One-Step Hydrothermal/Solvothermal Preparation of Pt/TiO2: An Efficient Catalyst for Biobutanol Oxidation at Room Temperature
Abstract
:1. Introduction
2. Results and Discuss
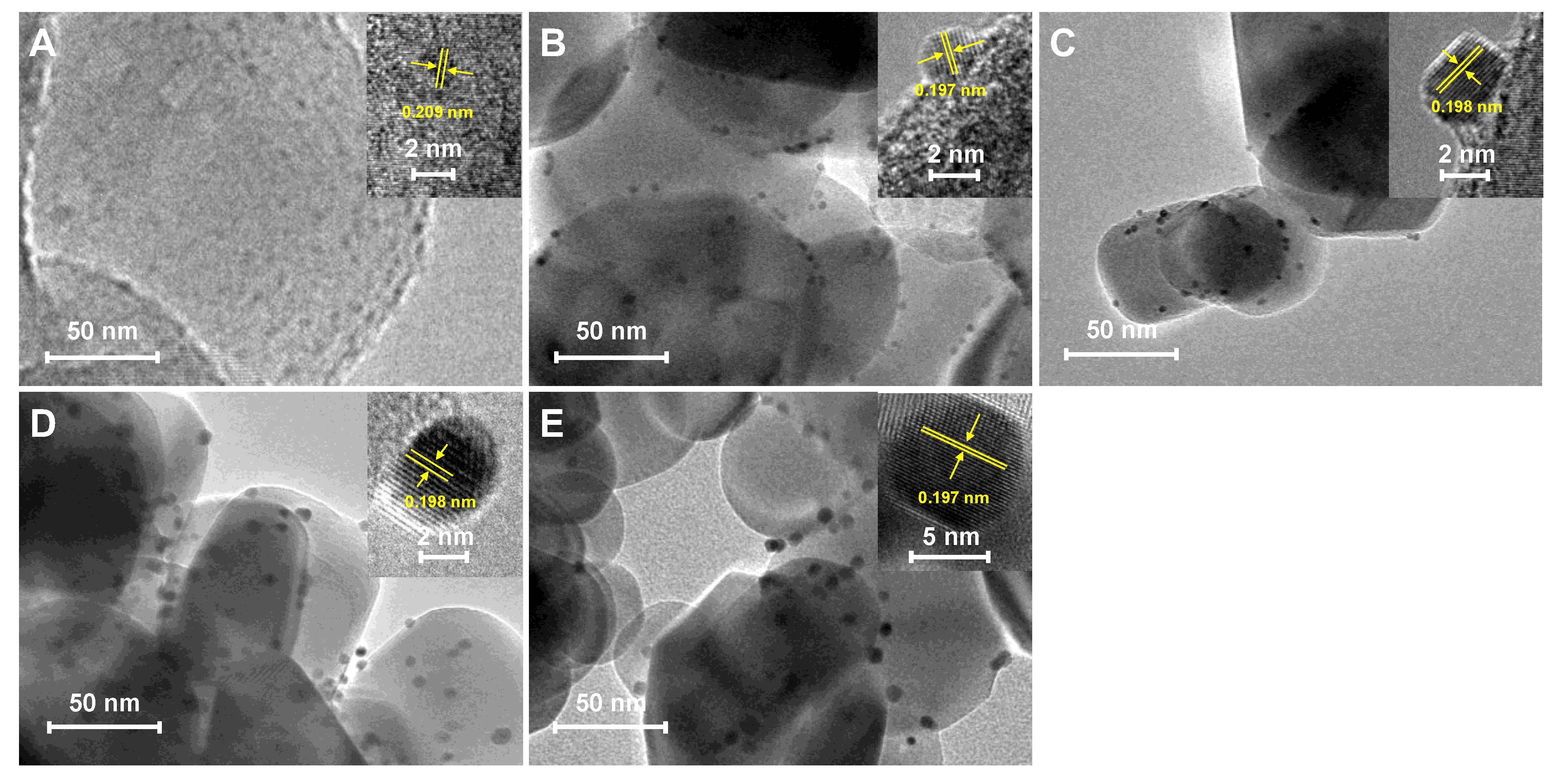
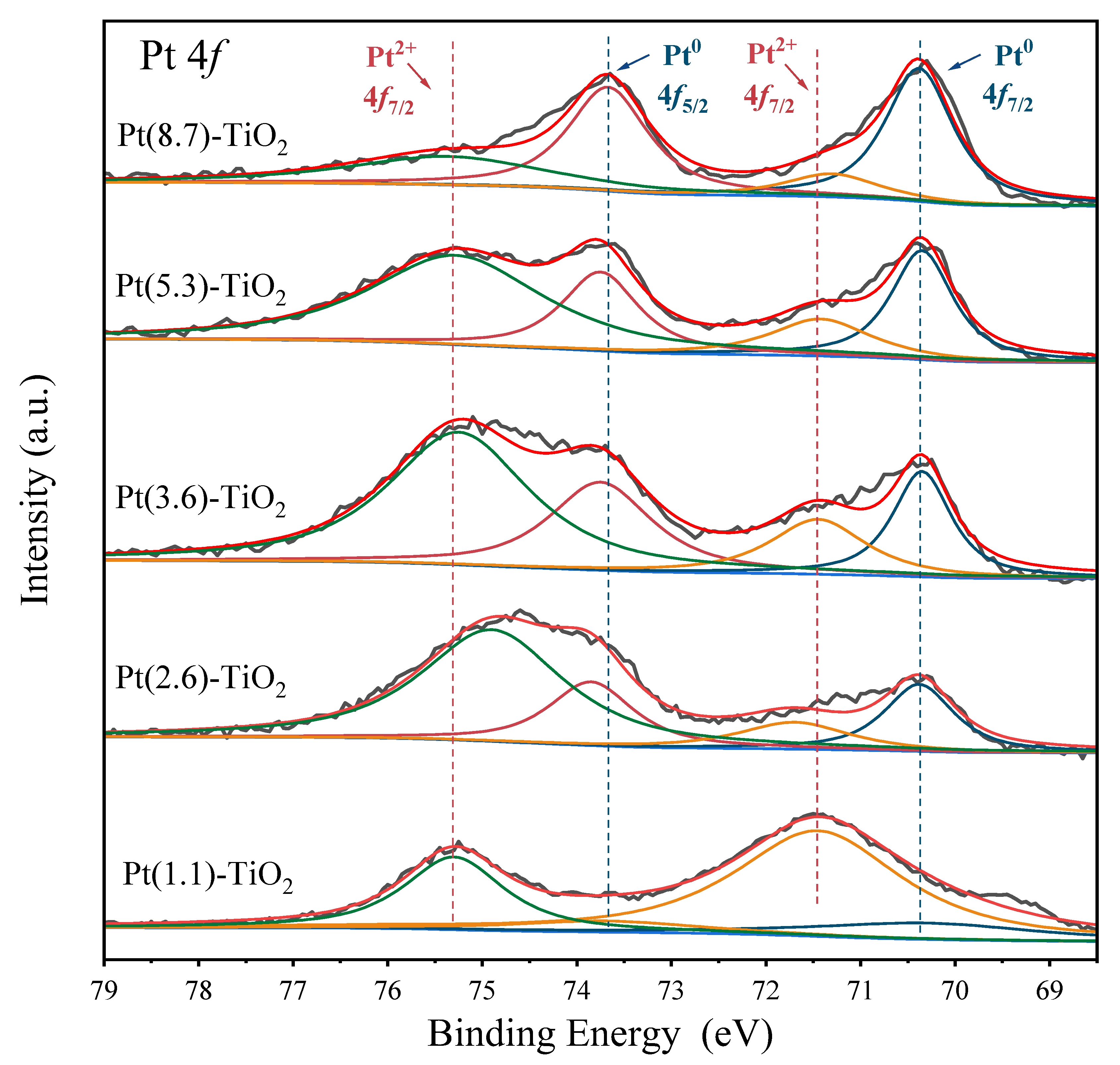
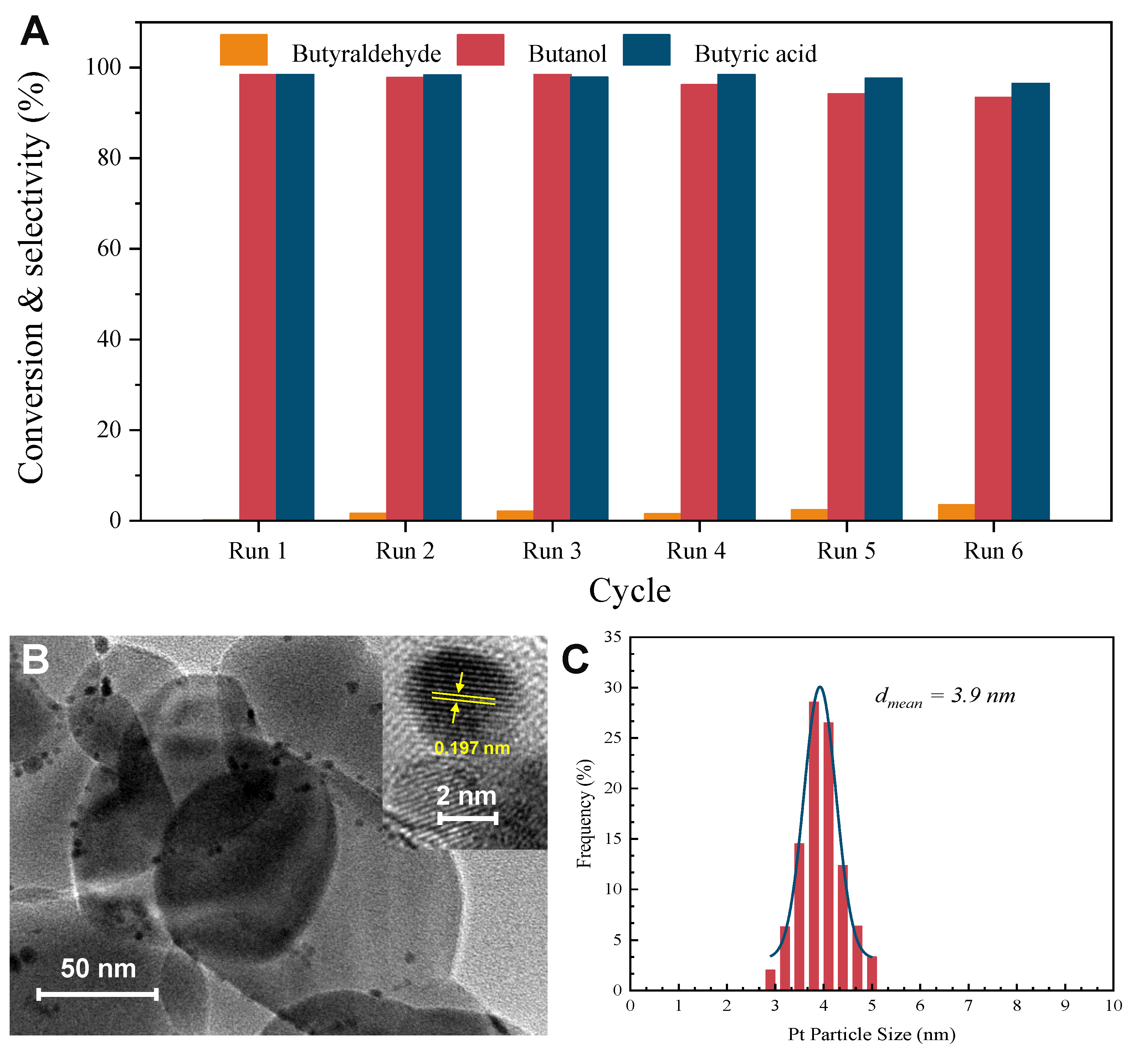
2.1. Characterization of the Pt-TiO2 Catalysts
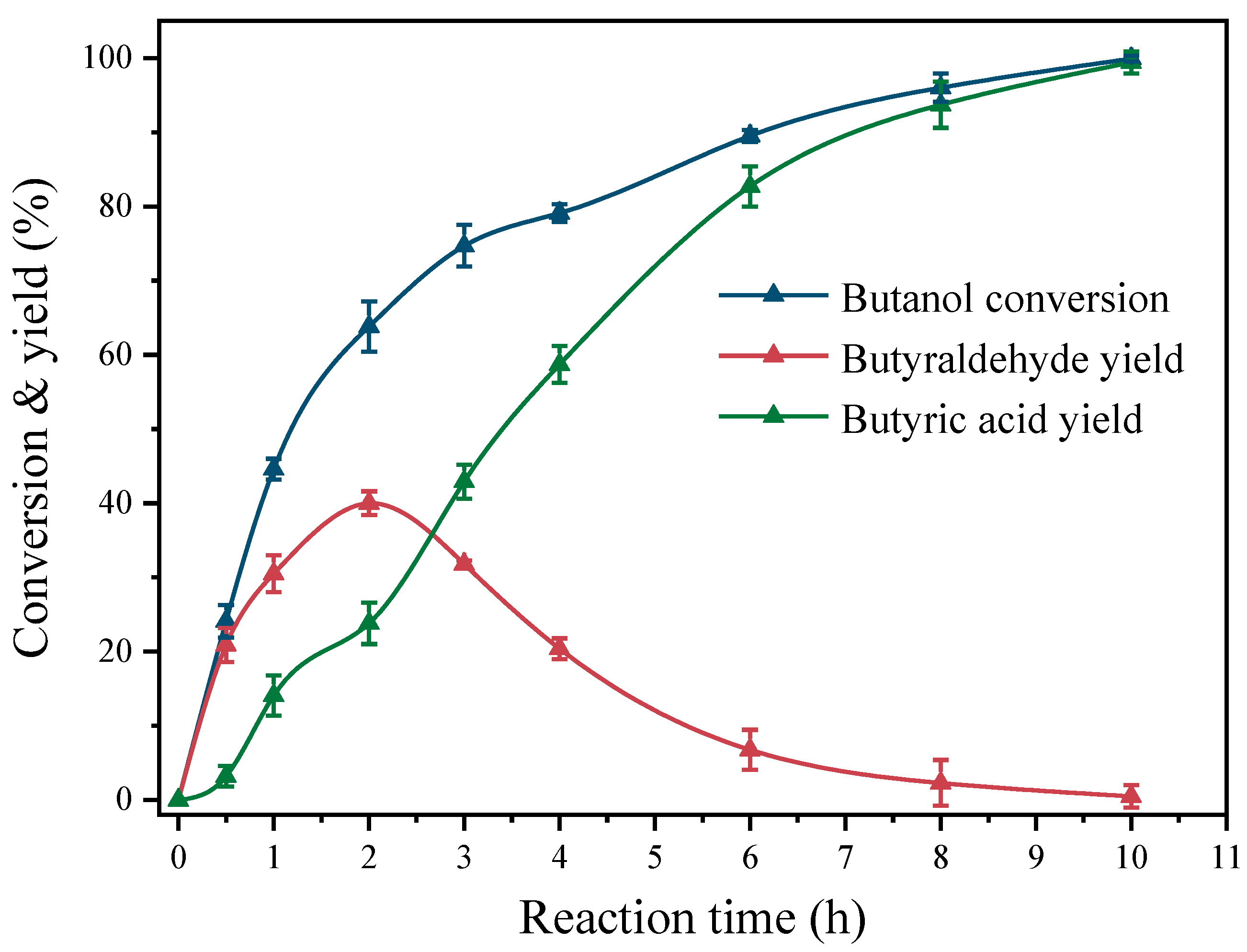
2.2. Catalytic Performance of Alcohol Oxidation
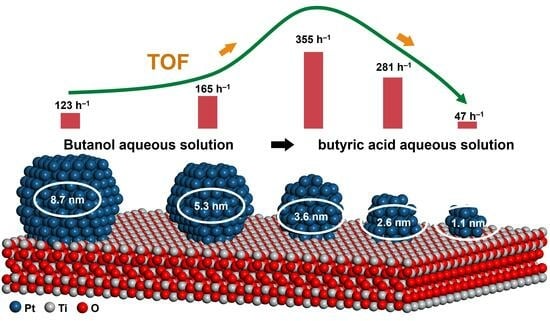
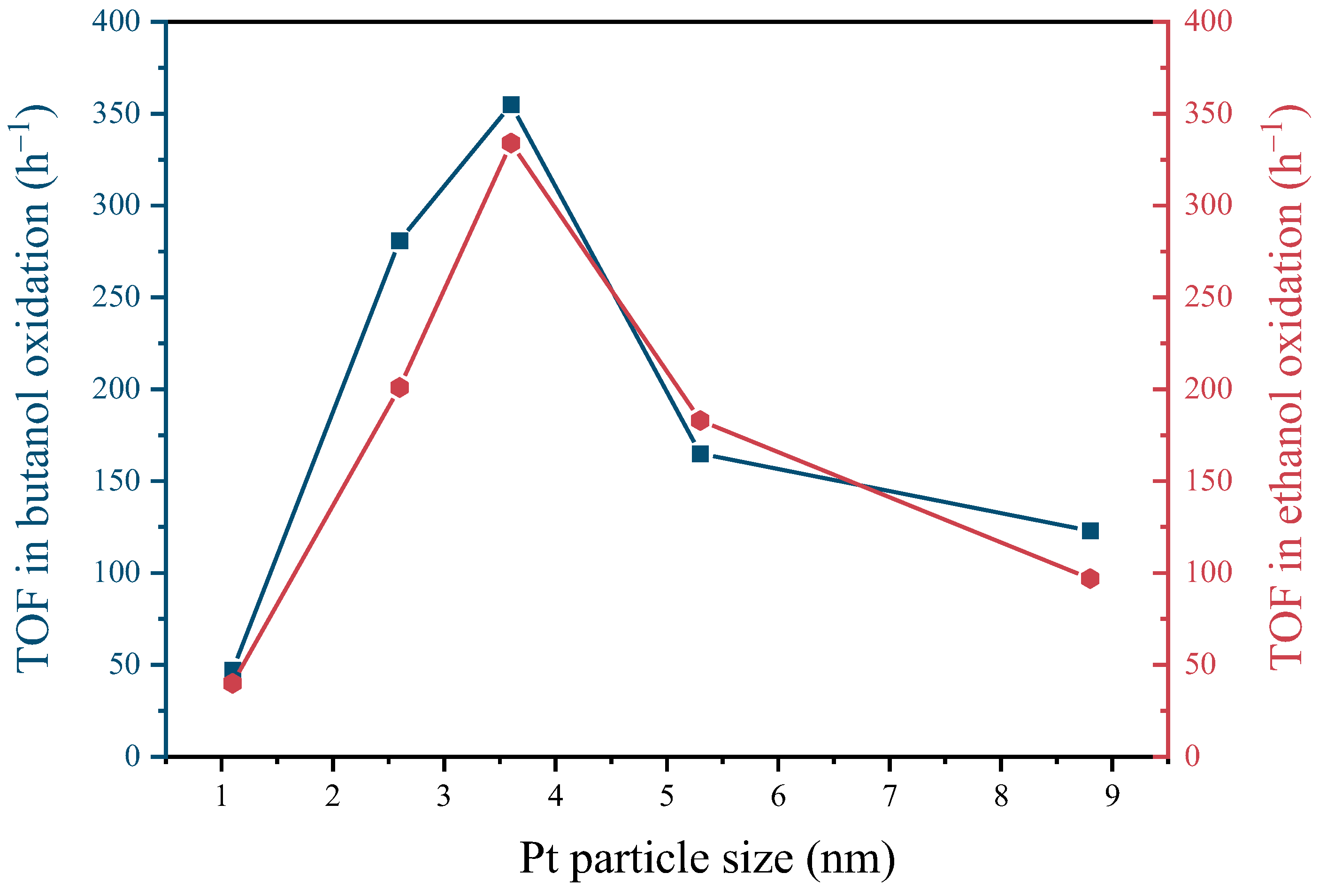
2.3. Pt Size Effect on Catalytic Performance of Butanol
2.4. Catalytic Performance of Other Non-Activated Alcohols and Biomass-Based Alcohols
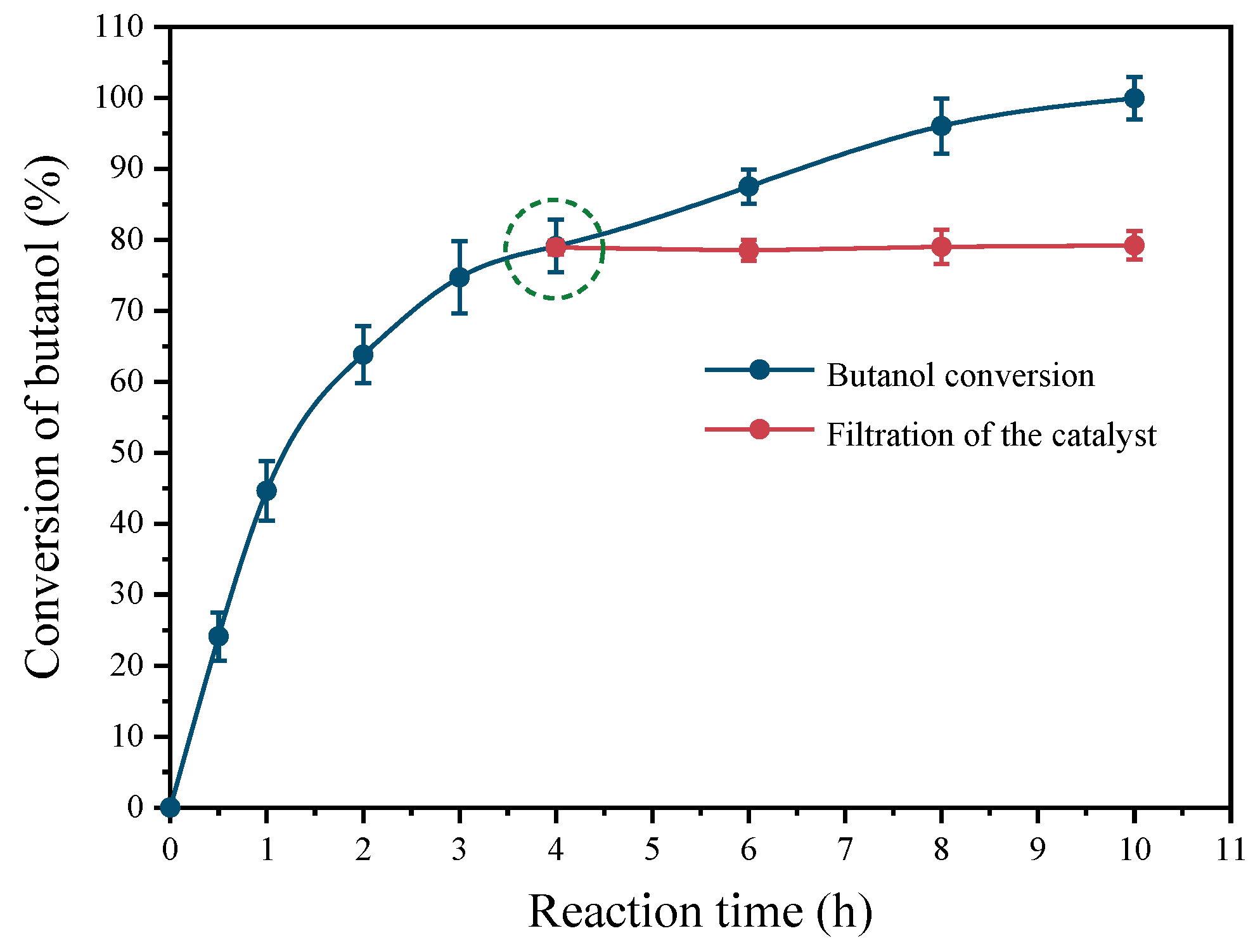
2.5. Reusability and Stability of Pt(3.6)-TiO2
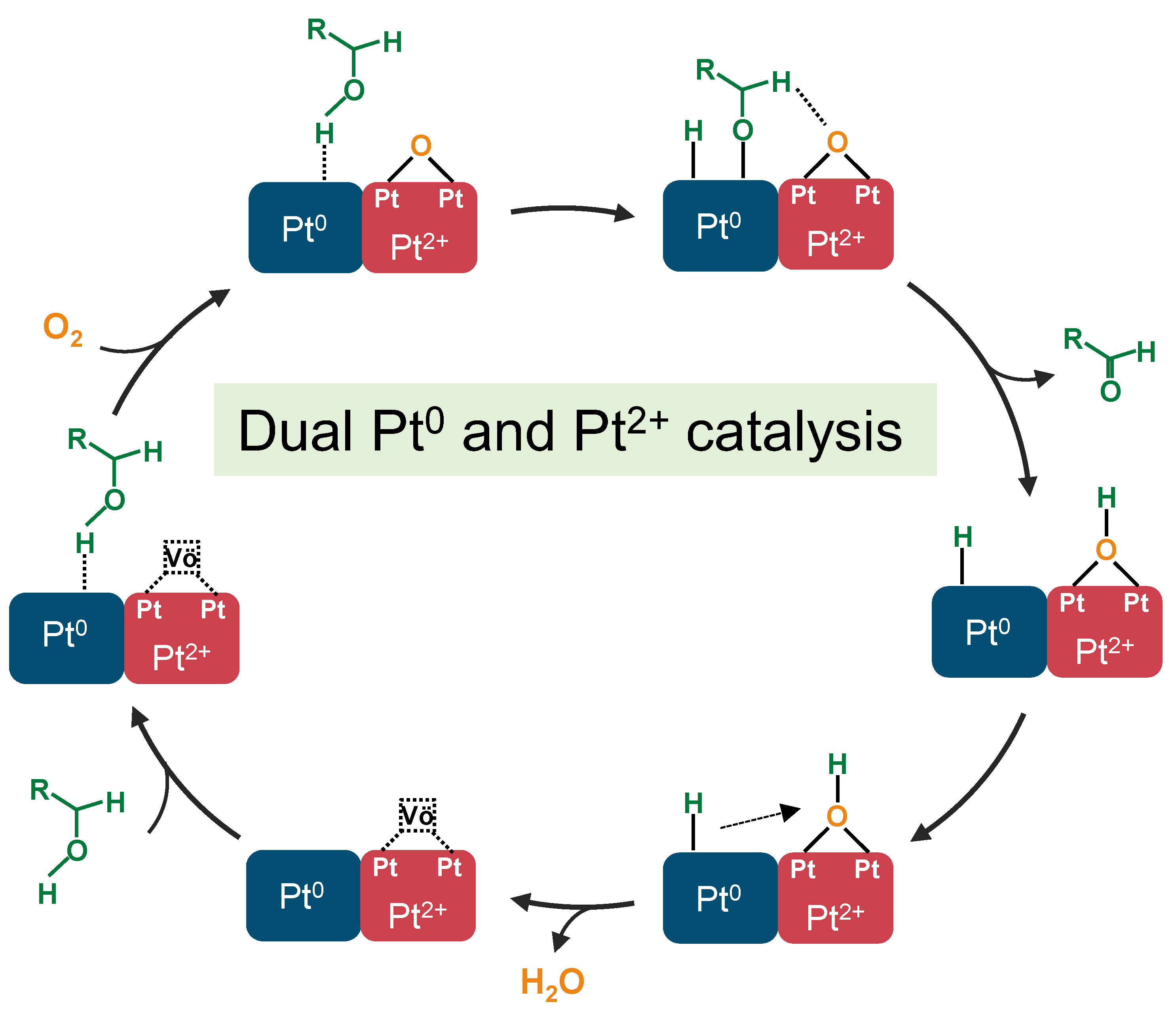
2.6. Discussion of the Catalytic Mechanism of Pt(3.6)-TiO2
3. Materials and Methods
3.1. Preparation of the Catalyst
3.2. Characterization Techniques
3.3. Catalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandarias, I.; Miedziak, P.J.; Nowicka, E.; Douthwaite, M.; Morgan, D.J.; Hutchings, G.J.; Taylor, S.H. Selective Oxidation of n-Butanol Using Gold-Palladium Supported Nanoparticles Under Base-Free Conditions. ChemSusChem 2015, 8, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.J.; Wang, Y.H.; Zhang, Z.X.; An, J.H.; Wang, F. Transformations of Biomass, Its Derivatives, and Downstream Chemicals over Ceria Catalysts. ACS Catal. 2020, 10, 8788–8814. [Google Scholar] [CrossRef]
- Gandarias, I.; Nowicka, E.; May, B.J.; Alghareed, S.; Armstrong, R.D.; Miedziak, P.J.; Taylor, S.H. The selective oxidation of n-butanol to butyraldehyde by oxygen using stable Pt-based nanoparticulate catalysts: An efficient route for upgrading aqueous biobutanol. Catal. Sci. Technol. 2016, 6, 4201–4209. [Google Scholar] [CrossRef]
- Melendez, J.R.; Mátyás, B.; Hena, S.; Lowy, D.A.; El Salous, A. Perspectives in the production of bioethanol: A review of sustainable methods, technologies, and bioprocesses. Renew. Sustain. Energy Rev. 2022, 160, 112260–112275. [Google Scholar] [CrossRef]
