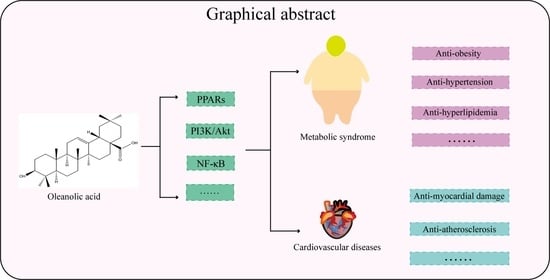
The Effect and Mechanism of Oleanolic Acid in the Treatment of Metabolic Syndrome and Related Cardiovascular Diseases
Abstract
:1. Introduction
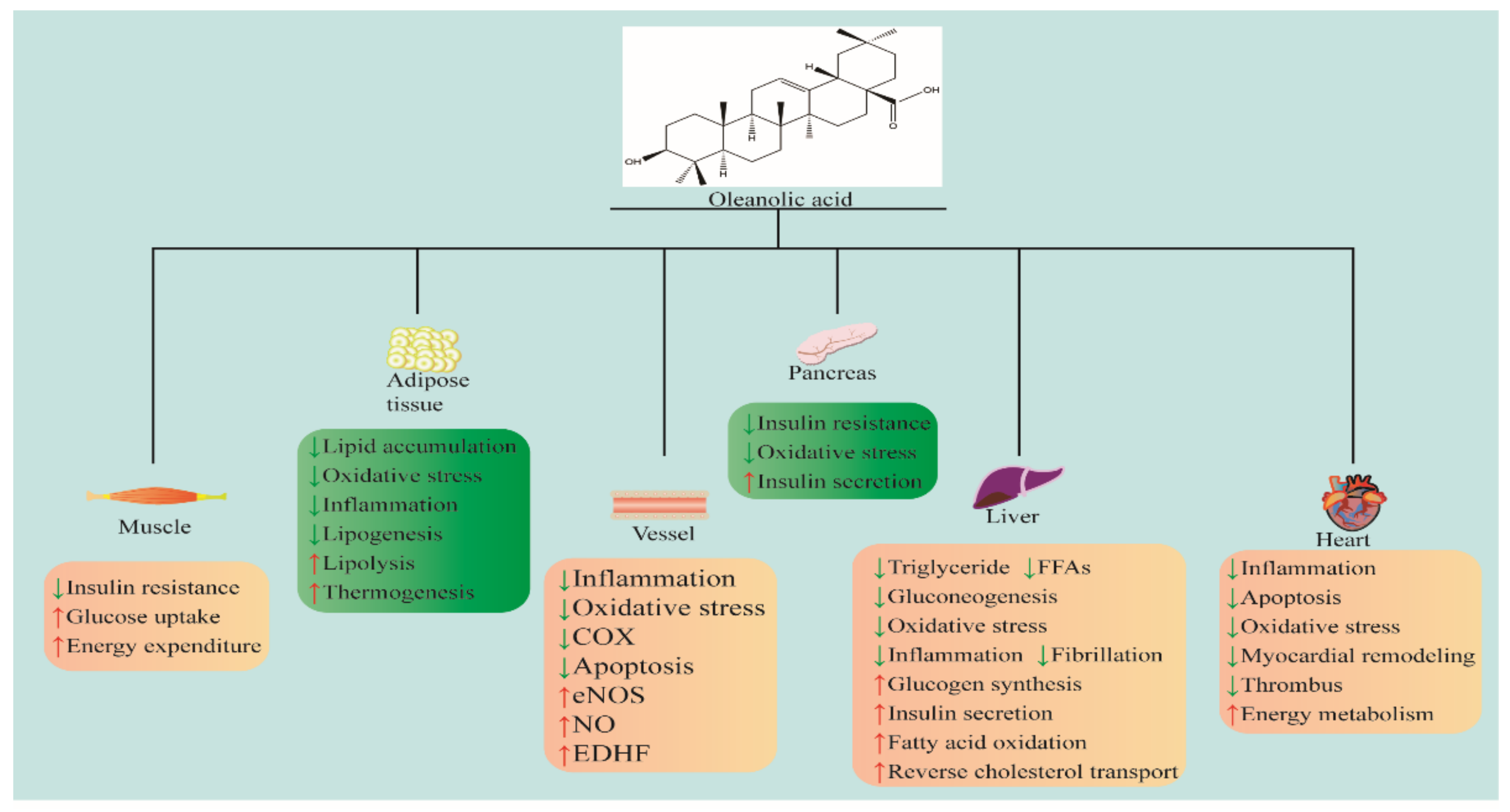
2. Anti-Metabolic Syndromes’ Effects
2.1. Anti-Obesity
2.2. Anti-Hyperlipidemia
2.3. Anti-Hypertension
2.4. Anti-Nonalcoholic Fatty Liver
2.5. Anti-Diabetes Mellitus
3. Anti-Cardiovascular Diseases Effects
3.1. Anti-Stroke
3.2. Heart Protection
3.3. Anti-Atherosclerosis
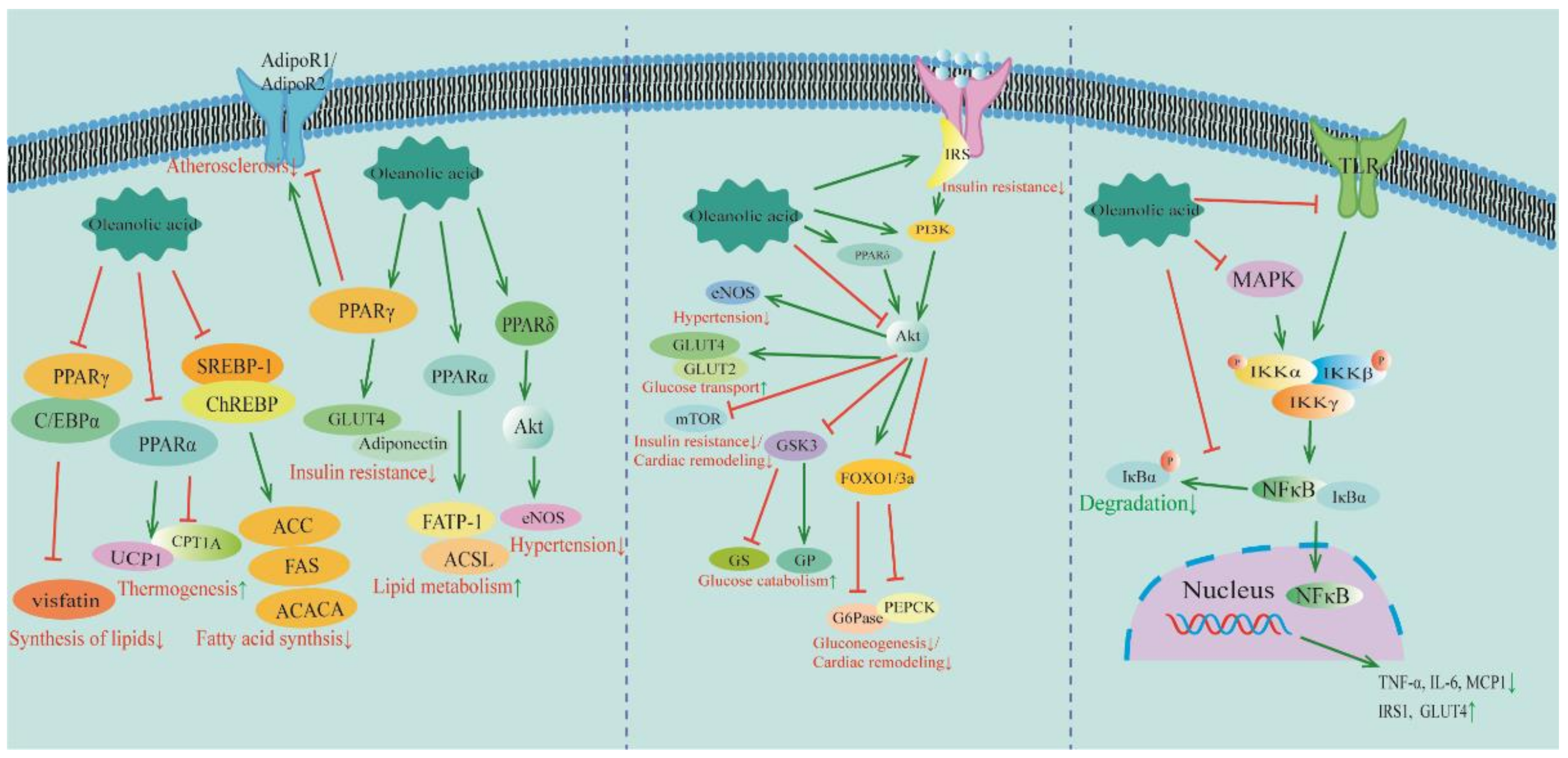
4. Signaling Pathways of OA for the Treatment of MetS and CVDs
4.1. PPAR Signaling Pathway
4.2. PI3K/Akt Signaling Pathway
4.3. NF-κB Signaling Pathway
5. Metabolism, Bioavailability, and Clinical Potential of OA
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef] [PubMed]
- Fishel Bartal, M.; Blackwell, S.C.; Pedroza, C.; Lawal, D.; Amro, F.; Samuel, J.; Chauhan, S.P.; Sibai, B.M. Oral combined hydrochlorothiazide and lisinopril vs nifedipine for postpartum hypertension: A comparative-effectiveness pilot randomized controlled trial. Am. J. Obstet. Gynecol. 2023, 228, 571.e1–571.e10. [Google Scholar] [CrossRef] [PubMed]
- Davidsen, L.; Jensen, M.H.; Cook, M.E.; Vestergaard, P.; Knop, F.K.; Drewes, A.M.; Olesen, S.S. Metformin treatment is associated with reduced risk of hypoglycaemia, major adverse cardiovascular events, and all-cause mortality in patients with post-pancreatitis diabetes mellitus: A nationwide cohort study. Eur. J. Endocrinol. 2024, 190, 44–53. [Google Scholar] [CrossRef]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef] [PubMed]
- Elizalde-Velázquez, G.A.; Herrera-Vázquez, S.E.; Gómez-Oliván, L.M.; García-Medina, S. Health impact assessment after Danio rerio long-term exposure to environmentally relevant concentrations of metformin and guanylurea. Chemosphere 2023, 341, 140070. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Yoo, S.R.; Jeong, S.J.; Lee, N.R.; Shin, H.K.; Seo, C.S. Quantification Analysis and In Vitro Anti-Inflammatory Effects of 20-Hydroxyecdysone, Momordin Ic, and Oleanolic Acid from the Fructus of Kochia scoparia. Pharmacogn. Mag. 2017, 13, 339–344. [Google Scholar] [CrossRef]
- De Stefani, C.; Vasarri, M.; Salvatici, M.C.; Grifoni, L.; Quintela, J.C.; Bilia, A.R.; Degl’Innocenti, D.; Bergonzi, M.C. Microemulsions Enhance the In Vitro Antioxidant Activity of Oleanolic Acid in RAW 264.7 Cells. Pharmaceutics 2022, 14, 2232. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol. Cell Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Q.; Yu, Y.; Zhang, Y.; Zhang, M.; Liu, Q.; Zhang, E.; Li, S.; Song, G. Oleanolic Acid, a Novel Endothelin A Receptor Antagonist, Alleviated High Glucose-Induced Cardiomyocytes Injury. Am. J. Chin. Med. 2018, 46, 1187–1201. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, F.; Song, Z.; Huang, H.; Chen, Y.; Shen, Y.; Jia, Y.; Chen, J. Oleanolic acid protects against pathogenesis of atherosclerosis, possibly via FXR-mediated angiotensin (Ang)-(1-7) upregulation. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 97, 1694–1700. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- White, U.A.; Stephens, J.M. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell Endocrinol. 2010, 318, 10–14. [Google Scholar] [CrossRef]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.Y.; Kang, S.W.; Kim, J.L.; Li, J.; Lee, E.S.; Gong, J.H.; Han, S.J.; Kang, Y.H. Oleanolic acid reduces markers of differentiation in 3T3-L1 adipocytes. Nutr. Res. 2010, 30, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Djeziri, F.Z.; Belarbi, M.; Murtaza, B.; Hichami, A.; Benammar, C.; Khan, N.A. Oleanolic acid improves diet-induced obesity by modulating fat preference and inflammation in mice. Biochimie 2018, 152, 110–120. [Google Scholar] [CrossRef]
- Acin, S.; Munoz, D.L.; Guillen, A.; Soscue, D.; Castano, A.; Echeverri, F.; Balcazar, N. Triterpene-enriched fractions from Eucalyptus tereticornis ameliorate metabolic alterations in a mouse model of diet-induced obesity. J. Ethnopharmacol. 2021, 265, 113298. [Google Scholar] [CrossRef] [PubMed]
- De Melo, C.L.; Queiroz, M.G.; Fonseca, S.G.; Bizerra, A.M.; Lemos, T.L.; Melo, T.S.; Santos, F.A.; Rao, V.S. Oleanolic acid, a natural triterpenoid improves blood glucose tolerance in normal mice and ameliorates visceral obesity in mice fed a high-fat diet. Chem. Biol. Interact. 2010, 185, 59–65. [Google Scholar] [CrossRef]
- Wan, Q.; Lu, H.; Liu, X.; Yie, S.; Xiang, J.; Yao, Z. Study of oleanolic acid on the estrodiol production and the fat production of mouse preadipocyte 3T3-L1 in vitro. Hum. Cell 2015, 28, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Horng, T. Lipid Metabolism in Regulation of Macrophage Functions. Trends Cell Biol. 2020, 30, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Shen, P.; Huang, Y.; Han, L.; Ba, X.; Huang, Y.; Yan, J.; Li, T.; Xu, L.; Qin, K.; et al. Wutou decoction attenuates the synovial inflammation of collagen-induced arthritis rats via regulating macrophage M1/M2 type polarization. J. Ethnopharmacol. 2023, 301, 115802. [Google Scholar] [CrossRef] [PubMed]
