Synthesis of 5-(4-X-Phenyl)-10,15,20-tris(Substituted Phenyl) Porphyrins Using Dipyrromethanes
Abstract
:Introduction
Results and Discussion
Dipyrromethane formation.
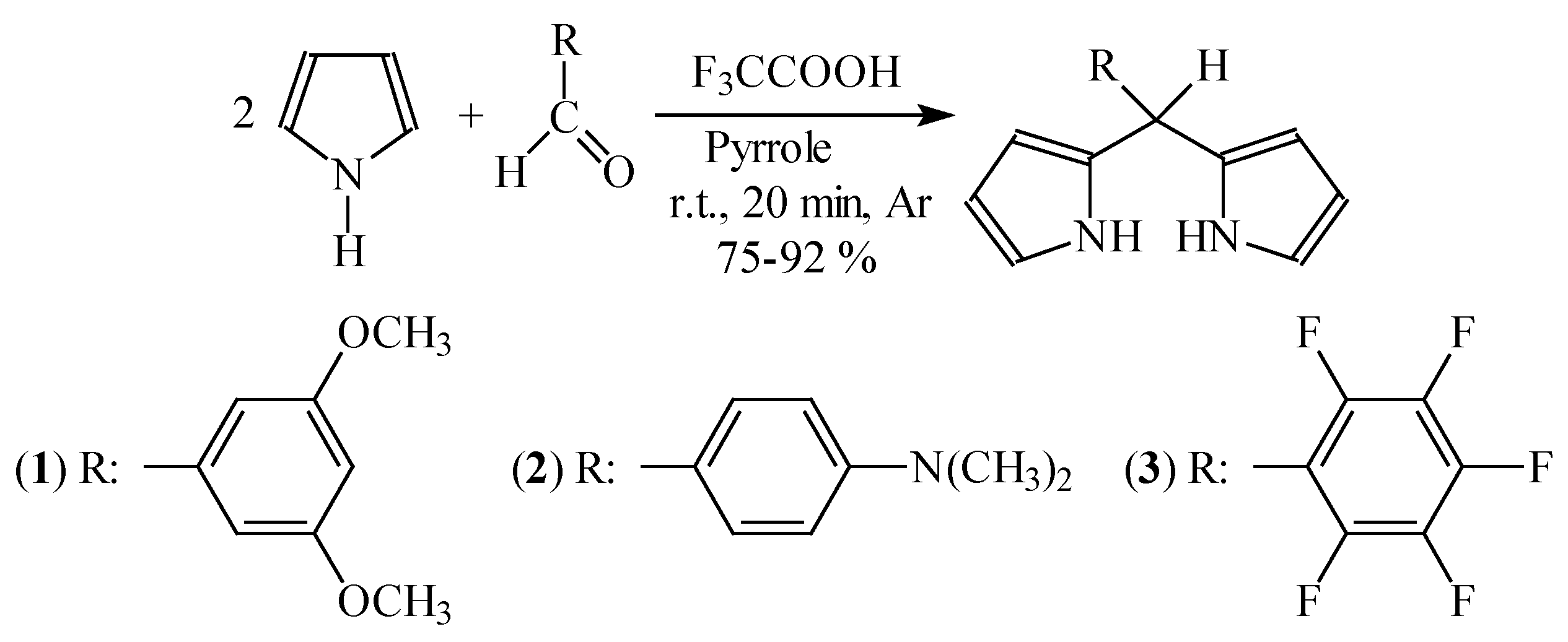
Synthesis of porphyrins

Acknowledgments
Experimental
General
Synthesis of dipyrromethane derivative.s
Synthesis of meso-substituted porphyrins
References
- Wasielewki, M. R. Photoinduced electron transfer in supramolecular systems for artificial photosynthesis. Chem. Rev. 1992, 92, 435–461. [Google Scholar] Gust, D.; Moore, T. A.; Moore, A. L. Molecular mimicry of photosynthetic energy and electron transfer. Acc. Chem. Res. 1993, 26, 198–205. [Google Scholar]
- Fungo, F.; Otero, L. A.; Sereno, L.; Silber, J. J.; Durantini, E. N. Synthesis of porphyrin dyads with potential use in solar energy conversion. J. Mater. Chem. 2000, 10, 645–650. [Google Scholar] [CrossRef]
- Durantini, E. N. Synthesis of meso-nitrophenyl porphyrins covalently linked to a polyphenylene chain bearing methoxy groups. J. Porphyrins Phthalocyanines 2000, 4, 233–242. [Google Scholar] [CrossRef]
- Gust, D.; Moore, T. A.; Moore, A. L.; Leggett, L.; Lin, S.; DeGraziano, J. M.; Hermant, R. M.; Nicodem, D.; Craig, P.; Seely, G. R.; Nieman, R. A. Photoinduced electron transfer in a porphyrin dyad. J. Phys. Chem. 1993, 97, 7926–7931. [Google Scholar] [CrossRef]
- Gust, D.; Moore, T. A.; Moore, A. L.; Gao, F.; Luttrull, D.; DeGraziano, J. M.; Ma, X. C.; Makings, L. R.; Lee, S-J.; Trier, T. T.; Bittersmann, E.; Seely, G. R.; Woodward, S.; Bensasson, R. V.; Rougée, M.; De Schryver, F. C.; Van der Auweraer, M. Long-lived photoinitiated charge separation in carotene-diporphyrin triad molecules. J. Am. Chem. Soc. 1991, 113, 3638–3649. [Google Scholar] [CrossRef]
- Lindsey, S. J.; Schreiman, Y. C.; Hsu, H. C.; Kearney, P. C.; Marguerettaz, A. M. Rothemund and Adler-Longo reactions revisited: synthesis of tetraphenylporphyrins under equilibrium condituions. J. Org. Chem. 1987, 52, 827–836. [Google Scholar] [CrossRef]
- Lee, C-H.; Lindsey, J. S. One-flask synthesis of meso-substituted dipyrromethanes and their application in the synthesis of trans-substituted porphyrin building blocks. Tetrahedron 1994, 50, 11427–11440. [Google Scholar] [CrossRef]
- Durantini, E. N.; Silber, J. J. Synthesis of 5-(4-acetamidophenyl)-10,15,20-tris(4-substituted phenyl) porphyrins using dipyrromethanes. Synth. Commun. 1999, 29, 3353–3368. [Google Scholar] [CrossRef]
- Lee, C-H.; Li, F.; Iwamoto, K.; Dadok, J.; Bothner-By, A. A.; Lindsey, J. S. Synthetic approaches to regioisomerically pure porphyrins bearing four different meso-substituteds. Tetrahedron 1995, 51, 11645–11672. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the author.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
Durantini, E.N. Synthesis of 5-(4-X-Phenyl)-10,15,20-tris(Substituted Phenyl) Porphyrins Using Dipyrromethanes. Molecules 2001, 6, 533-539. https://doi.org/10.3390/60600533
Durantini EN. Synthesis of 5-(4-X-Phenyl)-10,15,20-tris(Substituted Phenyl) Porphyrins Using Dipyrromethanes. Molecules. 2001; 6(6):533-539. https://doi.org/10.3390/60600533
Chicago/Turabian StyleDurantini, Edgardo N. 2001. "Synthesis of 5-(4-X-Phenyl)-10,15,20-tris(Substituted Phenyl) Porphyrins Using Dipyrromethanes" Molecules 6, no. 6: 533-539. https://doi.org/10.3390/60600533