- Jiang, D.H.; Fang, G.Q.; Tong, Y.Q.; Wu, X.Y.; Wang, Y.F.; Hong, D.S.; Leng, W.H.; Liang, Z.; Tu, P.X.; Liu, L.; et al. Multifunctional Pd@UiO-66 Catalysts for Continuous Catalytic Upgrading of Ethanol to n-Butanol. ACS Catal. 2018, 8, 11973–11978. [Google Scholar] [CrossRef]
- Jiang, L.; Fu, H.; Yang, H.K.; Xu, W.; Wang, J.; Yang, S.-T. Butyric acid: Applications and recent advances in its bioproduction. Biotechnol. Adv. 2018, 36, 2101–2117. [Google Scholar] [CrossRef] [PubMed]
- Najafishirtari, S.; Friedel Ortega, K.; Douthwaite, M.; Pattisson, S.; Hutchings, G.J.; Bondue, C.J.; Tschulik, K.; Waffel, D.; Peng, B.; Deitermann, M.; et al. A Perspective on Heterogeneous Catalysts for the Selective Oxidation of Alcohols. Chem. Eur. J. 2021, 27, 16809–16833. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, F.; Tang, Y.; Li, L.; Miao, S.; Su, Y.; Zhang, J.; Huang, J.; Sun, H.; Haruta, M.; et al. Maximizing the Number of Interfacial Sites in Single-Atom Catalysts for the Highly Selective, Solvent-Free Oxidation of Primary Alcohols. Angew. Chem. Int. Ed. 2018, 57, 7795–7799. [Google Scholar] [CrossRef]
- Kunene, A.; Van Heerden, T.; Gambu, T.G.; Van Steen, E. Liquid Phase, Aerobic Oxidation of Benzyl Alcohol over the Catalyst System (Pt/TiO2+H2O). ChemCatChem 2020, 12, 4760–4764. [Google Scholar] [CrossRef]
- Xin, P.; Li, J.; Xiong, Y.; Wu, X.; Dong, J.; Chen, W.; Wang, Y.; Gu, L.; Luo, J.; Rong, H.; et al. Revealing the Active Species for Aerobic Alcohol Oxidation by Using Uniform Supported Palladium Catalysts. Angew. Chem. Int. Ed. 2018, 57, 4642–4646. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, Y.; Li, H.; Chen, Z.; Wang, Y. Solvent-free aerobic oxidation of hydrocarbons and alcohols with Pd@N-doped carbon from glucose. Nat. Commun. 2013, 4, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Mori, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Creation of a Monomeric Ru Species on the Surface of Hydroxyapatite as an Efficient Heterogeneous Catalyst for Aerobic Alcohol Oxidation. J. Am. Chem. Soc. 2000, 122, 7144–7145. [Google Scholar] [CrossRef]