- Chylikova, J.; Dvorackova, J.; Tauber, Z.; Kamarad, V. M1/M2 macrophage polarization in human obese adipose tissue. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czechoslov. 2018, 162, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zeng, H.; Xu, M.; Huang, C.; Tao, L.; Li, J.; Zhang, T.; Chen, H.; Xia, J.; Li, C.; et al. Oleanolic Acid Improves Obesity-Related Inflammation and Insulin Resistance by Regulating Macrophages Activation. Front. Pharmacol. 2021, 12, 697483. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A. Resistin- and Obesity-associated metabolic diseases. Horm. Metab. Res. 2007, 39, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Sung, H.Y.; Kim, M.S.; Kim, J.L.; Kang, M.K.; Gong, J.H.; Park, H.S.; Kang, Y.H. Oleanolic acid suppresses resistin induction in adipocytes by modulating Tyk-STAT signaling. Nutr. Res. 2013, 33, 144–153. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; Yu, W.; Liu, J.; Peng, J.; Liao, N.; Zhang, J.; Zhang, X.; Hai, C. Hepatocyte nuclear factor 1b is a novel negative regulator of white adipocyte differentiation. Cell Death Differ. 2017, 24, 1588–1597. [Google Scholar] [CrossRef]
- Su, S.; Wu, G.; Cheng, X.; Fan, J.; Peng, J.; Su, H.; Xu, Z.; Cao, M.; Long, Z.; Hao, Y.; et al. Oleanolic acid attenuates PCBs-induced adiposity and insulin resistance via HNF1b-mediated regulation of redox and PPARgamma signaling. Free Radic. Biol. Med. 2018, 124, 122–134. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef]
- Yunoki, K.; Sasaki, G.; Tokuji, Y.; Kinoshita, M.; Naito, A.; Aida, K.; Ohnishi, M. Effect of dietary wine pomace extract and oleanolic acid on plasma lipids in rats fed high-fat diet and its DNA microarray analysis. J. Agric. Food Chem. 2008, 56, 12052–12058. [Google Scholar] [CrossRef]
- Lin, Y.; Vermeer, M.A.; Trautwein, E.A. Triterpenic Acids Present in Hawthorn Lower Plasma Cholesterol by Inhibiting Intestinal ACAT Activity in Hamsters. Evid. Based Complement. Altern. Med. Ecam 2011, 2011, 801272. [Google Scholar] [CrossRef]
- Lin, J.; Yang, R.; Tarr, P.T.; Wu, P.H.; Handschin, C.; Li, S.; Yang, W.; Pei, L.; Uldry, M.; Tontonoz, P.; et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell 2005, 120, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wen, X.; Zhang, W.; Wang, C.; Liu, J.; Liu, C. Hypolipidemic effect of oleanolic acid is mediated by the miR-98-5p/PGC-1beta axis in high-fat diet-induced hyperlipidemic mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 1085–1096. [Google Scholar] [CrossRef]
- Luo, H.Q.; Shen, J.; Chen, C.P.; Ma, X.; Lin, C.; Ouyang, Q.; Xuan, C.X.; Liu, J.; Sun, H.B.; Liu, J. Lipid-lowering effects of oleanolic acid in hyperlipidemic patients. Chin. J. Nat. Med. 2018, 16, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, D.; Hu, Y.; Zhang, L.; Li, Y.; Zhang, Z.; Li, C. Persistence of severe global inequalities in the burden of Hypertension Heart Disease from 1990 to 2019: Findings from the global burden of disease study 2019. BMC Public Health 2024, 24, 110. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R.; Perona, J.S.; Herrera, M.D.; Ruiz-Gutierrez, V. Triterpenic compounds from “orujo” olive oil elicit vasorelaxation in aorta from spontaneously hypertensive rats. J. Agric. Food Chem. 2006, 54, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R.; Herrera, M.D.; Perona, J.S.; Ruiz-Gutierrez, V. Potential vasorelaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in ‘orujo’ olive oil, on rat aorta. Br. J. Nutr. 2004, 92, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Madlala, H.P.; Metzinger, T.; van Heerden, F.R.; Musabayane, C.T.; Mubagwa, K.; Dessy, C. Vascular Endothelium-Dependent and Independent Actions of Oleanolic Acid and Its Synthetic Oleanane Derivatives as Possible Mechanisms for Hypotensive Effects. PLoS ONE 2016, 11, e0147395. [Google Scholar] [CrossRef] [PubMed]
- Bachhav, S.S.; Patil, S.D.; Bhutada, M.S.; Surana, S.J. Oleanolic acid prevents glucocorticoid-induced hypertension in rats. Phytother. Res. PTR 2011, 25, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R.; Herrera, M.D.; de Sotomayor, M.A.; Ruiz-Gutierrez, V. Pomace olive oil improves endothelial function in spontaneously hypertensive rats by increasing endothelial nitric oxide synthase expression. Am. J. Hypertens. 2007, 20, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R.; Herrera, M.D.; de Sotomayor, M.A.; Ruiz-Gutierrez, V. Effects of pomace olive oil-enriched diets on endothelial function of small mesenteric arteries from spontaneously hypertensive rats. Br. J. Nutr. 2009, 102, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Acelajado, M.C.; Hughes, Z.H.; Oparil, S.; Calhoun, D.A. Treatment of Resistant and Refractory Hypertension. Circ. Res. 2019, 124, 1061–1070. [Google Scholar] [CrossRef]
- Ahn, Y.M.; Choi, Y.H.; Yoon, J.J.; Lee, Y.J.; Cho, K.W.; Kang, D.G.; Lee, H.S. Oleanolic acid modulates the renin-angiotensin system and cardiac natriuretic hormone concomitantly with volume and pressure balance in rats. Eur. J. Pharmacol. 2017, 809, 231–241. [Google Scholar] [CrossRef]
- Kim, H.Y.; Cho, K.W.; Kang, D.G.; Lee, H.S. Oleanolic acid increases plasma ANP levels via an accentuation of cardiac ANP synthesis and secretion in rats. Eur. J. Pharmacol. 2013, 710, 73–79. [Google Scholar] [CrossRef]
