Efficient Recovery of Phosphate from Water Media by Iron-Magnesium Functionalized Lignite: Adsorption Evaluation, Mechanism Revelation and Potential Application Exploration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Adsorption Characteristic
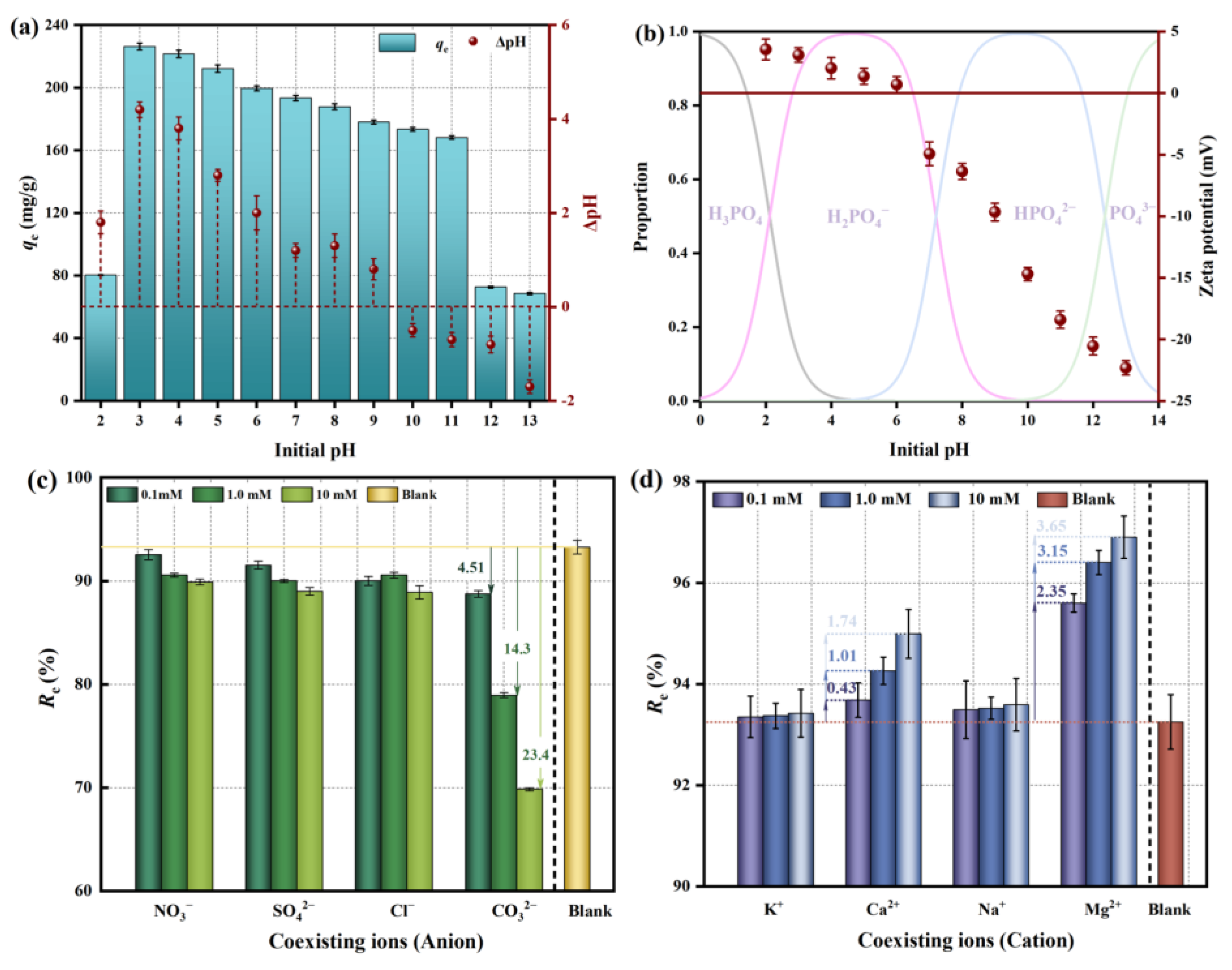
2.1.1. The Effect of pH
2.1.2. The Effect of Co-Existing Ions
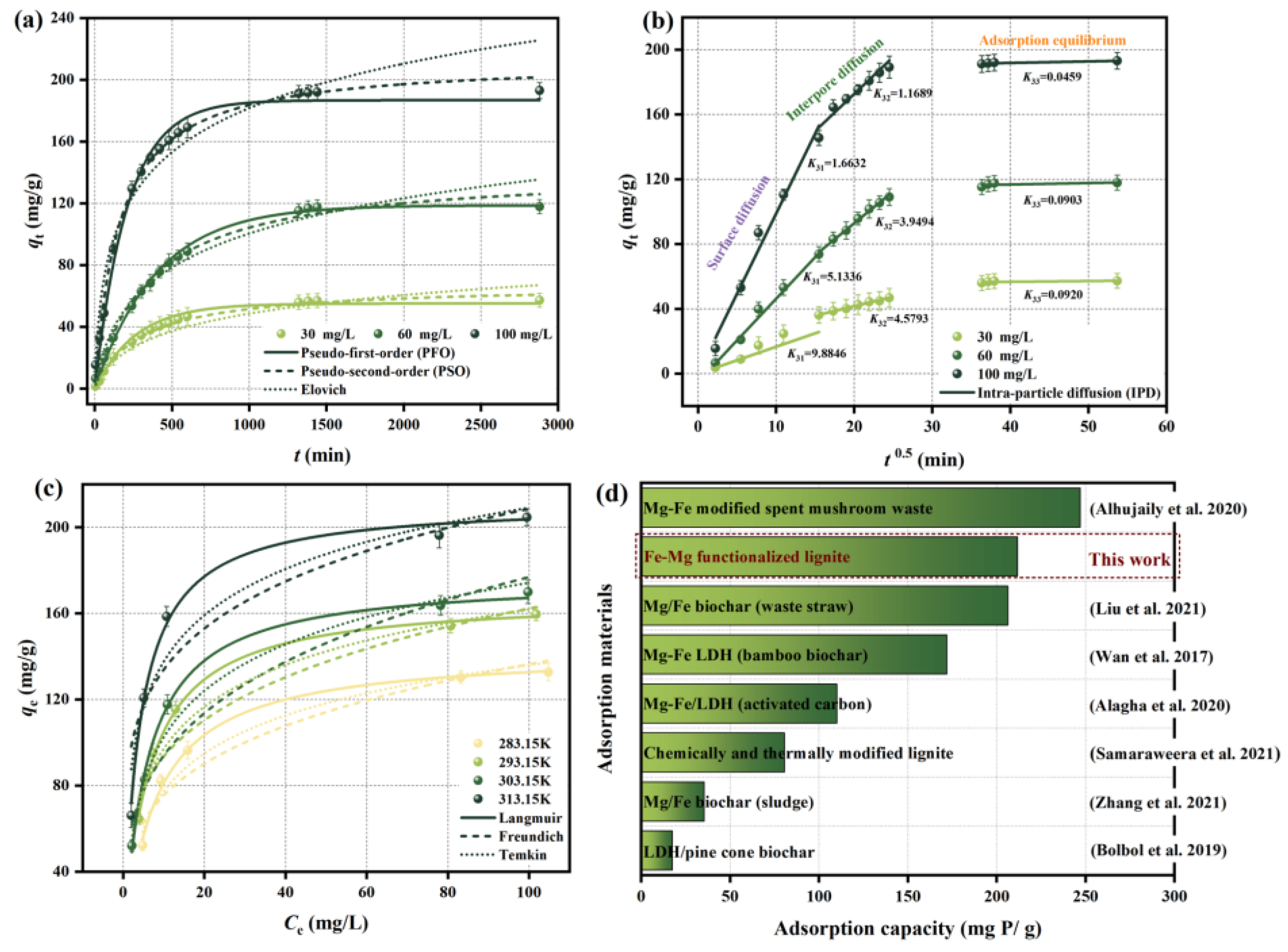
2.2. Adsorption Kinetics
2.3. Adsorption Isotherms
2.4. Adsorption Thermodynamics
2.5. Adsorption Mechanism
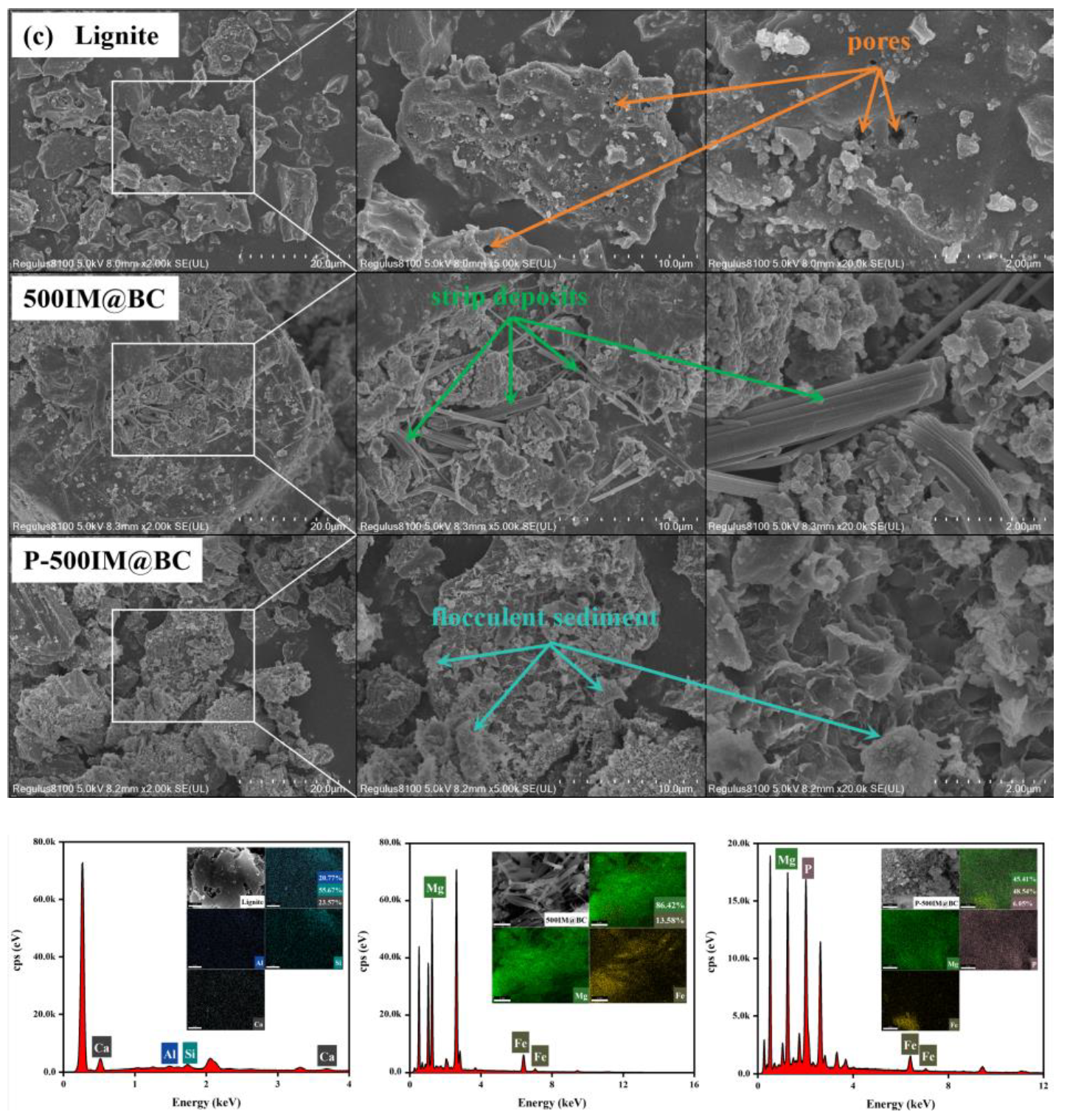
2.5.1. The Results of BET
2.5.2. The Results of FESEM-EDS
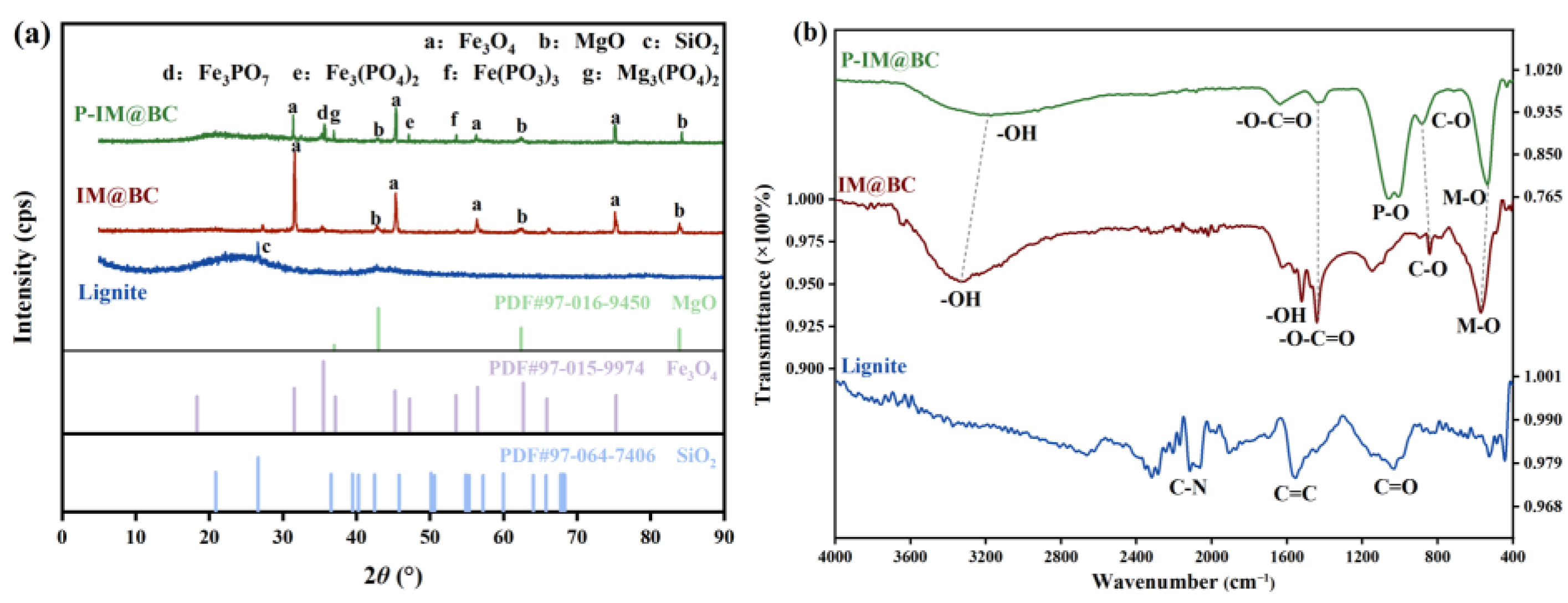
2.5.3. The Results of XRD
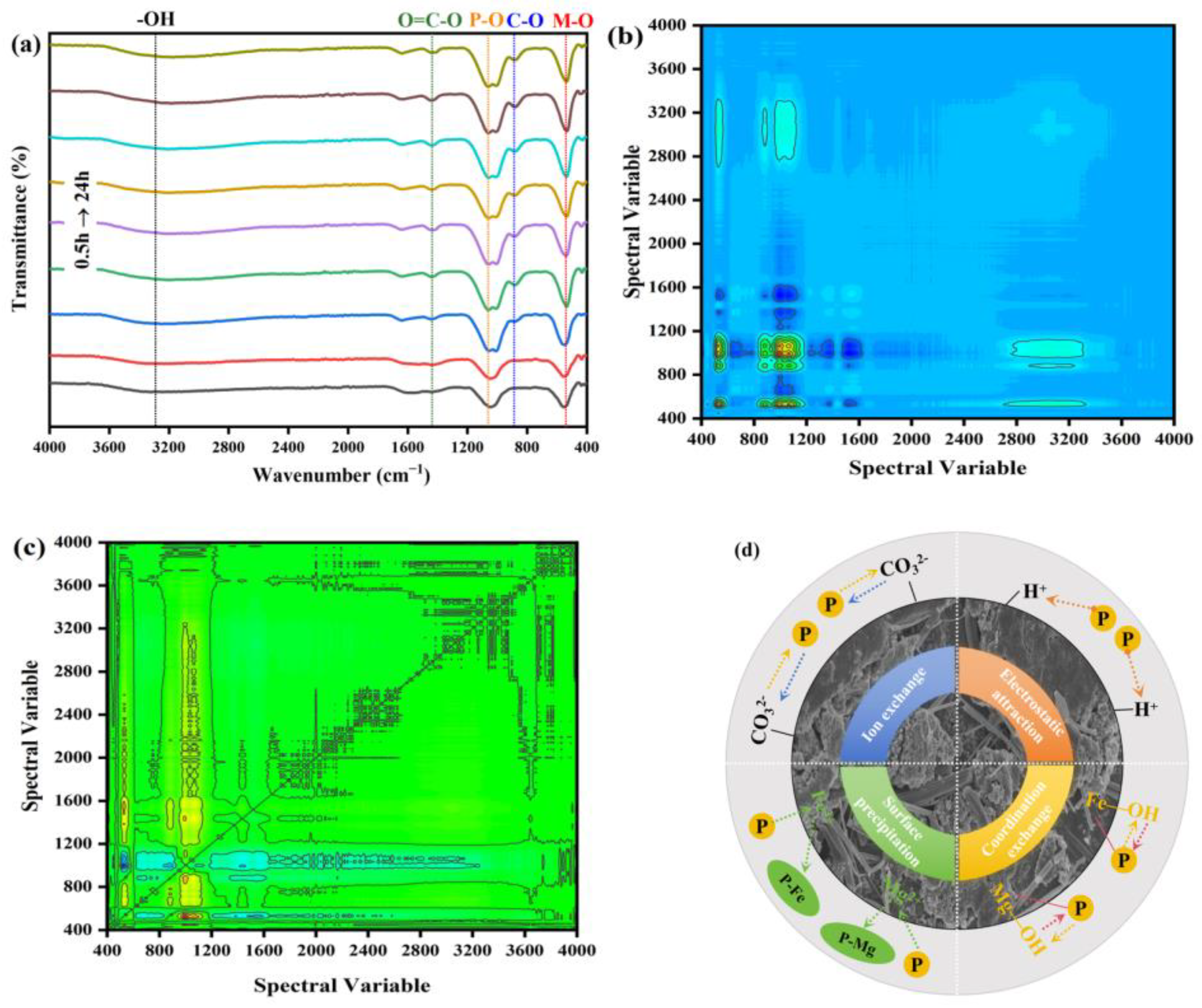
2.5.4. The Results of FTIR
2.5.5. The Results of XPS
2.5.6. The Results of 2D-COS
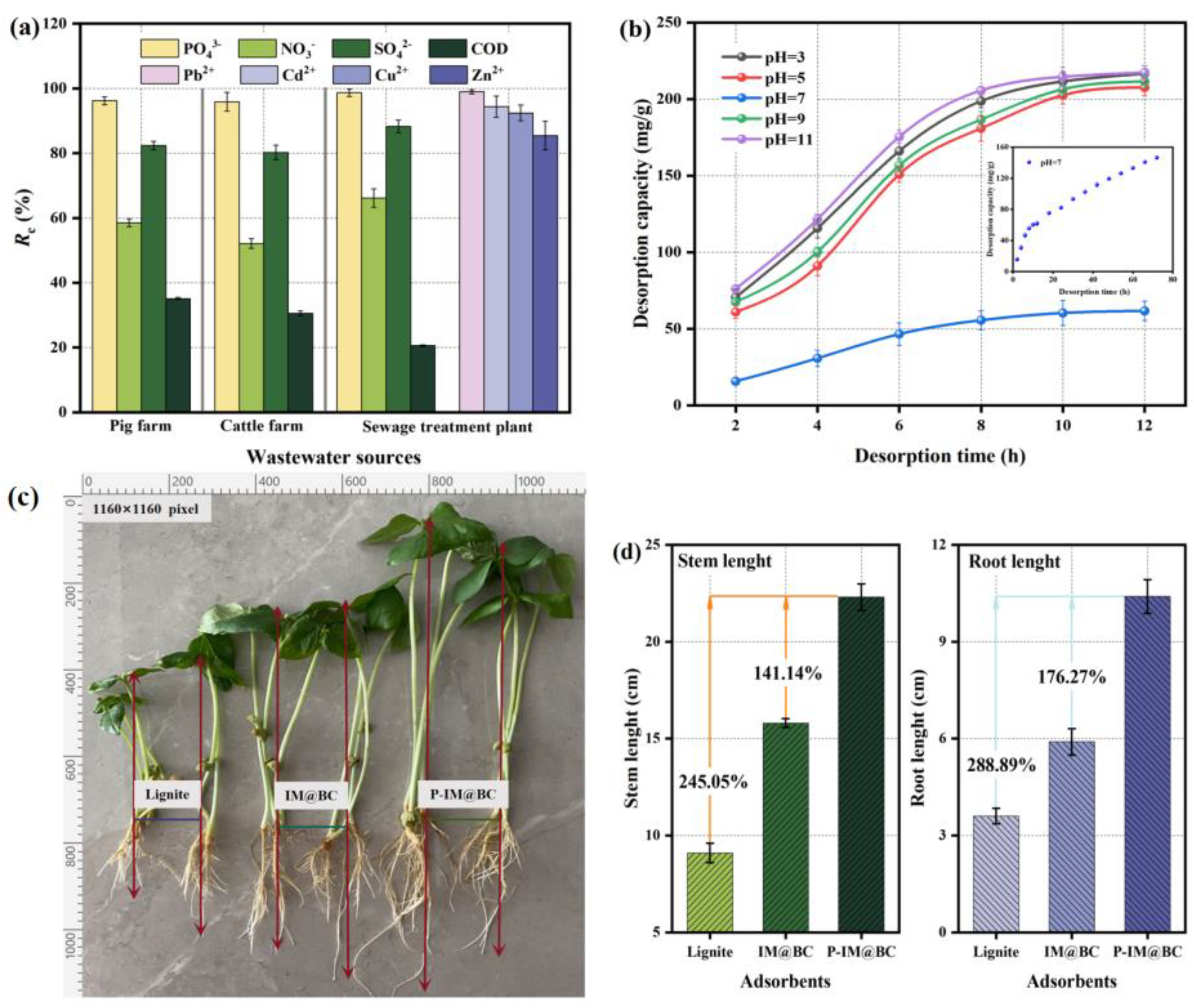
2.6. Application Potential of Saturated Adsorbents
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Iron–Magnesium Functionalized Lignite (IM@BC)
3.3. Batch Adsorption Experiments
3.4. Data Analysis and Modelling
3.5. Characterization of Adsorbents
3.6. Regeneration and Utilization Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, D.; Wang, L.Y.; Nan, H.Y.; Cao, Y.J.; Wang, H.; Kumar, T.V.; Wang, C.Q. Phosphorus adsorption by functionalized biochar: A review. Environ. Chem. Lett. 2023, 21, 497–524. [Google Scholar] [CrossRef]
- Sun, D.Q.; Hale, L.; Kar, G.; Soolanayakanahally, R.; Adl, S. Phosphorus recovery and reuse by pyrolysis: Applications for agriculture and environment. Chemosphere 2018, 194, 682–691. [Google Scholar] [CrossRef]
- Shaddel, S.; Grini, T.; Ucar, S.; Azrague, K.; Andreassen, J.P.; Osterhus, S.W. Struvite crystallization by using raw seawater: Improving economics and environmental footprint while maintaining phosphorus recovery and product quality. Water Res. 2020, 173, 115572. [Google Scholar] [CrossRef]
- Marella, T.K.; Saxena, A.; Tiwari, A.; Datta, A.; Dixit, S. Treating agricultural non-point source pollutants using periphyton biofilms and biomass volarization. J. Environ. Manag. 2022, 301, 113869. [Google Scholar] [CrossRef]
- Liu, B.; Gai, S.; Lan, Y.B.; Cheng, K.; Yang, F. Metal-based adsorbents for water eutrophication remediation: A review of performances and mechanisms. Environ. Res. 2022, 212, 113353. [Google Scholar] [CrossRef]