- Sofack Kreutzer, J.; Vanoye, L.; Guicheret, B.; Philippe, R.; Metay, E.; Duclos, M.-C.; Lemaire, M.; De Bellefon, C.; Fongarland, P.; Favre-Réguillon, A. Continuous flow aerobic alcohol oxidation using a heterogeneous Ru0 catalyst. React. Chem. Eng. 2019, 4, 550–558. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Zhao, Q.; Li, D.; Wang, J.-Q.; Cho, M.; Cho, H.; Terasaki, O.; Chen, S.; Wan, Y. Highly Active Heterogeneous 3 nm Gold Nanoparticles on Mesoporous Carbon as Catalysts for Low-Temperature Selective Oxidation and Reduction in Water. ACS Catal. 2015, 5, 797–802. [Google Scholar] [CrossRef]
- Ballarin, B.; Barreca, D.; Boanini, E.; Cassani, M.C.; Dambruoso, P.; Massi, A.; Mignani, A.; Nanni, D.; Parise, C.; Zaghi, A. Supported Gold Nanoparticles for Alcohols Oxidation in Continuous-Flow Heterogeneous Systems. ACS Sustain. Chem. Eng. 2017, 5, 4746–4756. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Xu, M.; Kunal, P.; Trewyn, B.G. Aerobic oxidative esterification of primary alcohols over Pd-Au bimetallic catalysts supported on mesoporous silica nanoparticles. Catal. Today 2018, 306, 81–88. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pandarus, V.; Béland, F.; Xu, Y.-J.; Pagliaro, M. Heterogeneously Catalyzed Alcohol Oxidation for the Fine Chemical Industry. Org. Process Res. Dev. 2015, 19, 1554–1558. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev. 2014, 43, 3480–3524. [Google Scholar] [CrossRef]
- Lei, L.; Wu, Z.; Wang, R.; Qin, Z.; Chen, C.; Liu, Y.; Wang, G.; Fan, W.; Wang, J. Controllable decoration of palladium sub-nanoclusters on reduced graphene oxide with superior catalytic performance in selective oxidation of alcohols. Catal. Sci. Technol. 2017, 7, 5650–5661. [Google Scholar] [CrossRef]
- Xiao, Q.; Liu, Z.; Bo, A.; Zavahir, S.; Sarina, S.; Bottle, S.; Riches, J.D.; Zhu, H.Y. Catalytic Transformation of Aliphatic Alcohols to Corresponding Esters in O2 under Neutral Conditions Using Visible-Light Irradiation. J. Am. Chem. Soc. 2015, 137, 1956–1966. [Google Scholar] [CrossRef]
- Lu, T.; Du, Z.; Liu, J.; Ma, H.; Xu, J. Aerobic oxidation of primary aliphatic alcohols over bismuth oxide supported platinum catalysts in water. Green Chem. 2013, 15, 2215. [Google Scholar] [CrossRef]