- Bachhav, S.S.; Bhutada, M.S.; Patil, S.P.; Sharma, K.S.; Patil, S.D. Oleanolic Acid Prevents Increase in Blood Pressure and Nephrotoxicity in Nitric Oxide Dependent Type of Hypertension in Rats. Pharmacogn. Res. 2014, 7, 385–392. [Google Scholar] [CrossRef]
- Somova, L.O.; Nadar, A.; Rammanan, P.; Shode, F.O. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine Int. J. Phytother. Phytopharm. 2003, 10, 115–121. [Google Scholar] [CrossRef]
- Bacon, S.L.; Sherwood, A.; Hinderliter, A.; Blumenthal, J.A. Effects of exercise, diet and weight loss on high blood pressure. Sport. Med. 2004, 34, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Alwardat, N.; Di Renzo, L.; de Miranda, R.C.; Alwardat, S.; Sinibaldi Salimei, P.; De Lorenzo, A. Association between hypertension and metabolic disorders among elderly patients in North Jordan. Diabetes Metab. Syndr. 2018, 12, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Wang, X.; Tian, Z.; Qi, D.; Li, Y.; Jiang, H. Antihypertensive activity of oleanolic acid is mediated via downregulation of secretory phospholipase A2 and fatty acid synthase in spontaneously hypertensive rats. Int. J. Mol. Med. 2020, 46, 2019–2034. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar] [PubMed]
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Nyakudya, T.T.; Mukwevho, E.; Nkomozepi, P.; Erlwanger, K.H. Neonatal intake of oleanolic acid attenuates the subsequent development of high fructose diet-induced non-alcoholic fatty liver disease in rats. J. Dev. Orig. Health Dis. 2018, 9, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Li, Y.; Lv, H.; Zhang, L.; Bi, C.; Dong, N.; Shan, A.; Wang, J. Oleanolic Acid Targets the Gut-Liver Axis to Alleviate Metabolic Disorders and Hepatic Steatosis. J. Agric. Food Chem. 2021, 69, 7884–7897. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. Plant-derived oleanolic acid ameliorates markers associated with non-alcoholic fatty liver disease in a diet-induced pre-diabetes rat model. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1953–1962. [Google Scholar] [CrossRef]
- Repa, J.J.; Liang, G.; Ou, J.; Bashmakov, Y.; Lobaccaro, J.M.; Shimomura, I.; Shan, B.; Brown, M.S.; Goldstein, J.L.; Mangelsdorf, D.J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000, 14, 2819–2830. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Chen, Y.; Huang, Z.; Li, M.; Jiang, W.; Chen, J.; Rao, W.; Luo, S.; Chen, Y.; et al. Dingxin Recipe IV attenuates atherosclerosis by regulating lipid metabolism through LXR-α/SREBP1 pathway and modulating the gut microbiota in ApoE (−/−) mice fed with HFD. J. Ethnopharmacol. 2021, 266, 113436. [Google Scholar] [CrossRef]
- Lin, Y.N.; Chang, H.Y.; Wang, C.C.N.; Chu, F.Y.; Shen, H.Y.; Chen, C.J.; Lim, Y.P. Oleanolic Acid Inhibits Liver X Receptor Alpha and Pregnane X Receptor to Attenuate Ligand-Induced Lipogenesis. J. Agric. Food Chem. 2018, 66, 10964–10976. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Zuo, G.; Xu, W.; Gao, H.; Yang, Y.; Yamahara, J.; Wang, J.; Li, Y. Oleanolic Acid diminishes liquid fructose-induced Fatty liver in rats: Role of modulation of hepatic sterol regulatory element-binding protein-1c-mediated expression of genes responsible for de novo Fatty Acid synthesis. Evid. Based Complement. Altern. Med. Ecam. 2013, 2013, 534084. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.C.; Philipson, L.H. Update on diabetes classification. Med. Clin. North Am. 2015, 99, 1–16. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef]
- Glass, C.K.; Olefsky, J.M. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef]
- Iskender, H.; Dokumacioglu, E.; Terim Kapakin, K.A.; Yenice, G.; Mohtare, B.; Bolat, I.; Hayirli, A. Effects of oleanolic acid on inflammation and metabolism in diabetic rats. Biotech. Histochem. Off. Publ. Biol. Stain Comm. 2022, 97, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Matumba, M.; Ayeleso, A.; Nyakudya, T.; Erlwanger, K.; Chegou, N.; Mukwevho, E. Long-Term Impact of Neonatal Intake of Oleanolic Acid on the Expression of AMP-Activated Protein Kinase, Adiponectin and Inflammatory Cytokines in Rats Fed with a High Fructose Diet. Nutrients 2019, 11, 226. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Xing, H.; Kang, L.; Chen, X.; Liu, B.; Niu, K. Renoprotective effects of oleanolic acid and its possible mechanisms in rats with diabetic kidney disease. Biochem. Biophys. Res. Commun. 2022, 636, 1–9. [Google Scholar] [CrossRef]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. Plant-derived oleanolic acid ameliorates markers of subclinical inflammation and innate immunity activation in diet-induced pre-diabetic rats. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820935771. [Google Scholar] [CrossRef]