- Bacelo, H.; Pintor, A.M.A.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566–122584. [Google Scholar] [CrossRef]
- Nguyen, T.; Ngo, H.; Guo, W.; Zhang, J.; Liang, S.; Lee, D.; Nguyen, P.; Bui, X. Modification of agricultural waste/by-products for enhanced phosphate removal and recovery: Potential and obstacles. Bioresour. Technol. 2014, 169, 750–762. [Google Scholar] [CrossRef]
- Oginni, O.; Yakaboylu, G.; Singh, K.; Sabolsky, E.; Unal-Tosun, G.; Jaisi, D.; Khanal, S.; Shah, A. Phosphorus adsorption behaviors of MgO modified biochars derived from waste woody biomass resources. J. Environ. Chem. Eng. 2020, 8, 103723. [Google Scholar] [CrossRef]
- Polat, H.; Molva, M.; Polat, M. Capacity and mechanism of phenol adsorption on lignite. Int. J. Miner. Process. 2006, 79, 264–273. [Google Scholar] [CrossRef]
- Samaraweera, H.; Sharp, A.; Edwards, J.; Pittman, C.; Zhang, X.; Hassan, E.; Warren, S.; Reid, C.; Mlsna, T. Lignite, thermally-modified and Ca/Mg-modified lignite for phosphate remediation. Sci. Total Environ. 2021, 773, 145631. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Ruan, Z.; Zhang, S.; Dong, Y.; Fu, S.; Li, H.; Jiang, G. Adsorption behaviors and mechanisms of Cu2+, Zn2+ and Pb2+ by magnetically modified lignite. Sci. Rep. 2022, 12, 1394. [Google Scholar] [CrossRef]
- Koh, K.Y.; Zhang, S.; Chen, J.P. Hydrothermally synthesized lanthanum carbonate nanorod for adsorption of phosphorus: Material synthesis and optimization, and demonstration of excellent performance. Chem. Eng. J. 2020, 380, 122153–122166. [Google Scholar] [CrossRef]
- Ahmed, S.; Lo, I.M.C. Phosphate removal from river water using a highly efficient magnetically recyclable Fe3O4/La(OH)3 nanocomposite. Chemosphere 2020, 261, 128118–128128. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Taddesse, A.M.; Kebede, T.; Teju, E.; Diaz, I. Fe-Al-Mn ternary oxide nanosorbent: Synthesis, characterization and phosphate sorption property. J. Environ. Chem. Eng. 2017, 5, 1330–1340. [Google Scholar] [CrossRef]
