Polymerization Mechanism of α,α’-bis(Tetrahydrothiophenio)-β-xylene Dichloride
Abstract
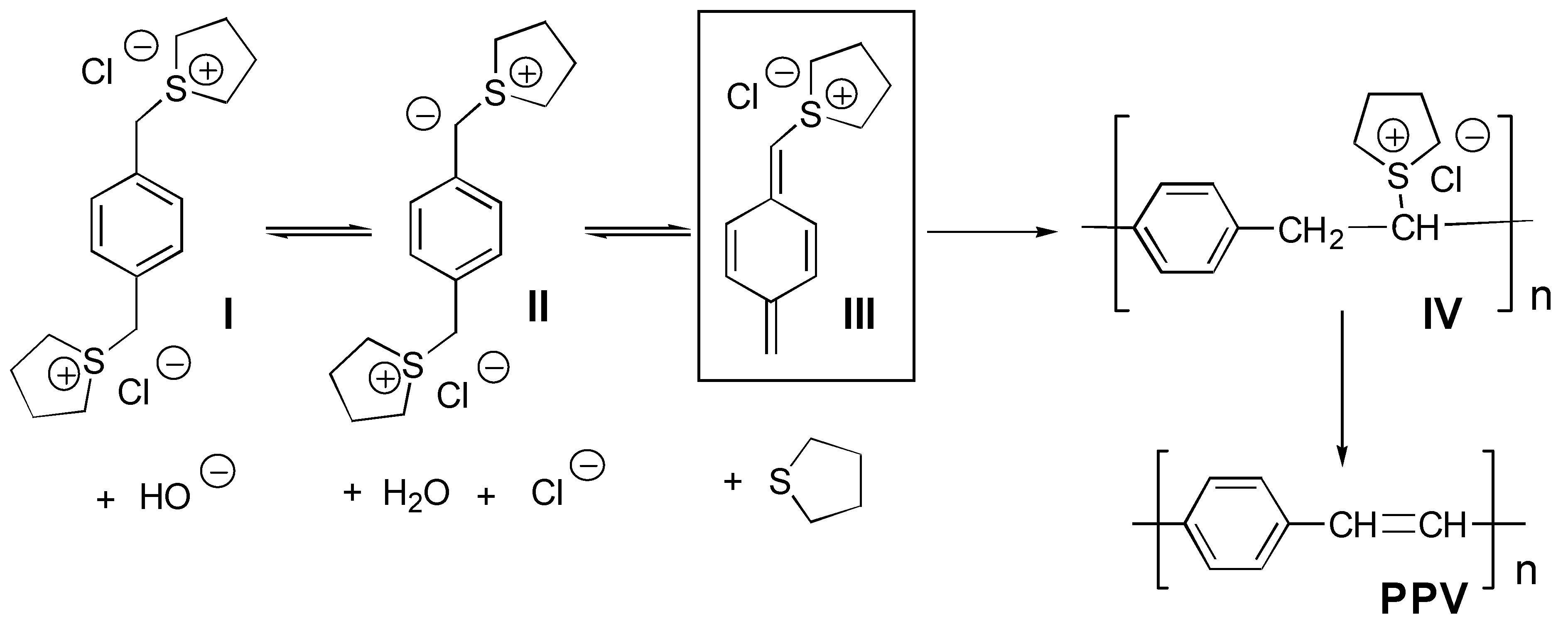
:Introduction
Experimental
Results and Discussion
Acknowledgments:
References and Notes
- Kraft, A.; Grimsdale, A. C.; Holmes, A. B. Electroluminescent conjugated polymers-Seeing polymers in a new light. Angew. Chem. Intern. Ed. 1998, 37, 402. [Google Scholar] [CrossRef]
- Denton, F. R., III; Lahti, P. M.; Karasz, F. E. J. Polym. Sci. A. 1992, 30, 2223.
- Sawyer, D. T.; Roberts, J. L. Hydroxide ion: an effective one-electron reducing agent? Acc. Chem. Res. 1988, 21, 31. [Google Scholar] [CrossRef]
| III | OH | OH | Radical Anion of III | ΔHR | |
|---|---|---|---|---|---|
| AM1 | 187.05 | -14.12 | 0.63 | 41.08 | -131.22 |
| PM3 | 208.13 | -17.52 | 2.82 | 63.52 | -124.27 |
| AM1* | 165.96 | -6.89 | -12.10 | 32.13 | -139.04 |
Share and Cite
Almassio, M.; Garay, R.O. Polymerization Mechanism of α,α’-bis(Tetrahydrothiophenio)-β-xylene Dichloride. Molecules 2000, 5, 596-597. https://doi.org/10.3390/50300596
Almassio M, Garay RO. Polymerization Mechanism of α,α’-bis(Tetrahydrothiophenio)-β-xylene Dichloride. Molecules. 2000; 5(3):596-597. https://doi.org/10.3390/50300596
Chicago/Turabian StyleAlmassio, Marcela, and Raúl O. Garay. 2000. "Polymerization Mechanism of α,α’-bis(Tetrahydrothiophenio)-β-xylene Dichloride" Molecules 5, no. 3: 596-597. https://doi.org/10.3390/50300596