- Li, M.; Han, Z.; Bei, W.; Rong, X.; Guo, J.; Hu, X. Oleanolic Acid Attenuates Insulin Resistance via NF-kappaB to Regulate the IRS1-GLUT4 Pathway in HepG2 Cells. Evid. Based Complement. Altern. Med. Ecam 2015, 2015, 643102. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Wang, S.; Du, L.B.; Jin, L.; Wang, Z.; Peng, J.; Liao, N.; Zhao, Y.Y.; Zhang, J.L.; Pauluhn, J.; Hai, C.X.; et al. Nano-oleanolic acid alleviates metabolic dysfunctions in rats with high fat and fructose diet. Biomed. Pharmacother. 2018, 108, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, S.; Gu, J.; Min, Z.; Wang, R. Effect of Nano-Oleanolic Acid Combined With Lipid-Lowering Ketones on Insulin Resistance in Rats with Gestational Diabetes. J. Biomed. Nanotechnol. 2022, 18, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.L.; Wu, H.; Liu, J.Z.; Hu, J.X.; Liao, N.; Peng, J.; Cao, P.P.; Liang, X.; Hai, C.X. Antidiabetic effect of oleanolic acid: A promising use of a traditional pharmacological agent. Phytother. Res. PTR 2011, 25, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, Q.; Li, Y.; Liu, Z.; Fan, Y.; Liu, Z.; Zhao, H.; Li, J.; Han, Z. Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan-induced diabetic rats. Phytother. Res. PTR 2009, 23, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Dubey, V.K.; Patil, C.R.; Kamble, S.M.; Tidke, P.S.; Patil, K.R.; Maniya, P.J.; Jadhav, R.B.; Patil, S.P. Oleanolic acid prevents progression of streptozotocin induced diabetic nephropathy and protects renal microstructures in Sprague Dawley rats. J. Pharmacol. Pharmacother. 2013, 4, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Nosadini, R.; Tonolo, G. Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. NMCD 2011, 21, 79–85. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Song, C.; Yuan, S.; Bian, X.; Lin, Z.; Yang, M.; Dou, K. Triglyceride-glucose index as a suitable non-insulin-based insulin resistance marker to predict cardiovascular events in patients undergoing complex coronary artery intervention: A large-scale cohort study. Cardiovasc. Diabetol. 2024, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Victoria Awolola, G.; Ibrahim, M.A.; Anthony Koorbanally, N.; Islam, M.S. Oleanolic acid as a potential antidiabetic component of Xylopia aethiopica (Dunal) A. Rich. (Annonaceae) fruit: Bioassay guided isolation and molecular docking studies. Nat. Prod. Res. 2021, 35, 788–791. [Google Scholar] [CrossRef]
- Teodoro, T.; Zhang, L.; Alexander, T.; Yue, J.; Vranic, M.; Volchuk, A. Oleanolic acid enhances insulin secretion in pancreatic beta-cells. FEBS Lett. 2008, 582, 1375–1380. [Google Scholar] [CrossRef]
- Gao, D.; Li, Q.; Li, Y.; Liu, Z.; Liu, Z.; Fan, Y.; Han, Z.; Li, J.; Li, K. Antidiabetic potential of oleanolic acid from Ligustrum lucidum Ait. Can. J. Physiol. Pharmacol. 2007, 85, 1076–1083. [Google Scholar] [CrossRef]
- Musabayane, C.T.; Tufts, M.A.; Mapanga, R.F. Synergistic antihyperglycemic effects between plant-derived oleanolic acid and insulin in streptozotocin-induced diabetic rats. Ren. Fail. 2010, 32, 832–839. [Google Scholar] [CrossRef]
- Khathi, A.; Masola, B.; Musabayane, C.T. Effects of Syzygium aromaticum-derived oleanolic acid on glucose transport and glycogen synthesis in the rat small intestine. J. Diabetes 2013, 5, 80–87. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. The Effects of Plant-Derived Oleanolic Acid on Selected Parameters of Glucose Homeostasis in a Diet-Induced Pre-Diabetic Rat Model. Molecules 2018, 23, 794. [Google Scholar] [CrossRef] [PubMed]
- Molepo, M.; Ayeleso, A.; Nyakudya, T.; Erlwanger, K.; Mukwevho, E. A Study on Neonatal Intake of Oleanolic Acid and Metformin in Rats (Rattus norvegicus) with Metabolic Dysfunction: Implications on Lipid Metabolism and Glucose Transport. Molecules 2018, 23, 2528. [Google Scholar] [CrossRef] [PubMed]
- Mukundwa, A.; Langa, S.O.; Mukaratirwa, S.; Masola, B. In vivo effects of diabetes, insulin and oleanolic acid on enzymes of glycogen metabolism in the skin of streptozotocin-induced diabetic male Sprague-Dawley rats. Biochem. Biophys. Res. Commun. 2016, 471, 315–319. [Google Scholar] [CrossRef]
- Loza-Rodriguez, H.; Estrada-Soto, S.; Alarcon-Aguilar, F.J.; Huang, F.; Aquino-Jarquin, G.; Fortis-Barrera, A.; Giacoman-Martinez, A.; Almanza-Perez, J.C. Oleanolic acid induces a dual agonist action on PPARgamma/alpha and GLUT4 translocation: A pentacyclic triterpene for dyslipidemia and type 2 diabetes. Eur. J. Pharmacol. 2020, 883, 173252. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Sato, H.; Genet, C.; Strehle, A.; Thomas, C.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J.; Saladin, R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007, 362, 793–798. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.Y.; Wang, Y.P.; Cantley, J.; Iseli, T.J.; Molero, J.C.; Hegarty, B.D.; Kraegen, E.W.; Ye, Y.; Ye, J.M. Oleanolic acid reduces hyperglycemia beyond treatment period with Akt/FoxO1-induced suppression of hepatic gluconeogenesis in type-2 diabetic mice. PLoS ONE 2012, 7, e42115. [Google Scholar] [CrossRef]
- Mukundwa, A.; Mukaratirwa, S.; Masola, B. Effects of oleanolic acid on the insulin signaling pathway in skeletal muscle of streptozotocin-induced diabetic male Sprague-Dawley rats. J. Diabetes 2016, 8, 98–108. [Google Scholar] [CrossRef]