- Du, W.Y.; Li, Y.R.; Xu, X.; Shang, Y.N.; Gao, B.Y.; Yue, Q.Y. Selective removal of phosphate by dual Zr and La hydroxide/ cellulose-based bio-composites. J. Colloid Interface Sci. 2019, 533, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, X.; Nie, G.Z.; Xu, L.J.; Hu, Y. Enhanced phosphate removal by using La-Zr binary metal oxide nanoparticles confined in millimeter-sized anion exchanger. J. Colloid Interface Sci. 2020, 580, 234–244. [Google Scholar] [CrossRef]
- Sun, H.; He, J.; Liu, Y.; Ji, X.; Wang, G.; Yang, X.; Zhang, Y. Removal Performance and Mechanism of Emerging Pollutant Chloroquine Phosphate from Water by Iron and Magnesium Co-Modified Rape Straw Biochar. Molecules 2023, 28, 3290. [Google Scholar] [CrossRef]
- Tang, J.; Ma, Y.; Cui, S.; Ding, Y.; Zhu, J.; Chen, X.; Zhang, Z. Insights on ball milling enhanced iron magnesium layered double oxides bagasse biochar composite for ciprofloxacin adsorptive removal from water. Bioresour. Technol. 2022, 359, 127468. [Google Scholar] [CrossRef]
- Wu, B.L.; Fang, L.P.; Fortner, J.D.; Guan, X.H.; Lo, I.M.C. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites. Water Res. 2017, 126, 179–188. [Google Scholar] [CrossRef]
- Mi, X.; Yu, F.; Zhang, H.N.; Chen, R.F.; Hu, X.L.; Zhang, W.; Wang, B. Lanthanum activated palygorskite for selective phosphate separation from aqueous media: Comprehensive understanding of adsorptive behavior and mechanism affected by interfering substances. Chem. Eng. J. 2022, 443, 136423. [Google Scholar] [CrossRef]
- Wang, B.; Hu, X.L.; Li, L.; Wang, H.Y.; Huang, H.Y.; Wang, R.R.; Zhou, D.; Yuan, J.P.; Chen, L. Application and functionalization of toxic waste sludge-derived biochar for efficient phosphate separation from aqueous media: Toxicity diminution, robust adsorption, and inner mechanism. Chem. Eng. J. 2023, 468, 143745. [Google Scholar] [CrossRef]
- Qiu, H.; Liang, C.; Yu, J.H.; Zhang, Q.R.; Song, M.X.; Chen, F.H. Preferable phosphate sequestration by nano-La(III) (hydr)oxides modified wheat straw with excellent properties in regeneration. Chem. Eng. J. 2017, 315, 345–354. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.J.; Huang, Y.K.; Zhu, J.Y.; Zhu, R.L. Role of phosphate concentration in control for phosphate removal and recovery by layered double hydroxides. Environ. Sci. Pollut. Res. 2020, 27, 16612–16623. [Google Scholar] [CrossRef] [PubMed]
