Introduction
Epoxides are among the most versatile intermediates in organic chemistry due to the particular polarity and strength of their three member ring. Those characteristics make them in a very suitable substrate to the attack or many nucleophilic and electrophilic reagents [
1].
The regioselective opening of oxiranes has been the subject of interest for many years, since these heterocyclics are frequently used as starting material for the preparation of natural products and biological active compounds [
2].
Many different organotin reagents have been employed to effect the regio- and stereoselective cleavage of oxiranes in the presence of nucleophiles affording 1,2-alcoxialcohols, 1,2-aminoalcohols, 1, 2-azidoalcohols, etc [
3].
Results and Discussion
As a part of our ongoing research work concerning the development of organotin reagents for the opening of epoxides [
4], the bis(tributyltin) oxide (
1) and bis(chlorodibutyltin) oxide (
2) were subjected to act as Lewis acids assisting the opening of epoxides in the presence of halogenated alcohols.
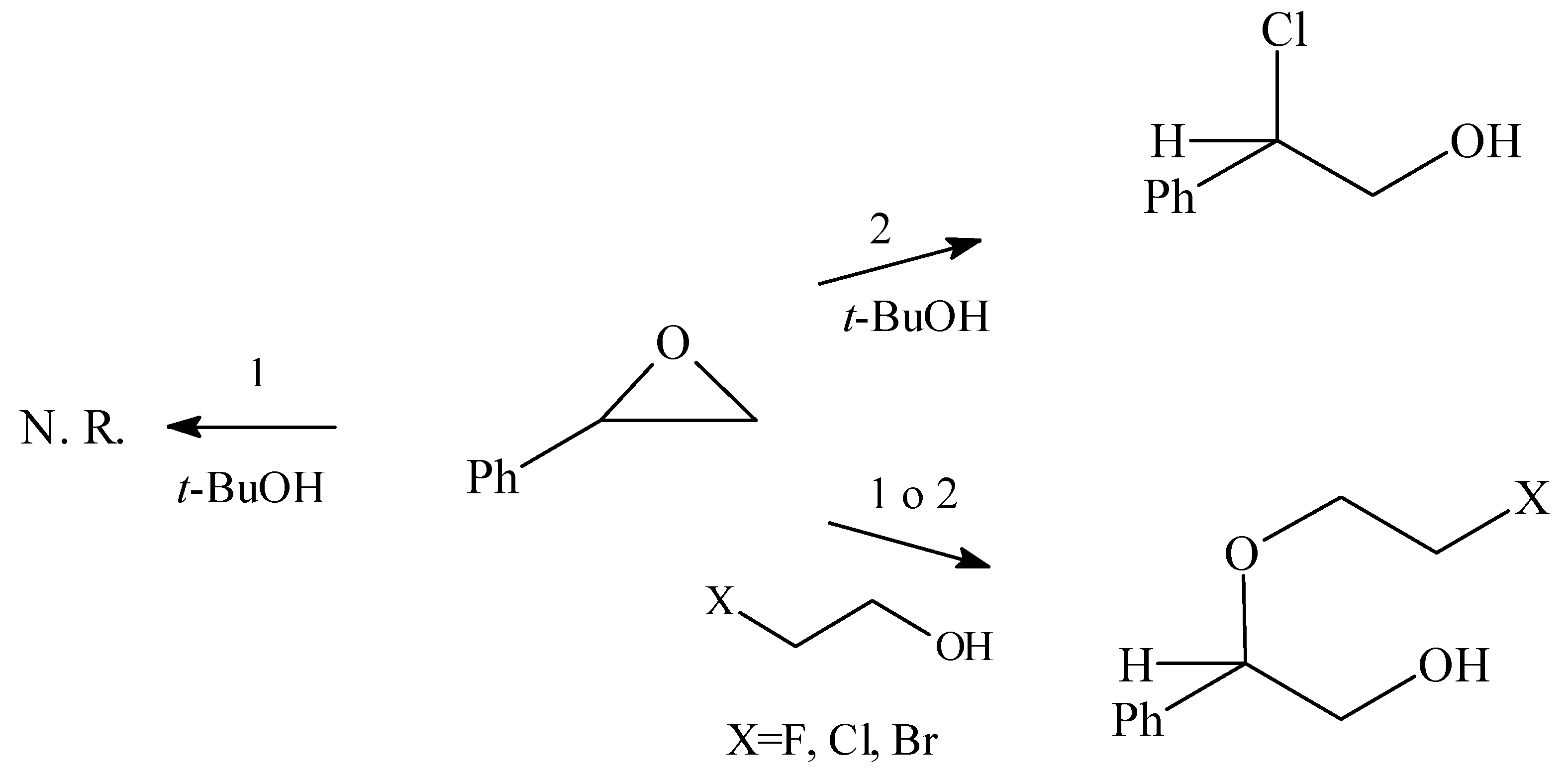
When styrene oxide was reacted with t-BuOH and 1, the starting material was recovered unchanged after 5 days, probably due to steric hindrances. However, when the same reaction was carried out using the oxide 2, after 3 days a compound was isolated and identified as the chlorohydrin. In this case, bis(chlorodibutyltin) oxide would act as a Lewis acid and, moreover, as a chloride donor.
In view of the result described above, we decided to extend the study using a series of halogenated alcohols as a nucleophiles in the presence of the organotin oxides 1 or 2.
These results outlined in
Scheme 1, shows the formation of the halohydrin derivatives in very good yield by the highly regioselective opening of styrene oxide.
However, when the same methodology was applied to other monosubstituted epoxides, the results obtained were different. While the oxide 1, showed a low reactivity towards aliphatic oxiranes, the oxide 2 was able to assist the cleavage of those heterocycles by the nucleophilic attack of the alcohol, or by the chloride anion originated from (ClBu2Sn)2O.
The unusual formation of the chlorohydrin compounds would be due to the reaction of the nucleophile with the reagent 2, and the subsequent nucleophilic attack of the chloride over the epoxide.
In summary, (ClBu2Sn)2O emerge as a new reagent for the regioselective opening of epoxides yielding the chlorohydrin derivatives, in mild and non-hydrolytic conditions.