- Ngubane, P.S.; Masola, B.; Musabayane, C.T. The effects of Syzygium aromaticum-derived oleanolic acid on glycogenic enzymes in streptozotocin-induced diabetic rats. Ren. Fail. 2011, 33, 434–439. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Abdelkader, D.; Hassan, W.; Sun, H.; Liu, J. Combination therapy with oleanolic acid and metformin as a synergistic treatment for diabetes. J. Diabetes Res. 2015, 2015, 973287. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Gu, T.; Yamahara, J.; Li, Y. Oleanolic acid supplement attenuates liquid fructose-induced adipose tissue insulin resistance through the insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt signaling pathway in rats. Toxicol. Appl. Pharmacol. 2014, 277, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Park, S.; Mariadoss, A.V.A.; Sathiyaseelan, A.; Veeraraghavan, V.P.; Kim, S.; Wang, M.H. Chemical composition, antioxidant, and anti-diabetic activities of ethyl acetate fraction of Stachys riederi var. japonica (Miq.) in streptozotocin-induced type 2 diabetic mice. Food Chem. Toxicol. 2021, 155, 112374. [Google Scholar] [CrossRef]
- Nyakudya, T.T.; Mukwevho, E.; Erlwanger, K.H. The protective effect of neonatal oral administration of oleanolic acid against the subsequent development of fructose-induced metabolic dysfunction in male and female rats. Nutr. Metab. 2018, 15, 82. [Google Scholar] [CrossRef]
- Mohandes, S.; Doke, T.; Hu, H.; Mukhi, D.; Dhillon, P.; Susztak, K. Molecular pathways that drive diabetic kidney disease. J. Clin. Investig. 2023, 133, jci165654. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, M.; Xie, X.; Yang, H.; Wang, X.; Xiao, L.; Wang, N. Oleanolic acid ameliorates high glucose-induced endothelial dysfunction via PPARdelta activation. Sci. Rep. 2017, 7, 40237. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G. Red Blood Cells Deserve Attention in Patients with Type 2 Diabetes. J. Am. Coll. Cardiol. 2018, 72, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Bashiru Shola, O.; Olatunde Olugbenga, F. Hyperglycaemic Environment: Contribution to the Anaemia Associated with Diabetes Mellitus in Rats Experimentally Induced with Alloxan. Anemia 2015, 2015, 848921. [Google Scholar] [CrossRef] [PubMed]
- Baloyi, C.M.; Khathi, A.; Sibiya, N.H.; Ngubane, P.S. The Haematological Effects of Oleanolic Acid in Streptozotocin-Induced Diabetic Rats: Effects on Selected Markers. J. Diabetes Res. 2019, 2019, 6753541. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Mao, X.; Zhang, Z.; Wu, H. Classification and Differential Diagnosis of Diabetic Nephropathy. J. Diabetes Res. 2017, 2017, 8637138. [Google Scholar] [CrossRef] [PubMed]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. Preventing the onset of diabetes-induced chronic kidney disease during prediabetes: The effects of oleanolic acid on selected markers of chronic kidney disease in a diet-induced prediabetic rat model. Biomed. Pharmacother. 2021, 139, 111570. [Google Scholar] [CrossRef]
- Gugliucci, A.; Bendayan, M. Renal fate of circulating advanced glycated end products (AGE): Evidence for reabsorption and catabolism of AGE-peptides by renal proximal tubular cells. Diabetologia 1996, 39, 149–160. [Google Scholar] [CrossRef]
- Wang, Z.H.; Hsu, C.C.; Huang, C.N.; Yin, M.C. Anti-glycative effects of oleanolic acid and ursolic acid in kidney of diabetic mice. Eur. J. Pharmacol. 2010, 628, 255–260. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, H.M.; Kang, J.S.; Lee, E.Y.; Yadav, D.; Kwon, M.H.; Kim, Y.M.; Kim, H.S.; Chung, C.H. Oleanolic acid and N-acetylcysteine ameliorate diabetic nephropathy through reduction of oxidative stress and endoplasmic reticulum stress in a type 2 diabetic rat model. Nephrol. Dial. Transplant. 2016, 31, 391–400. [Google Scholar] [CrossRef]
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef]
- Orellana-Urzúa, S.; Rojas, I.; Líbano, L.; Rodrigo, R. Pathophysiology of Ischemic Stroke: Role of Oxidative Stress. Curr. Pharm. Des. 2020, 26, 4246–4260. [Google Scholar] [CrossRef]
- Escobar-Peso, A.; Martínez-Alonso, E.; Masjuan, J.; Alcázar, A. Development of Pharmacological Strategies with Therapeutic Potential in Ischemic Stroke. Antioxidants 2023, 12, 2102. [Google Scholar] [CrossRef]
- Du, Y.; Ko, K.M. Oleanolic acid protects against myocardial ischemia-reperfusion injury by enhancing mitochondrial antioxidant mechanism mediated by glutathione and alpha-tocopherol in rats. Planta Med. 2006, 72, 222–227. [Google Scholar] [CrossRef]
- Rong, Z.T.; Gong, X.J.; Sun, H.B.; Li, Y.M.; Ji, H. Protective effects of oleanolic acid on cerebral ischemic damage in vivo and H2O2-induced injury in vitro. Pharm. Biol. 2011, 49, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.J.; Sun, L.L.; Ji, X.; Shi, R.; Xu, F.; Gu, J.H. Neuroprotective effects of oleanolic acid against cerebral ischemia-reperfusion injury in mice. Exp. Neurol. 2021, 343, 113785. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Kong, B.; Zheng, J.; Wang, X.; Li, L. Alprostadil Injection Attenuates Coronary Microembolization-Induced Myocardial Injury Through GSK-3β/Nrf2/HO-1 Signaling-Mediated Apoptosis Inhibition. Drug Des. Dev. Ther. 2020, 14, 4407–4422. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhang, Z.; Zhang, Z.; Zhu, P.; Jiang, X.; Wang, Y.; Deng, Q.; Lam Yung, K.K.; Zhang, S. Oleanolic Acid Alleviates Cerebral Ischemia/Reperfusion Injury via Regulation of the GSK-3beta/HO-1 Signaling Pathway. Pharmaceuticals 2021, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.W.; Wong, S.S.; Hong, Y.; Kanaya, A.M.; Khan, S.S.; Hayman, L.L.; Shah, S.H.; Welty, F.K.; Deedwania, P.C.; Khaliq, A.; et al. Epidemiology of Diabetes and Atherosclerotic Cardiovascular Disease Among Asian American Adults: Implications, Management, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2023, 148, 74–94. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Senthil, S.; Sridevi, M.; Pugalendi, K.V. Cardioprotective effect of oleanolic acid on isoproterenol-induced myocardial ischemia in rats. Toxicol. Pathol. 2007, 35, 418–423. [Google Scholar] [CrossRef]