- Alhujaily, A.; Mao, Y.Z.; Zhang, J.L.; Ifthikar, J.; Zhang, X.Y.; Ma, F.Y. Facile fabrication of Mg-Fe-biochar adsorbent derived from spent mushroom waste for phosphate removal. J. Taiwan Inst. Chem. Eng. 2020, 117, 75–85. [Google Scholar] [CrossRef]
- Liu, H.B.; Shan, J.H.; Chen, Z.B.; Lichtfouse, E. Efficient recovery of phosphate from simulated urine by Mg/Fe bimetallic oxide modified biochar as a potential resource. Sci. Total Environ. 2021, 784, 147546. [Google Scholar] [CrossRef]
- Wan, S.; Wang, S.S.; Li, Y.C.; Gao, B. Functionalizing biochar with Mg-Al and Mg-Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Ind. Eng. Chem. 2017, 47, 246–253. [Google Scholar] [CrossRef]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’azu, N.D.; Qureshi, A. Magnetic Mg-Fe/LDH Intercalated Activated Carbon Composites for Nitrate and Phosphate Removal from Wastewater: Insight into Behavior and Mechanisms. Nanomaterial 2020, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Yang, J.; Wang, H.X.; Lv, Q.; Xue, J.B. Enhanced removal of phosphate from aqueous solution using Mg/Fe modified biochar derived from excess activated sludge: Removal mechanism and environmental risk. Environ. Sci. Pollut. Res. 2021, 28, 16282–16297. [Google Scholar] [CrossRef]
- Bolbol, H.; Fekri, M.; Hejazi-Mehrizi, M. Layered double hydroxide-loaded biochar as a sorbent for the removal of aquatic phosphorus: Behavior and mechanism insights. Arab. J. Geosci. 2019, 12, 503. [Google Scholar] [CrossRef]
- Chang, Y.; Wei, Z.P.; Chang, X.; Ma, G.L.; Meng, L.L.; Liu, T.T.; Yang, L.; Guo, Y.M.; Ma, X.M. Hollow hierarchically porous La2O3 with controllable multishells: A highperformance adsorbent for phosphate removal. Chem. Eng. J. 2021, 421, 127816. [Google Scholar] [CrossRef]
- Mohammadi, R.; Hezarjaribi, M.; Ramasamy, D.L.; Sillanpää, M.; Pihlajamäki, A. Application of a novel biochar adsorbent and membrane to the selective separation of phosphate from phosphate-rich wastewaters. Chem. Eng. J. 2021, 407, 126494. [Google Scholar] [CrossRef]
- Zong, E.; Wang, X.; Zhang, L.; Yang, J.; Liu, X. A Recyclable Magnetic Aminated Lignin Supported Zr-La Dual-Metal Hydroxide for Rapid Separation and Highly Efficient Sequestration of Phosphate. Molecules 2023, 28, 2923. [Google Scholar] [CrossRef]
