2-Substituted-1,3,2-dithioborolans as Chiral Lewis Acid Catalysts
Abstract
:Introduction
Results and Discussion
Conclusions
Experimental
References and Notes
- Howarth, J.; Helmchen, G.; Kiefer, M. Tetrahedron Lett. 1993, 34, 4095.
- Maruoka, K.; Yamamoto, H. Catalytic Asymmetric Synthesis; Ojma, I., Ed.; VCH Publishers Inc., 1993; Chapter 9. [Google Scholar]
- Mikolajczyk, M.; Perlikowska, W.; Omelanczuk, J. Synthesis 1987, 1009.
- Sample Availability: Not applicable.
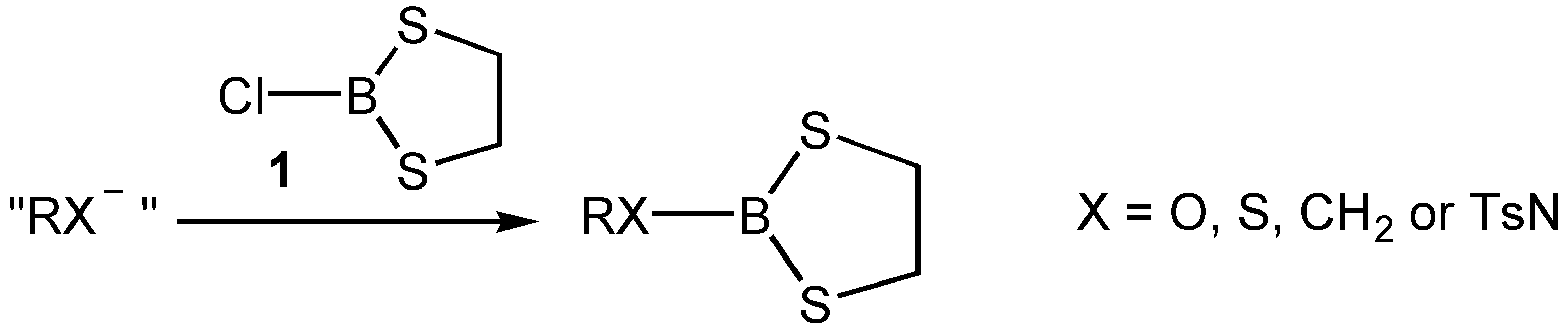
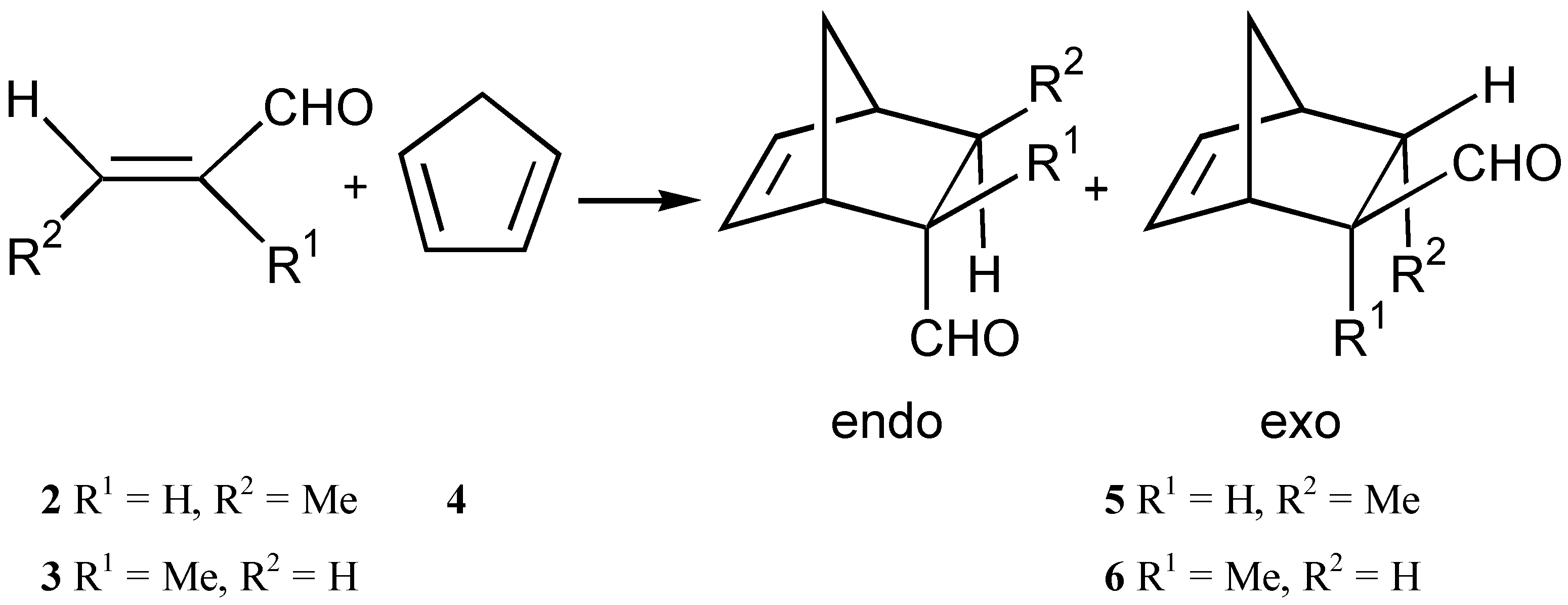
| Compound/Parent Chiral Group for RX | Crotonaldehyde | Methacrolein | ||||
|---|---|---|---|---|---|---|
| Yield% | ee% | Endo:Exo | Yield | ee% | Endo:Exo | |
| 7 Menthol | 61 | 33 | 90:10 | 69 | 54 | 8:92 |
| 8 Neothiomenthol | 64 | 35 | 94:6 | 35 | 42 | 9:91 |
| 9 (S)-(-)-N-Tosyl [3] methylbenzylamine | 51 | 38 | 90:10 | 43 | 31 | 11:89 |
| 10 (S)-(+)-Bromo-2-methylbutane | 42 | 18 | 89:11 | 34 | 20 | 11:89 |
| 11 (R)-(+)-BINOL | 25 | 41 | 90:10 | 30 | 56 | 11:89 |
| 12 (2R, 3R)-2,3-Butanediol | 40 | 24 | 90:10 | 70 | 31 | 8:92 |
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Howarth, J.; Glynn, P. 2-Substituted-1,3,2-dithioborolans as Chiral Lewis Acid Catalysts. Molecules 2000, 5, 1011-1013. https://doi.org/10.3390/50801011
Howarth J, Glynn P. 2-Substituted-1,3,2-dithioborolans as Chiral Lewis Acid Catalysts. Molecules. 2000; 5(8):1011-1013. https://doi.org/10.3390/50801011
Chicago/Turabian StyleHowarth, Joshua, and Paul Glynn. 2000. "2-Substituted-1,3,2-dithioborolans as Chiral Lewis Acid Catalysts" Molecules 5, no. 8: 1011-1013. https://doi.org/10.3390/50801011
APA StyleHowarth, J., & Glynn, P. (2000). 2-Substituted-1,3,2-dithioborolans as Chiral Lewis Acid Catalysts. Molecules, 5(8), 1011-1013. https://doi.org/10.3390/50801011