- Li, W.F.; Wang, P.; Li, H.; Li, T.Y.; Feng, M.; Chen, S.F. Oleanolic acid protects against diabetic cardiomyopathy via modulation of the nuclear factor erythroid 2 and insulin signaling pathways. Exp. Ther. Med. 2017, 14, 848–854. [Google Scholar] [CrossRef]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. Plant-Derived Oleanolic Acid (OA) Ameliorates Risk Factors of Cardiovascular Diseases in a Diet-Induced Pre-Diabetic Rat Model: Effects on Selected Cardiovascular Risk Factors. Molecules 2019, 24, 340. [Google Scholar] [CrossRef]
- Chan, C.Y.; Mong, M.C.; Liu, W.H.; Huang, C.Y.; Yin, M.C. Three pentacyclic triterpenes protect H9c2 cardiomyoblast cells against high-glucose-induced injury. Free Radic. Res. 2014, 48, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Mapanga, R.F.; Rajamani, U.; Dlamini, N.; Zungu-Edmondson, M.; Kelly-Laubscher, R.; Shafiullah, M.; Wahab, A.; Hasan, M.Y.; Fahim, M.A.; Rondeau, P.; et al. Oleanolic acid: A novel cardioprotective agent that blunts hyperglycemia-induced contractile dysfunction. PLoS ONE 2012, 7, e47322. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Yanagisawa, M. Endothelin: 30 Years From Discovery to Therapy. Hypertension 2019, 74, 1232–1265. [Google Scholar] [CrossRef] [PubMed]
- Swynghedauw, B. Molecular mechanisms of myocardial remodeling. Physiol. Rev. 1999, 79, 215–262. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Yang, Q.; Harada, M.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D.; Li, Y. Pomegranate flower extract diminishes cardiac fibrosis in Zucker diabetic fatty rats: Modulation of cardiac endothelin-1 and nuclear factor-kappaB pathways. J. Cardiovasc. Pharmacol. 2005, 46, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Volpe, M.; Sadoshima, J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ. Res. 2014, 114, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.H.; Zhang, N.; Feng, H.; Zhang, N.; Ma, Z.G.; Yang, Z.; Yuan, Y.; Bian, Z.Y.; Tang, Q.Z. Oleanolic acid alleviated pressure overload-induced cardiac remodeling. Mol. Cell. Biochem. 2015, 409, 145–154. [Google Scholar] [CrossRef]
- Lee, J.J.; Jin, Y.R.; Lim, Y.; Yu, J.Y.; Kim, T.J.; Yoo, H.S.; Shin, H.S.; Yun, Y.P. Oleanolic acid, a pentacyclic triterpenoid, induces rabbit platelet aggregation through a phospholipase C-calcium dependent signaling pathway. Arch. Pharmacal Res. 2007, 30, 210–214. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, G.; Wang, M.; Li, H.; Han, Z. Protective effect of oleanolic acid on oxidized-low density lipoprotein induced endothelial cell apoptosis. Biosci. Trends 2015, 9, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, D.; Han, Y.; Han, Z.; Zhong, W.; Wang, C. Modulation of oxidized-LDL receptor-1 (LOX1) contributes to the antiatherosclerosis effect of oleanolic acid. Int. J. Biochem. Cell Biol. 2015, 69, 142–152. [Google Scholar] [CrossRef]
- Luan, J.; Ji, X.; Liu, L. PPARγ in Atherosclerotic Endothelial Dysfunction: Regulatory Compounds and PTMs. Int. J. Mol. Sci. 2023, 24, 4494. [Google Scholar] [CrossRef]
- Luo, H.; Liu, J.; Ouyang, Q.; Xuan, C.; Wang, L.; Li, T.; Liu, J. The effects of oleanolic acid on atherosclerosis in different animal models. Acta Biochim. Biophys. Sin. 2017, 49, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, L.; Hu, X.; Wang, X.; Xu, F.; Chen, B.; Liang, X.; Xia, J.; Wang, P.; Aibara, D.; et al. Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J. Clin. Investig. 2021, 131, 2865. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhang, Y.H.; Dong, X.F.; Hao, Q.Q.; Zhou, X.M.; Yu, Q.T.; Li, S.Y.; Chen, X.; Tengbeh, A.F.; Dong, B.; et al. ACE2 and Ang-(1-7) protect endothelial cell function and prevent early atherosclerosis by inhibiting inflammatory response. Inflamm. Res. 2015, 64, 253–260. [Google Scholar] [CrossRef]
- Buus, N.H.; Hansson, N.C.; Rodriguez-Rodriguez, R.; Stankevicius, E.; Andersen, M.R.; Simonsen, U. Antiatherogenic effects of oleanolic acid in apolipoprotein E knockout mice. Eur. J. Pharmacol. 2011, 670, 519–526. [Google Scholar] [CrossRef]
- Sun, C.; Mao, S.; Chen, S.; Zhang, W.; Liu, C. PPARs-Orchestrated Metabolic Homeostasis in the Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 8974. [Google Scholar] [CrossRef]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Dentin, R.; Girard, J.; Postic, C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): Two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie 2005, 87, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Bjursell, M.; Ahnmark, A.; Bohlooly, Y.M.; William-Olsson, L.; Rhedin, M.; Peng, X.R.; Ploj, K.; Gerdin, A.K.; Arnerup, G.; Elmgren, A.; et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 2007, 56, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Katso, R.; Okkenhaug, K.; Ahmadi, K.; White, S.; Timms, J.; Waterfield, M.D. Cellular function of phosphoinositide 3-kinases: Implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2001, 17, 615–675. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Meyers, R.E.; Cantley, L.C. Phosphoinositide kinases. Annu. Rev. Biochem. 1998, 67, 481–507. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Su, S.; Xin, M.; Zhang, Z.; Nan, X.; Li, Z.; Lu, D. Luteolin ameliorates hypoxia-induced pulmonary hypertension via regulating HIF-2α-Arg-NO axis and PI3K-AKT-eNOS-NO signaling pathway. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 104, 154329. [Google Scholar] [CrossRef] [PubMed]
