs of substrate 1g are available from MDPI.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Mirjalili, B.F.; Zolfigol, M.A.; Bamoniri, A. Deprotection of Acetals and Ketals by Silica Sulfuric Acid and Wet SiO2. Molecules 2002, 7, 751-755. https://doi.org/10.3390/71000751
Mirjalili BF, Zolfigol MA, Bamoniri A. Deprotection of Acetals and Ketals by Silica Sulfuric Acid and Wet SiO2. Molecules. 2002; 7(10):751-755. https://doi.org/10.3390/71000751
Chicago/Turabian StyleMirjalili, BiBi Fathemeh, Mohamad Ali Zolfigol, and Abdolhamid Bamoniri. 2002. "Deprotection of Acetals and Ketals by Silica Sulfuric Acid and Wet SiO2" Molecules 7, no. 10: 751-755. https://doi.org/10.3390/71000751




