Stereoselective Synthesis of 8-Trialkylstannylmenthols
Abstract
:Introduction
Experimental
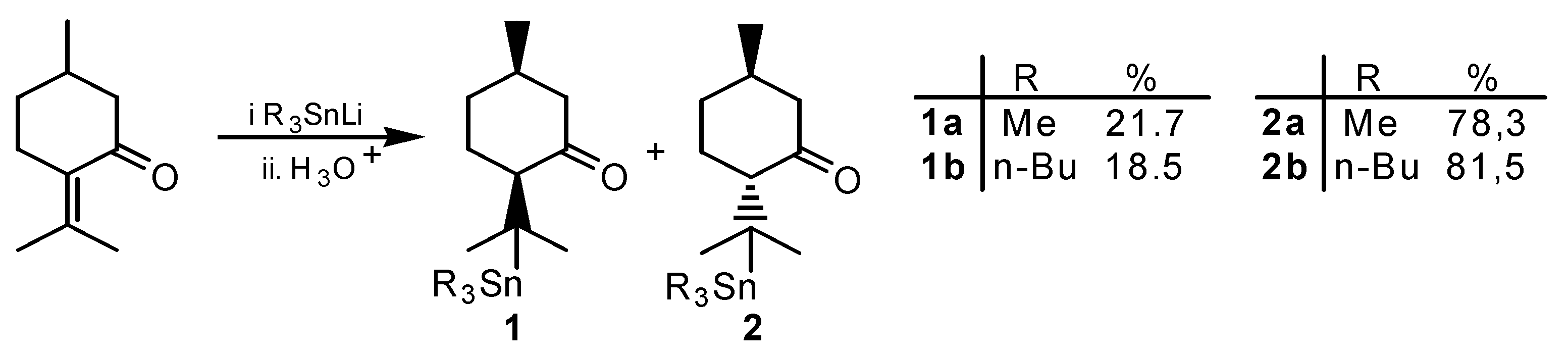
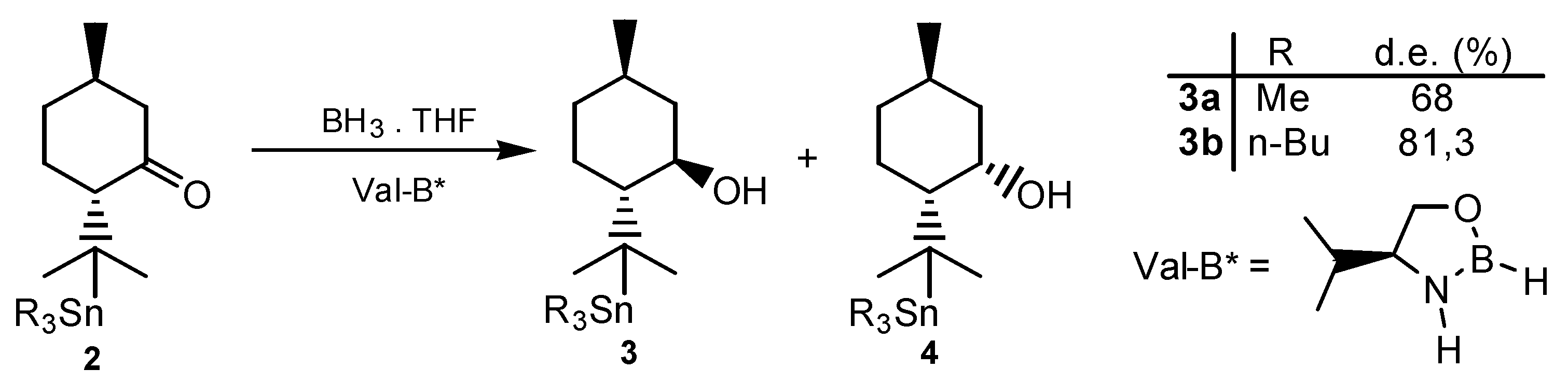
Results and Discussion
Acknowledgements:
References and Notes
- Radivoy, G.E. Doctor in Chemistry Thesis, Universidad Nacional del Sur, 1997.
- Itsuno, S.; Nakano, M.; Miyazaky, K; Masuda, H.; Ito, K.; Hirao, A; Nakahama, S. Asymmetric Synthesis Using Chirally Modified Borohydrides. Part 3. Enantioselective Reduction of Ketones and Oxime Ethers with Reagents Prepared from Borane and Chiral Amino Alcohols. J. Chem. Soc. Perkin Trans. I 1985, 2039. [Google Scholar] [CrossRef]
 | N° | δ C1(3J) | δ C2(2J) | δ C3(3J) | δ C8(1J) | 119Sn | [α]D20(conc.)b |
| 2a | 213.42 (17.8) | 61.25 (7.7) | 28.41 (31.0) | 32.59 (243,0) | 12.7 | -35.6° (0,874) | |
| 2b | 213.16 (16.1) | 61,40 (6.8) | 27.94 (NO) | 26.47 (388.2) | -8.3 | -22.2° (1,94) |
Share and Cite
Mandolesi, S.D.; Giagante, N.N.; Dodero, V.; Podestá, J.C. Stereoselective Synthesis of 8-Trialkylstannylmenthols. Molecules 2000, 5, 594-595. https://doi.org/10.3390/50300594
Mandolesi SD, Giagante NN, Dodero V, Podestá JC. Stereoselective Synthesis of 8-Trialkylstannylmenthols. Molecules. 2000; 5(3):594-595. https://doi.org/10.3390/50300594
Chicago/Turabian StyleMandolesi, Sandra D., Nelda N. Giagante, Verónica Dodero, and Julio C. Podestá. 2000. "Stereoselective Synthesis of 8-Trialkylstannylmenthols" Molecules 5, no. 3: 594-595. https://doi.org/10.3390/50300594