- Wang, T.; Shou, H.; Kou, Y.; Liu, H.C. Base-free aqueous-phase oxidation of non-activated alcohols with molecular oxygen on soluble Pt nanoparticles. Green Chem. 2009, 11, 562–568. [Google Scholar] [CrossRef]
- Oberhauser, W.; Poggini, L.; Capozzoli, L.; Bellini, M.; Filippi, J.; Vizza, F. Oxidation of Ethanol to Acetic Acid by Supported PtCu Nanoparticles Stabilized by a Diamine Ligand. Inorg. Chem. 2023, 62, 2848–2858. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.W.; Xia, W.; Xie, K.P.; Peng, B.X.; Muhler, M. Synergistic effect of potassium hydroxide and steam co-treatment on the functionalization of carbon nanotubes applied as basic support in the Pd-catalyzed liquid-phase oxidation of ethanol. Carbon 2017, 121, 452–462. [Google Scholar] [CrossRef]
- Wang, L.; Meng, X.J.; Wang, B.; Chi, W.Y.; Xiao, F.S. Pyrrolidone-modified SBA-15 supported Au nanoparticles with superior catalytic properties in aerobic oxidation of alcohols. Chem. Commun. 2010, 46, 5003–5005. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yan, X.; Liao, X.; Li, R.; Xu, S.; Xiao, L.; Fan, J. Platinum nanoparticles supported on Ca(Mg)-zeolites for efficient room-temperature alcohol oxidation under aqueous conditions. Chem. Commun. 2014, 50, 9679–9682. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zou, S.; Wu, J.; Kobayashi, H.; Zhao, H.; Fan, J. Green catalytic oxidation of benzyl alcohol over Pt/ZnO in base-free aqueous medium at room temperature. Chin. J. Catal. 2018, 39, 1081–1089. [Google Scholar] [CrossRef]
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Contescu, C.; Contescu, A. Methods for Preparation of Catalytic Materials. Chem. Rev. 1995, 95, 477–510. [Google Scholar] [CrossRef]
- Xie, Y.; Ding, K.; Liu, Z.; Tao, R.; Sun, Z.; Zhang, H.; An, G. In Situ Controllable Loading of Ultrafine Noble Metal Particles on Titania. J. Am. Chem. Soc. 2009, 131, 6648–6649. [Google Scholar] [CrossRef]
- O’neill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst Design with Atomic Layer Deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef]