- Nakae, J.; Kitamura, T.; Silver, D.L.; Accili, D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Investig. 2001, 108, 1359–1367. [Google Scholar] [CrossRef]
- Honma, M.; Sawada, S.; Ueno, Y.; Murakami, K.; Yamada, T.; Gao, J.; Kodama, S.; Izumi, T.; Takahashi, K.; Tsukita, S.; et al. Selective insulin resistance with differential expressions of IRS-1 and IRS-2 in human NAFLD livers. Int. J. Obes. 2018, 42, 1544–1555. [Google Scholar] [CrossRef]
- Matsui, T.; Tao, J.; del Monte, F.; Lee, K.H.; Li, L.; Picard, M.; Force, T.L.; Franke, T.F.; Hajjar, R.J.; Rosenzweig, A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 2001, 104, 330–335. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Lyon, A.R.; Volkmann, I.; Mills, A.M.; Bretthauer, J.; Pahuja, A.; Geers-Knörr, C.; Kraft, T.; Hajjar, R.J.; Macleod, K.T.; et al. SERCA2a gene therapy restores microRNA-1 expression in heart failure via an Akt/FoxO3A-dependent pathway. Eur. Heart J. 2012, 33, 1067–1075. [Google Scholar] [CrossRef]
- Kerkelä, R.; Woulfe, K.; Force, T. Glycogen synthase kinase-3beta—Actively inhibiting hypertrophy. Trends Cardiovasc. Med. 2007, 17, 91–96. [Google Scholar] [CrossRef]
- Napetschnig, J.; Wu, H. Molecular basis of NF-κB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, R.; Li, Y.; Cui, Y.; He, Y.; Wang, H.; Liu, Y.; Zhang, M.; Chen, Y.; Jia, M.; et al. Involvement of TLRs/NF-κB/ESE-1 signaling pathway in T-2 toxin-induced cartilage matrix degradation. Environ. Pollut. 2023, 342, 123114. [Google Scholar] [CrossRef]
- Shen, S.; Wu, G.; Luo, W.; Li, W.; Li, X.; Dai, C.; Huang, W.; Liang, G. Leonurine attenuates angiotensin II-induced cardiac injury and dysfunction via inhibiting MAPK and NF-κB pathway. Phytomedicine Int. J. Phytother. Phytopharm. 2023, 108, 154519. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Khan, Z.A.; Cukiernik, M.; Chakrabarti, S. Differential activation of NF-kappa B and AP-1 in increased fibronectin synthesis in target organs of diabetic complications. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E1089–E1097. [Google Scholar] [CrossRef]
- Song, M.; Hang, T.J.; Wang, Y.; Jiang, L.; Wu, X.L.; Zhang, Z.; Shen, J.; Zhang, Y. Determination of oleanolic acid in human plasma and study of its pharmacokinetics in Chinese healthy male volunteers by HPLC tandem mass spectrometry. J. Pharm. Biomed. Anal. 2006, 40, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.; Castellano, J.M.; Perona, J.S.; Guinda, Á. GC-FID determination and pharmacokinetic studies of oleanolic acid in human serum. Biomed. Chromatogr. BMC 2015, 29, 1687–1692. [Google Scholar] [CrossRef]
- De la Torre, R.; Carbó, M.; Pujadas, M.; Biel, S.; Mesa, M.D.; Covas, M.I.; Expósito, M.; Espejo, J.A.; Sanchez-Rodriguez, E.; Díaz-Pellicer, P.; et al. Pharmacokinetics of maslinic and oleanolic acids from olive oil-Effects on endothelial function in healthy adults. A randomized, controlled, dose-response study. Food Chem. 2020, 322, 126676. [Google Scholar] [CrossRef]
- García-González, A.; Espinosa-Cabello, J.M.; Cerrillo, I.; Montero-Romero, E.; Rivas-Melo, J.J.; Romero-Báez, A.; Jiménez-Andreu, M.D.; Ruíz-Trillo, C.A.; Rodríguez-Rodríguez, A.; Martínez-Ortega, A.J.; et al. Bioavailability and systemic transport of oleanolic acid in humans, formulated as a functional olive oil. Food Funct. 2023, 14, 9681–9694. [Google Scholar] [CrossRef]
- Yin, M.C.; Lin, M.C.; Mong, M.C.; Lin, C.Y. Bioavailability, distribution, and antioxidative effects of selected triterpenes in mice. J. Agric. Food Chem. 2012, 60, 7697–7701. [Google Scholar] [CrossRef]
- Pozo, O.J.; Pujadas, M.; Gleeson, S.B.; Mesa-García, M.D.; Pastor, A.; Kotronoulas, A.; Fitó, M.; Covas, M.I.; Navarro, J.R.F.; Espejo, J.A.; et al. Liquid chromatography tandem mass spectrometric determination of triterpenes in human fluids: Evaluation of markers of dietary intake of olive oil and metabolic disposition of oleanolic acid and maslinic acid in humans. Anal. Chim. Acta 2017, 990, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Stelling-Férez, J.; López-Miranda, S.; Gabaldón, J.A.; Nicolás, F.J. Oleanolic Acid Complexation with Cyclodextrins Improves Its Cell Bio-Availability and Biological Activities for Cell Migration. Int. J. Mol. Sci. 2023, 24, 4860. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yang, X.; Du, P.; Zhang, H.; Zhang, T. Dual strategies to improve oral bioavailability of oleanolic acid: Enhancing water-solubility, permeability and inhibiting cytochrome P450 isozymes. Eur. J. Pharm. Biopharm. 2016, 99, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Huang, X.; Dou, J.; Zhai, G.; Su, L. Self-microemulsifying drug delivery system for improved oral bioavailability of oleanolic acid: Design and evaluation. Int. J. Nanomed. 2013, 8, 2917–2926. [Google Scholar] [CrossRef]