- Li, H.; Mahyoub, S.A.A.; Liao, W.J.E.; Xia, S.Q.; Zhao, H.C.; Guo, M.Y.; Ma, P.S. Effect of pyrolysis temperature on characteristics and aromatic contaminants adsorption behavior of magnetic biochar derived from pyrolysis oil distillation residue. Bioresour. Technol. 2017, 223, 20–26. [Google Scholar] [CrossRef]
- Jung, K.W.; Lee, S.Y.; Choi, J.W.; Lee, Y.J. A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites: Adsorption behavior and mechanisms for the removal of copper(II) from aqueous media. Chem. Eng. J. 2019, 369, 529–541. [Google Scholar] [CrossRef]
- Simoes, M.C.; Hughes, K.J.; Ingham, D.B.; Ma, L.; Pourkashanian, M. Estimation of the thermochemical radii and ionic volumes of complex ions. Inorg. Chem. 2017, 56, 7566–7573. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.B.; Chen, J.B.; Zhang, Y.L.; Zhou, X.F. Enhancing efficient reclaim of phosphorus from simulated urine by magnesium-functionalized biochar: Adsorption behaviors, molecular-level mechanistic explanations and its potential application. Sci. Total Environ. 2023, 906, 167293. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, D.; Yan, B.; Li, W.; Guan, Y. Experimental and numerical investigations of phosphorus release under carbonate and variable flow in soil with Mg-Al lay-ered double hydroxides. Chem. Eng. J. 2021, 406, 1385–8947. [Google Scholar] [CrossRef]
- Lasch, P.; Noda, I. Two-Dimensional Correlation Spectroscopy (2D-COS) for Analysis of Spatially Resolved Vibrational Spectra. Appl. Spectrosc. 2019, 73, 359–379. [Google Scholar] [CrossRef]
- Dilxat, D.; Liang, T.; Wang, Y.; Habibul, N. Insights into the interaction mechanism of ofloxacin and functionalized nano-polystyrene. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 284, 121792. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.L.; Guo, X.R.; Li, R.; Hu, H.; Yuan, J.W.; Liu, B.M.; Hei, S.Q.; Zhang, Y.J. Activation of peroxymonosulfate via a novel UV/hydrated Fe(III) oxide coupling strategy for norfloxacin removal: Performance and mechanism. Sep. Purif. Technol. 2022, 300, 121909. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered biochar reclaiming phosphate from aqueous solutions: Mechanisms and potential application as a slow-release fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Wang, B.; Zhang, X.Y.; Xu, H.J.; Cheng, N.; Feng, Q.W.; Zhao, R.H.; Gao, Y.N.; Wei, M. Release characteristics of phosphate from ball-milled biochar and its potential effects on plant growth. Sci. Total Environ. 2022, 821, 153256. [Google Scholar] [CrossRef]
- Li, Z.W.; Niu, R.Y.; Yu, J.H.; Yu, L.Y.; Cao, D. Removal of cadmium from aqueous solution by magnetic biochar: Adsorption characteristics and mechanism. Environ. Sci. Pollut. Res. 2023, 31, 6543–6557. [Google Scholar] [CrossRef]
- Li, H.Y.; Kong, J.; Zhang, H.T.; Gao, J.L.; Fang, Y.; Shi, J.Q.; Ge, T.; Fang, T.; Shi, Y.H.; Zhang, R.; et al. Mechanisms and adsorption capacities of ball milled biomass fly ash/ biochar composites for the adsorption of methylene blue dye from aqueous solution. J. Water Process. Eng. 2023, 53, 103713. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Akdemir Evrendilek, G.; Zerrouq, F.; Lairini, S. Recent advances in adsorption kinetic models: Their application to dye types. Arab. J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Lütke, S.F.; Igansi, A.V.; Pegoraro, L.; Dotto, G.L.; Pinto, L.A.A.; Cadaval, T.R.S. Preparation of activated carbon from black wattle bark waste and its application for phenol adsorption. J. Environ. Chem. Eng. 2019, 7, 103396. [Google Scholar] [CrossRef]
- Adedeji, O.M.; Jahan, K. Removal of pollutants from aqueous product of Co-hydrothermal liquefaction: Adsorption and isotherm studies. Chemosphere 2023, 321, 138165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, L.B.; Chen, J.B.; Yin, W.J.; Zhang, Y.L.; Zhou, X.F.; Gao, F.; Zhao, J. Adsorption of Congo red and methylene blue onto nanopore-structured ashitaba waste and walnut shell-based activated carbons: Statistical thermodynamic investigations, pore size and site energy distribution studies. Nanomaterials 2022, 12, 3831. [Google Scholar] [CrossRef] [PubMed]