- Corbel, G.; Topic, M.; Gibaud, A.; Lang, C.I. Selective dry oxidation of the ordered Pt-11.1 at.% V alloy surface evidenced by in situ temperature-controlled X-ray diffraction. J. Alloys Compd. 2011, 509, 6532–6538. [Google Scholar] [CrossRef]
- Guo, L.-W.; Du, P.-P.; Fu, X.-P.; Ma, C.; Zeng, J.; Si, R.; Huang, Y.-Y.; Jia, C.-J.; Zhang, Y.-W.; Yan, C.-H. Contributions of distinct gold species to catalytic reactivity for carbon monoxide oxidation. Nat. Commun. 2016, 7, 13481. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.C.; Wang, X.; Chen, Q.; Ye, Y.F.; Zheng, H.Q.; Guo, J.H.; Yin, Y.D.; Gao, C.B. Explaining the Size Dependence in Platinum-Nanoparticle-Catalyzed Hydrogenation Reactions. Angew. Chem. Int. Ed. 2016, 55, 15656–15661. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.L.; Sun, W.M.; Shang, H.S.; Chen, W.X.; Sun, T.T.; Li, H.J.; Dong, J.C.; Zhou, J.; Li, Z.; Wang, Y.; et al. Atomic interface effect of a single atom copper catalyst for enhanced oxygen reduction reactions. Energy Environ. Sci. 2019, 12, 3508–3514. [Google Scholar] [CrossRef]
- Van Spronsen, M.A.; Frenken, J.W.M.; Groot, I.M.N. Observing the oxidation of platinum. Nat. Commun. 2017, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.Y.; Qi, X.; Yun, X.H.; Ge, J.M.; Tian, Y.; Lei, X.D.; Zhang, F.Z. Confined Ruthenium Nanoparticles as an Effective Catalyst for Aerobic Oxidation of Aqueous Ethanol to Acetic Acid. ACS Sustain. Chem. Eng. 2022, 10, 9687–9696. [Google Scholar] [CrossRef]
- Ishida, T.; Ogihara, Y.; Ohashi, H.; Akita, T.; Honma, T.; Oji, H.; Haruta, M. Base-Free Direct Oxidation of 1-Octanol to Octanoic Acid and its Octyl Ester over Supported Gold Catalysts. ChemSusChem 2012, 5, 2243–2248. [Google Scholar] [CrossRef]
- Mannel, D.S.; King, J.; Preger, Y.; Ahmed, M.S.; Root, T.W.; Stahl, S.S. Mechanistic Insights into Aerobic Oxidative Methyl Esterification of Primary Alcohols with Heterogeneous PdBiTe Catalystsy. ACS Catal. 2018, 8, 1038–1047. [Google Scholar] [CrossRef]
