Polyalkoxy Nitrones as Chiral Building Blocks in Asymmetric Synthesis
Abstract
:Introduction

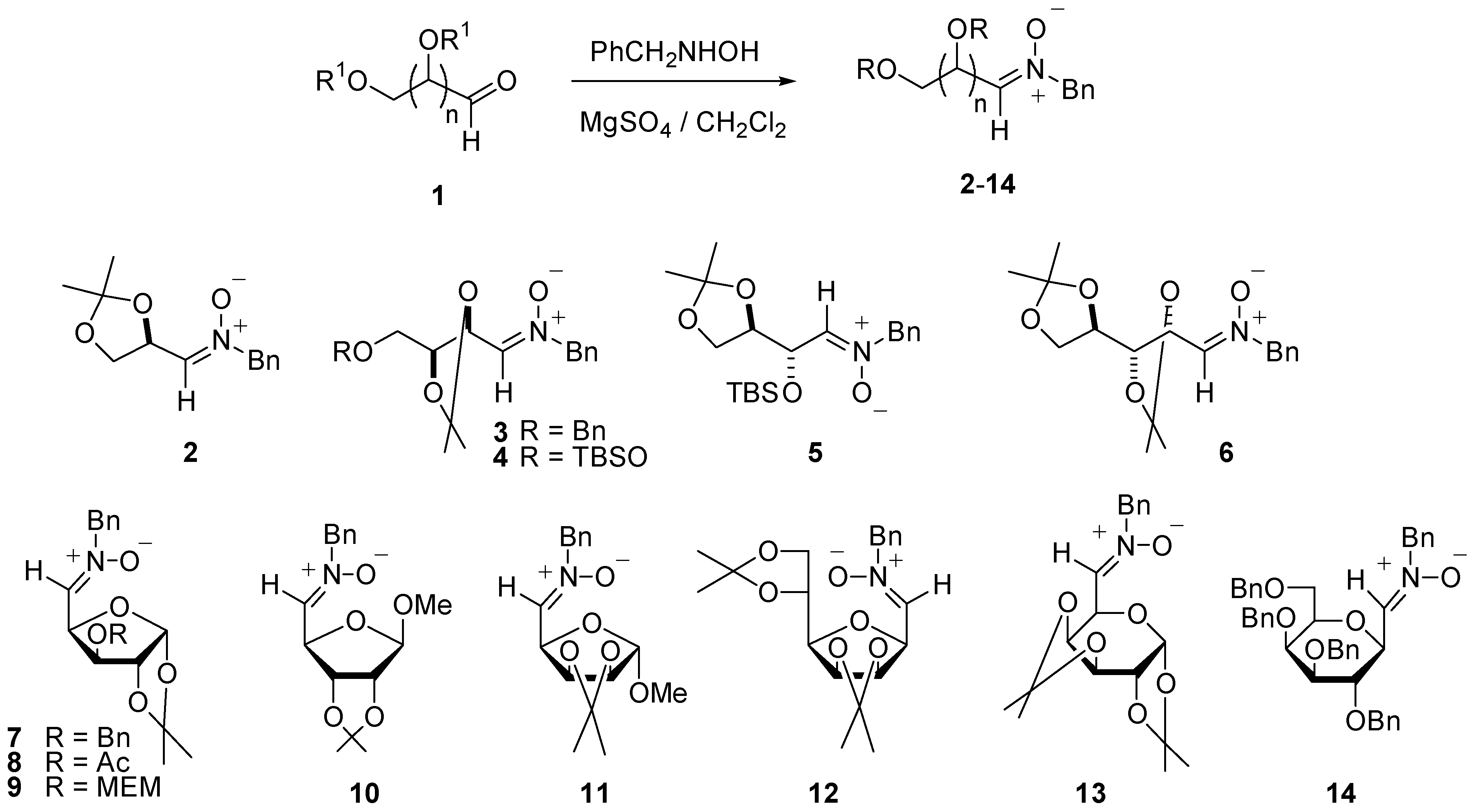
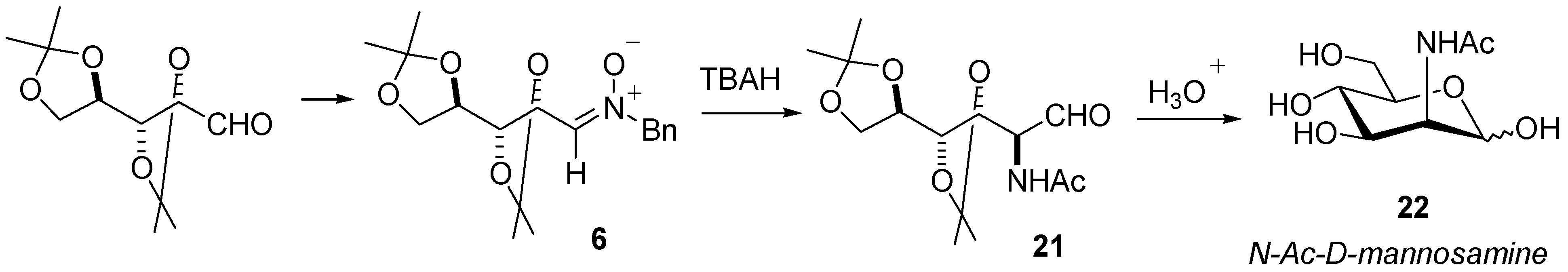