- Amrhar, O.; El Gana, L.; Mobarak, M. Calculation of adsorption isotherms by statistical physics models: A review. Environ. Chem. Lett. 2021, 19, 4519–4547. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.B.; Chen, J.B.; Zhou, X.F. Adsorption of SO2 and NH3 onto copper / graphene nanosheets composites: Statistical physics interpretations, thermodynamic investigations, and site energy distribution analyses. Chem. Eng. J. 2022, 446, 137224. [Google Scholar] [CrossRef]
- Zamojc, K.; Wiczk, W.; Zaborowski, B.; Makowski, M.; Pranczk, J.; Jacewicz, D.; Chmurzynski, L. Fluorescence quenching of fluoroquinolone antibiotics by 4-hydroxy-TEMPO in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 887–891. [Google Scholar] [CrossRef] [PubMed]
- An, W.B.; Wang, Q.Q.; Chen, H.; Di, J.Z.; Hu, X.C. Recovery of ammonia nitrogen and phosphate from livestock farm wastewater by iron-magnesium oxide coupled lignite and its potential for resource utilization. Environ. Sci. Pollut. R. 2024, 31, 8930–8951. [Google Scholar] [CrossRef] [PubMed]


| Initial Concentration of Phosphate (mg/L) | Model | ||||||||
| Pseudo-First-Order (PFO) | Pseudo-Second-Order (PSO) | Elovich | |||||||
| K1 | qe | R2 | K2 | qe | R2 | α | β | R2 | |
| 30 | 0.00376 | 55.1172 | 0.9286 | 4.90 × 10−5 | 67.2843 | 0.9347 | 0.2447 | 0.0549 | 0.9325 |
| 60 | 0.00250 | 118.7606 | 0.9910 | 1.97 × 10−5 | 141.6509 | 0.9913 | 0.6093 | 0.0292 | 0.9842 |
| 100 | 0.00472 | 186.7815 | 0.9870 | 2.97 × 10−5 | 212.5615 | 0.9948 | 3.1541 | 0.0239 | 0.9509 |
| Initial Concentration of Phosphate (mg/L) | Intra-Particle Diffusion (IPD) | ||||||||
| K31 | C1 | R12 | K32 | C2 | R22 | K33 | C3 | R32 | |
| 30 | 1.6632 | −0.0325 | 0.9831 | 1.1689 | 18.2824 | 0.9911 | 0.0459 | 54.8854 | 0.9483 |
| 60 | 5.1336 | −5.1105 | 0.9902 | 3.9494 | 13.8898 | 0.9929 | 0.0903 | 113.1805 | 0.9974 |
| 100 | 9.8846 | −0.0239 | 0.9792 | 4.5793 | 81.1255 | 0.9299 | 0.0920 | 188.1738 | 0.9916 |
| Temperature (K) | Model | ||||||||
| Langmuir | Freundlich | Temkin | |||||||
| qmax | KL | R2 | KF | 1/n | R2 | A | B | R2 | |
| 283.15 | 142.8071 | 0.1316 | 0.9940 | 41.7358 | −0.2572 | 0.9358 | 0.0812 | 25.4969 | 0.9688 |
| 293.15 | 167.8054 | 0.1678 | 0.9983 | 53.9760 | −0.2390 | 0.9457 | 0.1124 | 28.1210 | 0.9749 |
| 303.15 | 176.5077 | 0.1784 | 0.9948 | 49.8908 | −0.2748 | 0.9583 | 0.0858 | 31.1722 | 0.9928 |
| 313.15 | 211.6025 | 0.2503 | 0.9959 | 85.9176 | −0.1926 | 0.9157 | 0.2716 | 31.0343 | 0.9544 |