- Karimi, B.; Naderi, Z.; Khorasani, M.; Mirzaei, H.M.; Vali, H. Ultrasmall Platinum Nanoparticles Supported Inside the Nanospaces of Periodic Mesoporous Organosilica with an Imidazolium Network: An Efficient Catalyst for the Aerobic Oxidation of Unactivated Alcohols in Water. ChemCatChem 2016, 8, 906–910. [Google Scholar] [CrossRef]
- Wang, H.; Fan, W.; He, Y.; Wang, J.; Kondo, J.N.; Tatsumi, T. Selective oxidation of alcohols to aldehydes/ketones over copper oxide-supported gold catalysts. J. Catal. 2013, 299, 10–19. [Google Scholar] [CrossRef]
- An, J.; Wang, Y.; Lu, J.; Zhang, J.; Zhang, Z.; Xu, S.; Liu, X.; Zhang, T.; Gocyla, M.; Heggen, M.; et al. Acid-Promoter-Free Ethylene Methoxycarbonylation over Ru-Clusters/Ceria: The Catalysis of Interfacial Lewis Acid–Base Pair. J. Am. Chem. Soc. 2018, 140, 4172–4181. [Google Scholar] [CrossRef] [PubMed]
- Somorjai, G.A.; Park, J.Y. Molecular Factors of Catalytic Selectivity. Angew. Chem. Int. Ed. 2008, 47, 9212–9228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-G.; Yoon, Y.; Glezakou, V.-A.; Li, J.; Rousseau, R. The Role of Reducible Oxide–Metal Cluster Charge Transfer in Catalytic Processes: New Insights on the Catalytic Mechanism of CO Oxidation on Au/TiO2 from ab Initio Molecular Dynamics. J. Am. Chem. Soc. 2013, 135, 10673–10683. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.; Rana, R.K. A rational design of a Pd-based catalyst with a metal–metal oxide interface influencing molecular oxygen in the aerobic oxidation of alcohols. Green Chem. 2019, 21, 2494–2503. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B.; Yang, S.; Yang, X.; Meng, F.; Zhai, L.; Li, Z.; Zhao, S.; Zhang, G.; Qin, Y. Dual Pd2+ and Pd0 sites on CeO2 for benzyl alcohol selective oxidation. J. Catal. 2022, 414, 385–393. [Google Scholar] [CrossRef]
- Zhang, Z.-C.; Hui, J.-F.; Guo, Z.-G.; Yu, Q.-Y.; Xu, B.; Zhang, X.; Liu, Z.-C.; Xu, C.-M.; Gao, J.-S.; Wang, X. Solvothermal synthesis of Pt–Pd alloys with selective shapes and their enhanced electrocatalytic activities. Nanoscale 2012, 4, 2633–2639. [Google Scholar] [CrossRef]
- Yuan, Z.-F.; Zhao, W.-N.; Liu, Z.-P.; Xu, B.-Q. NaOH alone can be a homogeneous catalyst for selective aerobic oxidation of alcohols in water. J. Catal. 2017, 353, 37–43. [Google Scholar] [CrossRef]




