Results and Discussion
For the present work a series of similar compounds was chosen to study the electronic and steric effects of substituents in the aromatic macrocycles. They are CuPc(R)m (Formula I), where R = 3-NO2; 4-NO2; 3-COOH; 4-COOH; (4-Br, 5-NO2); 3,5-COOH; 4,5-COOH, and m = 4 or 8 and copper(II)-octaethylporphyrin CuOEP (Formula II). The coordination group CuN4 was chosen as the reaction center. The kinetics of dissociation of copper(II)phthalocyanines in hot concentrated sulfuric acid into the solvated metal cation and protonated macrocycle (destroyed under the experimental conditions) was studied (Equations 1, 2).
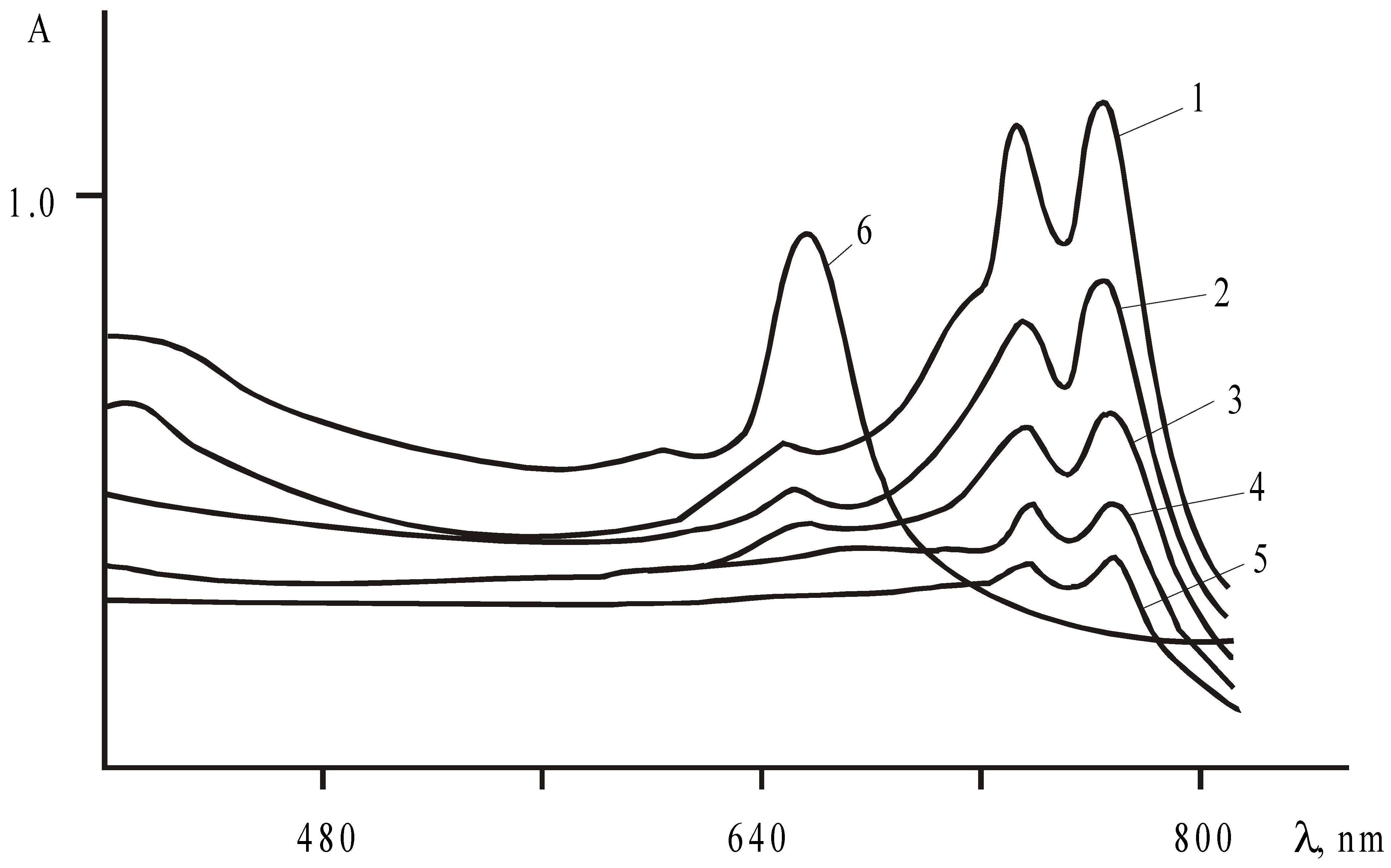
The kinetics of Reaction 1 were studied spectrophotometrically. The decrease of concentration of the copper(II)phthalocyanines was measured as the decrease of optical density at certain wavelengths during heating their sulphuric acid solutions at a defined temperature (
Fig.1).