| Pollutants | ΔH (kJ/mol) | ΔS (J/mol·K) | ΔG (kJ/mol) | lnK | ||||||
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 283.15 K | 293.15 K | 303.15 K | 313.15 K | |||
| Phosphate | 23.546 | 98.493 | −2.714 | −3.378 | −4.169 | −4.270 | 1.875 | 2.125 | 2.530 | 2.806 |
| Wave Number (cm−1) | Functional Group | Wave Number (cm−1) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synchronous Spectral Cross-Peak Symbols | Asynchronous Spectral Cross-Peak Symbols | Multiplication Results of Synchronous and Asynchronous Spectra | ||||||||||||||
| 1525 | 1437 | 1150 | 1056 | 564 | 1525 | 1437 | 1150 | 1056 | 564 | 1525 | 1437 | 1150 | 1056 | 564 | ||
| 1525 | -OH | + | - | - | - | + | - | + | + | + | + | - | - | |||
| 1437 | O=C-O | - | - | - | - | + | + | + | - | - | ||||||
| 1150 | C-O | - | - | + | + | - | - | |||||||||
| 1056 | P-O | + | + | + | ||||||||||||
| 564 | M-O | |||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, W.; Wang, Q.; Chen, H.; Liu, Y.; Hu, X.; Di, J. Efficient Recovery of Phosphate from Water Media by Iron-Magnesium Functionalized Lignite: Adsorption Evaluation, Mechanism Revelation and Potential Application Exploration. Molecules 2024, 29, 1252. https://doi.org/10.3390/molecules29061252
An W, Wang Q, Chen H, Liu Y, Hu X, Di J. Efficient Recovery of Phosphate from Water Media by Iron-Magnesium Functionalized Lignite: Adsorption Evaluation, Mechanism Revelation and Potential Application Exploration. Molecules. 2024; 29(6):1252. https://doi.org/10.3390/molecules29061252
Chicago/Turabian StyleAn, Wenbo, Qiqi Wang, He Chen, Yifan Liu, Xuechun Hu, and Junzhen Di. 2024. "Efficient Recovery of Phosphate from Water Media by Iron-Magnesium Functionalized Lignite: Adsorption Evaluation, Mechanism Revelation and Potential Application Exploration" Molecules 29, no. 6: 1252. https://doi.org/10.3390/molecules29061252
APA StyleAn, W., Wang, Q., Chen, H., Liu, Y., Hu, X., & Di, J. (2024). Efficient Recovery of Phosphate from Water Media by Iron-Magnesium Functionalized Lignite: Adsorption Evaluation, Mechanism Revelation and Potential Application Exploration. Molecules, 29(6), 1252. https://doi.org/10.3390/molecules29061252





