Thermal Decomposition Reaction of cis-6-Phenyl-5,6-(2-phenylpropilydene)-3,3-tetramethylene-1,2,4-trioxacyclohexane in Different Solvents
Abstract
:Introduction
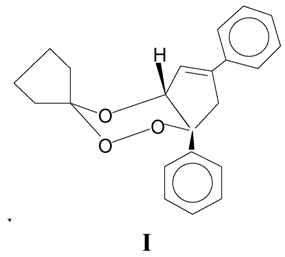
Experimental
Materials
Kinetic methods
Results and Discussion
Acknowledgements
References and Notes
- (a) Jefford, C. W.; Rossier, J. C.; Boukouvalas, J. J. Chem. Soc., Chem. Commun. 1987, 713. (b) Jefford, C. W.; Rossier, J. C.; Boukouvalas, J. J. Chem. Soc., Chem. Commun 1987, 1593. and references cited therein.
- Jefford, C. W.; Cafferata, L. F. R.; Mateo, C. M. Unpublished.
- Huyberechts, S.; Halleux, A.; Kruys, P. Bull. Soc. Chim. Belg. 1955, 64, 203. [CrossRef]
| SOLVENT | 103 x [I], mol/L | 106 x kexp, s-1 |
|---|---|---|
| n-hexane | 0.60 | 4.00 |
| benzene | 0.50 | 93.3 |
| acetonitrile | 0.65 | 173 |
| metanol | 0.36 | 390 |
Share and Cite
Cafferata, L.F.R.; Eyler, G.N.; Cañizo, A.I.; Mateo, C.M.; Rimada, R.S. Thermal Decomposition Reaction of cis-6-Phenyl-5,6-(2-phenylpropilydene)-3,3-tetramethylene-1,2,4-trioxacyclohexane in Different Solvents. Molecules 2000, 5, 362-364. https://doi.org/10.3390/50300362
Cafferata LFR, Eyler GN, Cañizo AI, Mateo CM, Rimada RS. Thermal Decomposition Reaction of cis-6-Phenyl-5,6-(2-phenylpropilydene)-3,3-tetramethylene-1,2,4-trioxacyclohexane in Different Solvents. Molecules. 2000; 5(3):362-364. https://doi.org/10.3390/50300362
Chicago/Turabian StyleCafferata, L. F. R., G. N. Eyler, A. I. Cañizo, C. M. Mateo, and R. S. Rimada. 2000. "Thermal Decomposition Reaction of cis-6-Phenyl-5,6-(2-phenylpropilydene)-3,3-tetramethylene-1,2,4-trioxacyclohexane in Different Solvents" Molecules 5, no. 3: 362-364. https://doi.org/10.3390/50300362
APA StyleCafferata, L. F. R., Eyler, G. N., Cañizo, A. I., Mateo, C. M., & Rimada, R. S. (2000). Thermal Decomposition Reaction of cis-6-Phenyl-5,6-(2-phenylpropilydene)-3,3-tetramethylene-1,2,4-trioxacyclohexane in Different Solvents. Molecules, 5(3), 362-364. https://doi.org/10.3390/50300362