To determine the form(s) of the phthalocyanines present in sulphuric acid, the dependence of their UV-vis spectra on the solvent nature was investigated (
Table 1). The first absorption band Q(0,0) in the studied copper(II)phthalocyanines spectra practically retains its shape and the maximum position in the sulphuric acid of different concentrations within the Brand region. Only for CuPc(4-NO
2)
4 and CuPc(3-NO
2)
4 this band shifts hypsochromically and becomes single when the H
2SO
4 concentration is decreased up to 12 mole/L. It means that the copper(II)phthalocyanines in the concentrated sulphuric acid are in the same form for all investigated substituents R and for all acid concentrations in the Brand region where their reactivity was studied. A substantial hypsochromic shift of the Q(0,0) band is observed in aprotic solvents compared with sulphuric acid solutions (
Table 1). This testifies to protonation of the substituted compounds at outer-cycle N atoms and to a cation form CuPc(R)
mH
+ in the sulphuric acid solutions, analogous with non-substituted CuPc [
1]. Thus in Equation (1) [CuPc(R)
m]
Solv = CuPc(R)
mH
+. The rate law (Equation 3) and the rate constants formally obeyed the first order (
Table 2,
Table 3) and describe Reaction 1 satisfactorily for all the investigated compounds.
Constants kobs decrease with an increase of the initial acid concentration non-linearly, in a smooth descending curve for all studied phthalocyanine complexes (except CuPc(4-Br)4(5-NO2)4). Also, there is no linear correlation between kobs and H0 values. We tried to correlate the rate constant values with the equilibrium concentrations of proton-donor particles in H2SO4.
In concentrated H
2SO
4 there are several proton-donor particles in the equilibrium concentrations: non-ionized H
2SO
4 molecules, H
3O
+ cations and also HSO
4-.H
3O
+ ion pairs and H
2O molecules in trifling amounts [
8]. The latter may be left out on account of the considered solvent concentrations (
>15.8 mole/L). Non-ionized H
2SO
4 molecules and H
3O
+ cations may be active particles when copper(II)phthalocyanines dissociate because the process demands protonation of donor N-atoms. It is known that the ratio of the activity coefficients of the acid and base forms remains constant in this range of H
2SO
4 concentrations. This allows us to use for our calculation the equilibrium concentrations instead of activities. The equilibrium concentrations of particles in H
2SO
4 at 298K, calculated from the Hammet equation [
9], were taken from [
8]. The temperature dependencies of H
0 and
were taken from [
10,
11]. As
increases the concentration of non-ionized H
2SO
4 molecules reduces and concentration of H
3O
+ increases. Thus
kobs constants, which reduce in value as
increases, correlate with the equilibrium concentration of H
3O
+ (
Figure 2). The dependence is linear (Equation 4) for all investigated compounds at the temperatures shown in
Table 2,
Table 3 (the correlation coefficient is equal to 0.96÷0.99). The reaction order dependence on the H
3O
+ concentration (
n) which is equal numerically to tangent of the angle of inclination of the lines in
Figure 2 is found to be close to 2 for three investigated nitro derivatives CuPc(3-NO
2)
4, CuPc(4-NO
2)
4 and CuPc(4-Br)
4(5-NO
2)
4.
For CuPc(4-Br)
4(5-NO
2)
4 maximums of the k
obs vs. dependencies at temperatures 410K and 379K are observed at the same
values as maximums of the
vs. dependence [
8,
12]. The extreme character of
kobs vs. dependence for CuPc(4-Br)
4(5-NO
2)
4 can be accounted for by participation of H
3O
+ cations as active particles in the dissociation processes. For CuPc(3-NO
2)
4, CuPc(4-NO
2)
4 the rate constants decrease with increasing solution acidity is considerably larger (
Table 2). Perhaps because of this the
kobs vs. dependence becomes insensitive to the mentioned extreme of
vs. dependence.
In equation (4)
kdis is the real rate constant of Reaction 1. Its values and corresponding values of the activation parameters are shown in
Table 4. Data for non-substituted CuPc are taken from [
1].
The dissociation reaction of carboxy derivatives of copper(II)phthalocyanine is more complex because the dependence of the order of Reaction 1 on
changes for different carboxy derivatives and temperature conditions (
Table 5). It makes it impossible to determine the activation parametres of the dissociation of CuPc(COOH)
m.