 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst | Butanol/Pt | Temperature (°C) | Time (h) | Conversion (%) | Selectivity (%) | Yield (%) Butyric Acid | TOF 3 (h−1) | |
| Butyraldehyde | Butyric Acid | ||||||||
| 1 | Pt(1.1)-TiO2 | 500 | 80 | 10 | 16.4 | 92.3 | 7.7 | 1.3 | 47 |
| 2 | Pt(2.6)-TiO2 | 500 | 80 | 10 | 78.9 | 31.7 | 68.3 | 53.9 | 281 |
| 3 | Pt(3.6)-TiO2 | 500 | 80 | 10 | 99.5 | 0.7 | 99.3 | 98.8 | 355 |
| 4 | Pt(5.3)-TiO2 | 500 | 80 | 10 | 67.2 | 40.7 | 59.3 | 39.8 | 165 |
| 5 | Pt(8.7)-TiO2 | 500 | 80 | 10 | 42.8 | 57.3 | 42.7 | 18.3 | 123 |
| 6 | Pt(3.6)-TiO2 | 50 | 30 | 40 | 98.9 | 1.6 | 98.4 | 97.3 | 36 |
| 7 | Pt(3.6)-TiO2 | 100 | 40 | 24 | 99.8 | 0.9 | 99.1 | 98.9 | 54 |
| 8 | Pt(3.6)-TiO2 | 200 | 60 | 16 | 98.7 | 0.5 | 99.5 | 98.2 | 135 |
| 9 | Pt(3.6)-TiO2 | 1000 | 90 | 10 | 99.0 | 1.2 | 98.8 | 97.8 | 619 |
| 10 | Pt(3.6)-TiO2 1 | 500 | 80 | 10 | 75.2 | 42.1 | 57.9 | 43.5 | 287 |
| 11 | Pt(3.6)-TiO2 2 | 500 | 80 | 10 | 3.1 | 99.5 | – | – | – |
| 12 | Pt-SiO2 | 500 | 80 | 10 | 41.6 | 37.4 | 62.6 | 32.9 | 125 |
| 13 | Pt-CeO2 | 500 | 80 | 10 | 90.7 | 13.8 | 86.2 | 74.7 | 328 |
| 14 | Pt-ZrO2 | 500 | 80 | 10 | 88.1 | 15.9 | 84.1 | 69.0 | 296 |
| 15 | Pt-Al2O3 | 500 | 80 | 10 | 58.4 | 28.8 | 71.2 | 48.7 | 143 |
| Entry | Substrate | Conversion (%) | Selectivity (%) | Yield (%) Acid | TOF (h−1) 1 | |
|---|---|---|---|---|---|---|
| Aldehyde/Ketone | Acid | |||||
| 1 | Ethanol | 92.5 | 5.6 | 94.4 | 87.3 | 334 |
| 2 | Propanol | 91.6 | 6.4 | 93.6 | 85.7 | 341 |
| 3 | Butanol | 99.5 | 0.7 | 99.3 | 98.8 | 355 |
| 4 | Pentanol | 92.1 | 9.3 | 90.7 | 83.5 | 320 |
| 5 | Hexanol | 93.4 | 3.7 | 96.3 | 89.9 | 317 |
| 6 | Octanol | 95.8 | 1.6 | 98.4 | 94.3 | 346 |
| 7 | 2-Octanol | 99.7 | 99.9 2 | – | 99.6 | 416 |
| 8 | Cyclohexanol | 99.3 | 99.9 2 | – | 99.2 | 403 |
| 9 3 | Furfural | 99.6 | – | 99.3 | 98.9 | 435 |
| 10 4 | Phenol | 81.2 | 76.4 | – | 62.0 | 314 |
| 11 4 | 5-Hydroxymethylfurfural | 96.4 | – | 99.4 5 | 95.8 | 346 |
| 12 4 | Furfuryl alcohol | 99.2 | 1.3 | 98.7 | 97.9 | 394 |