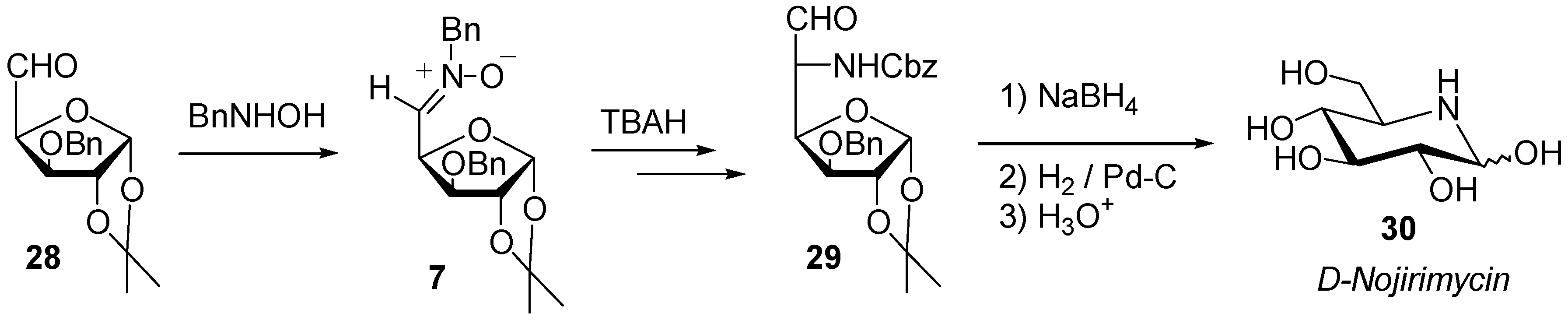
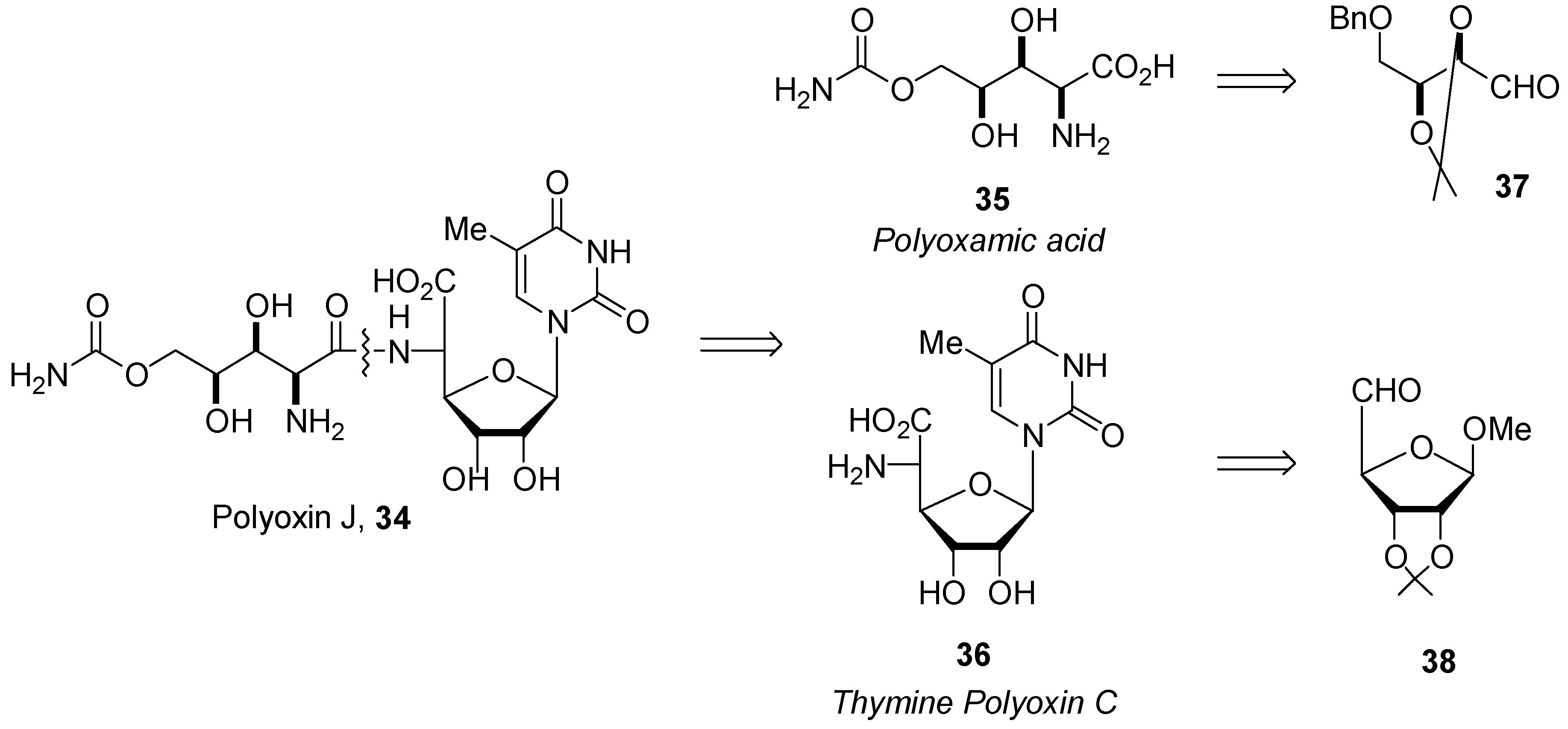
Synthesis
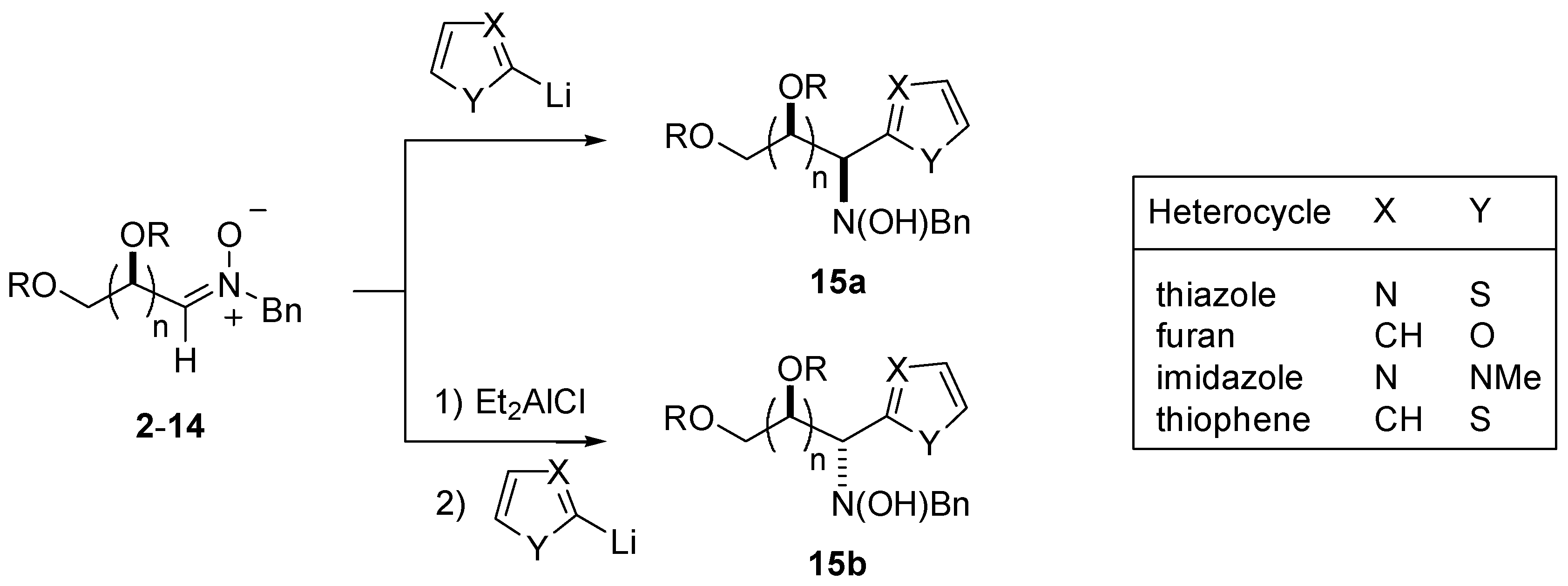
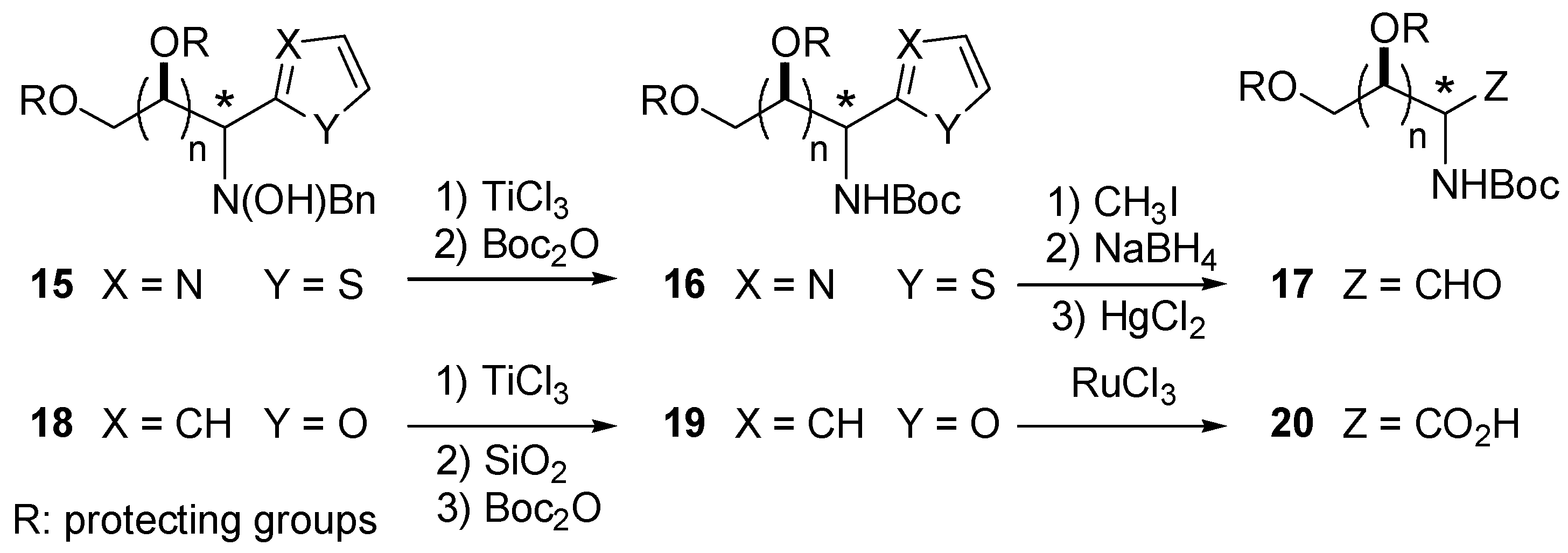