Taking into account the ability of carboxy groups to act as weak organic acids for protonation in concentrated H
2SO
4 we suppose that reaction (1) of carboxy substituted copper(II)phthalocyanine is forestalled with the pre-equilibrium depicted in Equation 5 and its stoichiometric mechanism depends on nature of the compound and temperature.
Therefore the complete kinetic equations 6 and 7 correspond to the dissociation reactions of nitro and carboxy substituted copper(II)phthalocyanines, respectively.
Thus only nitro derivatives and non-substituted CuPc may be placed in a series of kinetic stability to the hydroxonium cation action as far as they are compounds of the same type and dissociate according to one and the same mechanism. Value of
kdis298K increases in series shown in Equation 8:
Values of the activation energy of reaction (1) change in the same order (
Table 4). This can mean that in the series 8 the compounds are placed in accordance with destabilization of the Cu-N bonds from beginning to end of the series. Taking account of the mixed σπ character of the Cu-N bonds with the π-dative bond direction from Cu to N [
1] the order of the compounds in the series (8) can be explained from the point of view of the electron influence of NO
2 and Br substituents on the electron density of the bonds. Obviously the negative inductive effect of the substituents has little influence on state of the M-N bonds because of remote location of the substituents from the coordination center (Formula I). In reality, not all the complexes are less stable than CuPc.
The nitro groups in CuPc(4-NO2)4 are para-substituents with regard to the C atom in position 1 and meta-substituents with regard to the C atom in position 2. Inasmuch as the H3O+ attack on the reaction centre CuN4 is a complex nucleophilic-electrophilic process with two types of interaction (O→Cu and H←N), the π-withdrawing effect of the nitro groups can appear from para- and meta-substitution as well. Then 4-NO2 groups can stabilize the copper(II)phthalocyanine due to strengthening of the dative π-bonds Cu→N. This stabilization effect takes place in reality (Series 8). The result of analogous analysis for 3-NO2 substituted copper(II)phthalocyanine in which NO2 groups are simultaneously ortho- and para-substituents with regard to C atoms in positions 2 and 1 is the same. However, 3-NO2 groups are less conjugated with the benzene ring because of close positions of mezo C atoms of the macrocycle. Observed destabilization of CuPc when 3-NO2 is the substitutent (Series 8) shows that the negative Ieffect of the substituents takes place all the same. In the 4-NO2 derivative the substituents are farther from the reaction center and the induction effect is imperceptible.
CuPc(4-Br)4(5-NO2)4 is less stable compared with CuPc due to mutual steric hindrances of adjacent Br- and NO2- groups to conjugation with the macrocycle and their negative induction effect.
Regularities of the change of the activation parameters of the dissociation reaction of copper(II)phthalocyanine with functional substitution (
Table 4) are in good correspondence with the considered mechanism of the electronic influence. Larger values of E and ∆S
≠ correspond to the more stable complex CuPc(4-NO
2)
4 as compared with CuPc. An increase in strength of the donor-acceptor bonds Cu-N because of π-electron withdrawing effect of NO
2 groups leads to a sharp increase of the activation energy value, which is not compensated by changes of effectiveness of the nucleophilic interaction O→Cu. (∆S
≠ increases). For less stable CuPc(3-NO
2)
4 on the contrary, a decrease of E and ∆S
≠ values is observed. For CuPc(4-Br)
4(5-NO
2)
4 the activation parameters are close to those for the non-substituted complex.
An analogous mechanism for the electronic influence of substituents on reactivity in dissociation by dint of influence of the electronic state of the metal - nitrogen bonds was observed also for the earlier investigated metalloporphyrins substituted in β-positions of the macrocycle [
13].
The CuOEP studied in the present work is significantly less stable compared with coppertetraphenylporphyrin (CuTPP) (for CuOEP is equal 1.3.10-5, s-1 in mixed solvent 0.15 M H2SO4 in CH3COOH; for CuTPP is equal 1.5.10-6, s-1 in 0.5 M H2SO4 in CH3COOH) due to an electron donating action of alkyl groups onto the reverse dative M−N π-bond.
For numerous functional derivatives of metallotetraphenylporphyrins substituted in the benzene rings, the mechanism of the electron influence of the substituents is essentially different: substitution leads basically to changes of state of the n-electron pairs of the N atoms of the metalloporphyrins [
14,
15]. Our data show that eight alkyl groups introduced into phenyl rings of CuTPP instead of β-positions of the macrocycle decreases the complex stability only within the limits of an order of magnitude (
are equal 6.96
.10
-4, s
-1 for CuTP(3,4-di-CH
3)
4P and 1.08
.10
-4, s
-1 for CuTPP in 1 M H
2SO
4 in C
3H
7COOH).