| 13 4 | Tetrahydrofurfuryl alcohol | 90.3 | 7.6 | 92.4 | 83.4 | 327 |
| 14 6 | Glycerol | 62.0 | – | 99.8 | 61.9 | 179 |
| 15 7 | Glucose | 64.5 | – | 71.9 | 46.4 | 162 |
| Entry | Catalyst | Temp. (°C) | Molar Ratio | Base | Time (h) | Conv. (%) | Sel. (%) | TOF (h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pt/Bi2O3 | 90 | 75 | free | 5 | 99.0 | 99.0 | – | [21] |
| 2 | Au-Pd/TiO2 | 100 | 550 | free | 6 | 89.7 | 92.5 | 250 | [1] |
| 3 | Pt sol | 80 | 20 | free | 24 | 100 | 99.7 | 37 | [22] |
| 4 | Au/SBA-15-Py | 130 | 2190 | NaAc 4 | 24 | 50.7 | 98.0 | – | [25] |
| 5 | Pt-Pd/TiO2 | 100 | 163 | free | 6 | 75 | 53 | – | [3] |
| 6 1 | Pd/CK05-550 | 160 | 200 | free | 5 | 96 | 71 | – | [24] |
| 7 1 | (PtCu)L@CK | 140 | 14,600 | free | 17 | 40.9 | 7.3 | – | [23] |
| 8 1 | Ru/Mg1−xFexO | 150 | 400 | free | 4 | 94.1 | 95.6 | – | [37] |
| 9 2 | AuPd@HT-PO43− | 55 | 10 | free | 24 | 62 | 42 | – | [20] |
| 10 2 | Au/NiO | 100 | 1000 | free | 18 | 90 | 68 | – | [38] |
| 11 2 | PdBiTe/C | 60 | 100 | K2CO3 | 8 | >90 | 90 | – | [39] |
| 12 3 | Pt(NP)@PMO-IL-2 | 90 | 20 | K2CO3 | 21 | 83 | >99 5 | – | [40] |
| 13 | Pt(3.6)/TiO2 | 30 | 50 | free | 40 | 98.9 | 98.4 | 36 | This work |
| 14 | Pt(3.6)/TiO2 | 80 | 500 | free | 10 | 99.5 | 99.3 | 355 | |
| 15 | Pt(3.6)/TiO2 | 90 | 1000 | free | 10 | 99 | 98.8 | 619 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, L.; Cao, Q.; Ma, J.; Hou, F. One-Step Hydrothermal/Solvothermal Preparation of Pt/TiO2: An Efficient Catalyst for Biobutanol Oxidation at Room Temperature. Molecules 2024, 29, 1450. https://doi.org/10.3390/molecules29071450
Lei L, Cao Q, Ma J, Hou F. One-Step Hydrothermal/Solvothermal Preparation of Pt/TiO2: An Efficient Catalyst for Biobutanol Oxidation at Room Temperature. Molecules. 2024; 29(7):1450. https://doi.org/10.3390/molecules29071450
Chicago/Turabian StyleLei, Lijun, Qianyue Cao, Jiachen Ma, and Fengxiao Hou. 2024. "One-Step Hydrothermal/Solvothermal Preparation of Pt/TiO2: An Efficient Catalyst for Biobutanol Oxidation at Room Temperature" Molecules 29, no. 7: 1450. https://doi.org/10.3390/molecules29071450