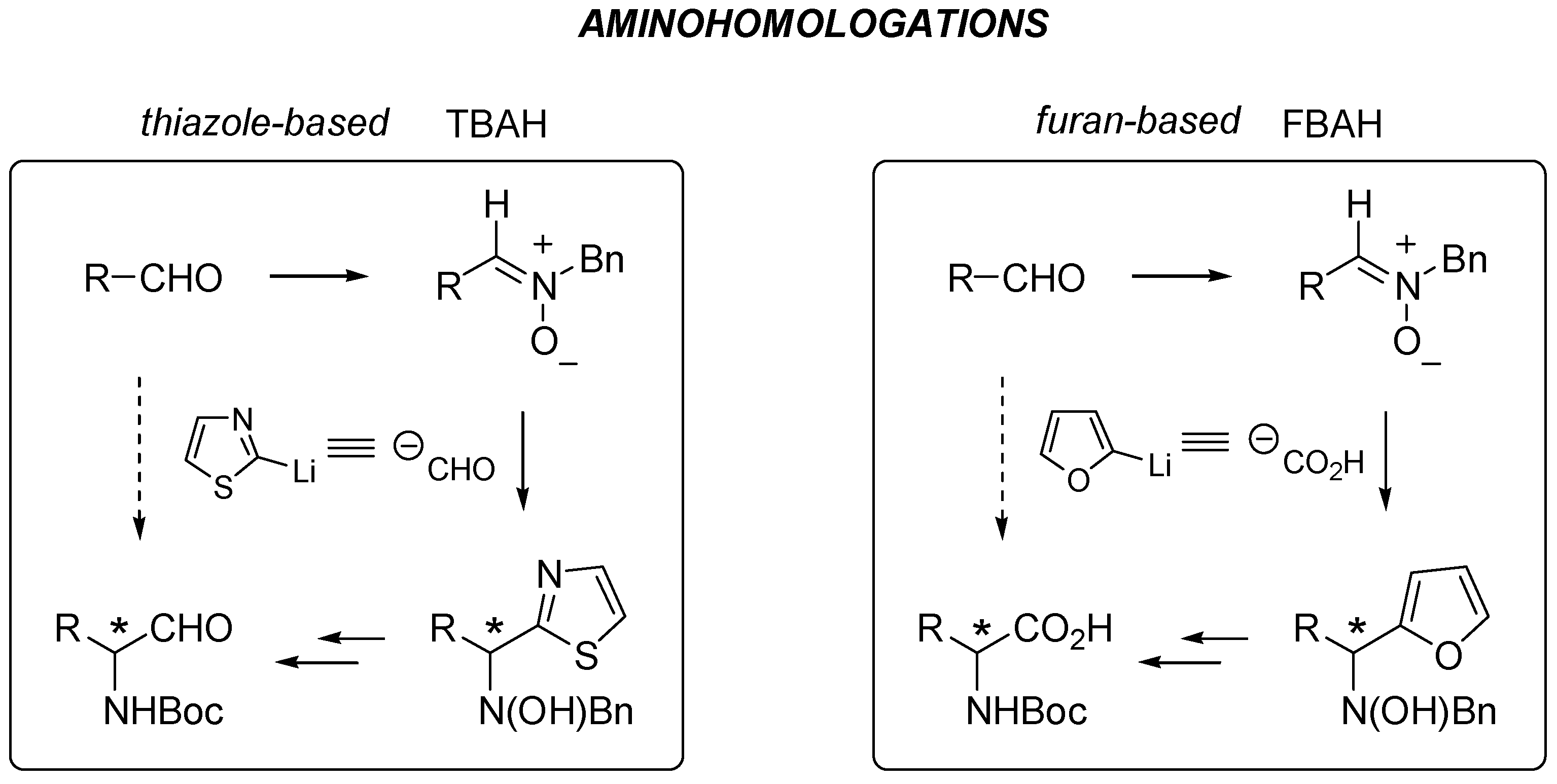
Reactivity
Concluding Remarks
Acknowledgments
References and Notes
- For reviews see: Inch, T.D. Tetrahedron 1984, 40, 3161–3213. Dueholm, K.L.; Pedersen, E.B. Synthesis 1992, 1–22. Ferrier, R.J.; Middleton, S. Chem. Rev. 1993, 93, 2779–2831. Casiraghi, G.; Zanardi, F.; Rassu, G.; Spanu, P. Chem. Rev. 1995, 95, 1677–1716. Knapp, S. Chem. Rev. 1995, 95, 1859–1876. Hultin, P.G.; Earle, M.A.; Sudharshan, M. Tetrahedron 1997, 53, 14823–14870.
- For reviews see: Fleet, G.W.J. Chem. Brit 1989, 287–292. Cintas, P. Tetrahedron 1991, 47, 6079–6111. Kaluza, Z.; Abramski, W.; Chmielewski, M. Ind. J. Chem. 1994, 33B, 913–940. Martinez-Grau, A.; Marco-Contelles, J. Chem. Soc. Rev. 1998, 27, 155–162.
- DeShong, P.; Leginus, J.M. J. Am. Chem. Soc. 1983, 105, 1686–1688. DeShong, P.; Dicken, C.M.; Leginus, J.M.; Whittle, R.R. J. Am. Chem. Soc. 1984, 106, 5598–5602. Vasella, A.; Voeffray, R. Helv. Chim. Acta 1982, 65, 1134–1144. Vasella, A.; Voeffray, R.; Pless, J.; Huguenin, R. Helv. Chim. Acta 1983, 66, 11241–1252. DeShong, P.; Li, W.; Kennington, J.W.; Ammon, H.L. J. Org. Chem. 1991, 56, 1364–1373. Iida, H.; Kasahara, K.; Kibayashi, C. J. Am. Chem. Soc. 1986, 108, 4647–4648. Iida, H.; Kasahara, K.; Kibayashi, C. J. Org. Chem. 1989, 54, 2225–2233. Herczeg, P.; Kovacs, I.; Szilagyi, L.; Varga, T.; Dinya, Z.; Sztaricskai, F. Tetrahedron Lett. 1993, 34, 1211–1214. Cordero, F.M.; Cicchi, S.; Goti, A.; Brandi, A. tetrahedron Lett. 1994, 35, 949–952.
- Tufariello, J.J. 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984; vol. 2, pp. 83–168. [Google Scholar] Padwa, A. Comprehensive Organic Synthesis; Trost, B.M., Ed.; Pergamon: Oxford, 1991; vol. 4, chap. 4.9. [Google Scholar] and references cited therein. Frederickson, M. Tetrahedron 1997, 53, 403–425.
- Tuffariello, J.J.; Lee, G.E. J. Am. Chem. Soc. 1980, 102, 373–374. Aurich, H.G.; Schmidt, M.; Schwerzel, T. Chem. Ber. 1985, 118, 1086–1104.
- Christensen, D.; Jorgensen, K.A. J. Org. Chem. 1989, 54, 126–131. Abou-Gharbia, M.A.; Joullie, M.M. Org. Prep. Proc. Int. 1979, 11, 95–96.
- Murahashi, S.-I.; Mitsui, H.; Shiota, T.; Tsuda, T.; Watanabe, S. J. Org. Chem. 1990, 55, 1736–1744.
- Zschiesche, R.; Reissig, H.V. Tetrahedron Lett. 1988, 29, 1685–1686.
- Franco, S.; Merchan, F.L.; Merino, P.; Tejero, T. Synth. Commun. 1995, 25, 2275–2284. Dondoni, A.; Franco, S.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Synth. Commun. 1994, 24, 2537–2550.
- Franco, S. Ph.D. Thesis, University of Zaragoza (Spain), 1994.
- Dondoni, A.; Franco, S.; Merchan, F.L.; Merino, P.; Tejero, T. Tetrahedron Lett. 1993, 34, 5475–5478.
- Dondoni, A.; Franco, S.; Merchan, F.L.; Merino, P.; Tejero, T. Tetrahedron Lett. 1993, 34, 5479–5482.
- Merino, P.; Franco, S.; Martinez, I.; Merchan, F.L.; Tejero, T. Electronic Conference on Heterocyclic Chemistry (ECHET98). Internet: http://www.ch.ic.ac.uk/ectoc/echet98/.
- Dondoni, A.; Junquera, F.; Franco, S.; Merchan, F.L.; Merino, P.; Tejero, T.; Bertolasi, V. Chem. Eur. J. 1995, 1, 505–520.
- Dondoni, A.; Merino, P. Org. Synth. 72, 21–31.
- Mukaiyama, T.; Tsuzuki, R.; Kato, J. Chem. Lett. 1985, 837–840. Danishefksy, S.J.; DeNinno, M.P.; Chen, S. J. Am. Chem. Soc. 1988, 110, 3929–3940. Marshall, J.A.; Luke, G.P. J. Org. Chem. 1993, 58, 6229–6234.
- Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Synthesis 1994, 1450–1456.
- Merchan, F.L.; Merino, P.; Rojo, I.; Tejero, T.; Dondoni, A. Tetrahedron: Asymmetry 1995, 6, 2145–2148.
- Tejero, T.; Franco, S.; Junquera, F.; Lanaspa, A.; Merchan, F.L.; Merino, P.; Rojo, I. Tetrahedron: Asymmetry 1996, 7, 667–670.
- Merino, P.; Merchan, F.L.; Tejero, T. Acta Cryst. Sect. C. 1995, 51, 2400–2402. Merino, P.; Junquera, F.; Merchan, F.L.; Tejero, T. Acta Cryst. Sect. C. 1996, 52, 3197–3198. Merino, P.; Franco, S.; Junquera, F.; Merchan, F.L.; Tejero, T. Zeits. Krist. 1997, 212, 321–322. Merino, P.; Franco, S.; Merchan, F.L.; Tejero, T. Zeits. Krist. 1998, 213, 133–134. Merino, P.; Franco, S.; Merchan, F.L.; Tejero, T. Zeits. Krist. 1998, 213, 135–136.
- Lipshutz, B.H. Chem. Rev. 1986, 86, 795–819. Shipman, M. Contemp. Org. Synth. 1995, 2, 1–18. Padwa, A. Progress in Heterocyclic Chemistry; Suschitzky, H., Scriven, E.F.V., Eds.; Pergamon: Oxford, 1994; Vol. 6, pp. 36–55. [Google Scholar] Novel Applications of Heterocycles in Synthesis; Tetrahedron Symposia-in-Print number 59; Katritzky, A.R. (Ed.) 1996; vol. 52, No. 9.
- Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Tetrahedron Lett. 1992, 33, 4221–4224.
- Dondoni, A.; Franco, S.; Merchan, F.L.; Merino, P.; Tejero, T. Synlett 1993, 78–80.
- Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Scherrmann, M.-C.; Tejero, T. J. Org. Chem. 1997, 62, 5484–5496.
- Dondoni, A.; Junquera, F.; Merchan, F.L.; Tejero, T. Chem. Commun. 1995, 2127–2128.
- Dondoni, A.; Franco, S.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. J. Org. Chem. 1997, 60, 5497–5507.
- Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Tetrahedron Lett. 1994, 35, 9439–9442. and references cited therein.
- Samples Availability: Available from the authors.



© 1999 by the authors. Reproduction of this article, by any means, is permitted for noncommercial purposes.
Share and Cite
Merino, P.; Tejero, T. Polyalkoxy Nitrones as Chiral Building Blocks in Asymmetric Synthesis. Molecules 1999, 4, 169-179. https://doi.org/10.3390/40700169
Merino P, Tejero T. Polyalkoxy Nitrones as Chiral Building Blocks in Asymmetric Synthesis. Molecules. 1999; 4(7):169-179. https://doi.org/10.3390/40700169
Chicago/Turabian StyleMerino, Pedro, and Tomás Tejero. 1999. "Polyalkoxy Nitrones as Chiral Building Blocks in Asymmetric Synthesis" Molecules 4, no. 7: 169-179. https://doi.org/10.3390/40700169