- Santos-Lozano, J.M.; Rada, M.; Lapetra, J.; Guinda, Á.; Jiménez-Rodríguez, M.C.; Cayuela, J.A.; Ángel-Lugo, A.; Vilches-Arenas, Á.; Gómez-Martín, A.M.; Ortega-Calvo, M.; et al. Prevention of type 2 diabetes in prediabetic patients by using functional olive oil enriched in oleanolic acid: The PREDIABOLE study, a randomized controlled trial. Diabetes Obes. Metab. 2019, 21, 2526–2534. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Espejo-Calvo, J.A.; Gil-Extremera, B.; de la Torre, R.; Fito, M.; Covas, M.I.; Vilchez, P.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Biomarkers of Oxidative Stress and Inflammation in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2019, 11, 561. [Google Scholar] [CrossRef] [PubMed]
| Experimental Models | Dose of OA | Signaling Pathways | Pharmacologic Action | Refs. |
|---|---|---|---|---|
| Obesity | ||||
| 3T3-L1 cells | 1 to 25 μM/L OA for 6 days | ↓PPARγ, ↓C/EBPα, ↓adiponectin | ↓Lipid accumulation | [18] |
| Female C57BL/6J mice induced by high-fat diet (HFD) | OA in water feeders at 0.005% for 16 weeks | ↑↑CD36, ↑PPARα, ↑↑SREBP1, ↓↓FAS | ↓↓Adipose tissue weights, ↓↓↓TG | [19] |
| HFD-induced C57BL6/J male mice | 300 mg/kg OA for 10 weeks | ↓PPARα, ↓↓CPT1A, ↓SERBP1, ↑↑UCP1 | ↓↓TC, ↓↓LDL, ↑↑HDL, ↓VLDL | [20] |
| HFD-induced male Swiss mice | 5, 10, or 20 mg/kg OA for 7 days | ↑Blood glucose tolerance | ↓Plasma lipids, ↓blood glucose | [21] |
| 3T3-L1 cells | 3 μg/mL OA for 16 days | ↓ACC, ↑CYP11A1, ↑↑CYP17, ↑↑CYP19, ↓↓CYP1A1 | ↓↓Fat production, ↑estrogen homeostasis | [22] |
| C57BL/6J mice were fed with HFD | 25 and 50 mg/kg OA for 4 weeks | ↓↓↓ROS, ↓NLRP3 | ↓↓↓Adipose tissue hypertrophy | [27] |
| 3T3-L1 cells | 1 to 25 μM OA for 2 days | ↓STAT1/3, ↓Tyk2, ↑SOCS3, ↓resistin | ↓Adipogenesis | [29] |
| Polychlorinated biphenyls-induced male C57B6/J mice | 50 mg/kg OA for 10 weeks | ↑HNF1b, ↓ROS, ↓NOX4, ↑SOD1/2, ↑GPx1 | ↓TG, ↓TC, ↓FFAs, ↓adipocyte size | [31] |
| Hyperlipidemia | ||||
| HFD-male Sprague-Dawley (SD) rats | 50 mg/kg OA for 4 weeks | ↓↓Levels of acetyl-CoA carboxylase, ↓↓glycerol-3-phosphate acyltransferase, ↓↓Srebf1 | ↓TG, ↓TC, ↓phospholipid | [33] |
| Human colorectal adenocarcinoma cells and typical western-diet-induced male Lakeview Golden Syrian hamsters | 50 μg OA in vitro; 0.01% OA for 4 weeks in vivo | ↓Enzyme cholesterol acyltransferase activity | ↓VLDL, ↓LDL, ↓TC | [34] |
| Male C57BL/6 mice were fed HFD | 20 mg/kg for 4 weeks | ↓↓PGC-1β | ↓↓TG, ↓↓TC, ↓↓LDL-c | [36] |
| Patients with hyperlipidemia | OA 4 tablets once, three times a day for 4 weeks | ↑↑↑CACNA1B, ↓FCN, ↑STEAP3, ↑AMPH, ↑NR6A1 | ↓TC, ↓TG, ↓HDL-c | [37] |
| Hypertension | ||||
| Male spontaneously hypertensive rats (SHR) | 10−7 to 10−4 M OA | ↑NO | ↑↑↑Vasorelaxation | [39] |
| Male Wistar and Dahl salt-sensitive rats induced by a high-salt Na+ diet | 160 μM OA | ↑NO, ↓COX | ↑↑↑Relaxation in aortic rings | [41] |
| Dexamethasone-induced male Wistar rats | 60 mg/kg for 5 days | ↑Plasma nitrate/nitrite, ↑NO | ↓↓Systolic pressure | [42] |
| HFD-induced Wistar Kyoto rats and SHR | 800 parts per million OA for 12 weeks | ↑eNOS | ↑↑↑Relaxation aorta | [43] |
| Male Wistar Kyoto rats, and HFD-induced SHR | 800 parts per million OA for 12 weeks | ↑NO/EDHF | ↓↓Endothelial dysfunction | [44] |
| Male SD rats induced by two-kidney, one-clip hypertensive | 20 and 30 mg/kg/day OA for 7 days | ↓↓Renin activity, ↓↓angiotensin II type-1/2 receptor, ↓aldosterone, ↑↑↑ANP | ↑↑↑Glomerular filtration rate, ↑↑↑electrolyte excretion, ↑↑↑urinary volume, ↓↓↓arterial blood pressure | [46] |
| Isoproterenol-induced male SD rats | 10, 20, or 30 mg/kg/day OA for 2 weeks | ↑↑↑ANP | ↓Atrial pressure, ↓pulse pressure | [47] |
| Glucocorticoid-induced male Wistar rats | 60 mg/kg/day OA for 4 weeks | ↑↑Urine volume, ↑↑urine sodium, ↑potassium | ↓↓↓Blood pressure | [48] |
| Dahl salt-sensitive genetically hypertensive rats and normotensive Dahl salt-resistant rats | 60 mg/kg OA for 6 weeks | ↑GPx, ↑SOD | ↑Systolic and diastolic blood pressure | [49] |
| SHR and Wistar Kyoto rats | 1.08 mg/kg OA for 4 weeks | ↓FAS, ↓sPLA2 | ↓↓TG, ↓LDL-c, ↓↓systolic blood pressure and diastolic blood pressure | [52] |
| Nonalcoholic fatty liver | ||||
| Fructose-induced male and female SD rats | 60 mg/kg for 7 days | ↓Inflammation, ↓steatosis and fibrosis | ↓↓Body mass, ↓liver mass, ↓hepatic lipid storage | [55] |
| HFD with 60 kcal% fat-induced rats | 25, 50, or 100 mg/kg for 8 weeks | ↓↓IL-6, ↓↓TLR4, ↓↓IL-1β, ↓↓TNF-α | ↓↓↓Body weight, ↓↓↓fatty liver score, ↓↓fasting blood glucose, ↓↓TG, ↓↓TC, ↓ALT, ↓AST | [57] |
| Male SD rats induced by a high-fat high-carbohydrate diet | 80 mg/kg for 12 weeks | ↓SREBP1, ↓MDA, ↑GPX, ↑SOD | ↓Body/liver weight ratio, ↓TG, ↓VLDL, ↑total bilirubin, ↓ALT, ↓AST | [59] |
| HepG2 cells | 5, 10, or 20 μM OA | ↓↓↓LXR, ↓↓↓SREBP-1c, ↑↑↑ABCA1, ↑↑↑ABCG1 | ↓↓↓Lipogenesis | [62] |
| Liquid fructose-induced male SD rats | 5 or 25 mg/kg OA for 10 weeks | ↓SREBP-1 | ↓TG, ↓lipid accumulation | [63] |
| Diabetes mellitus | ||||
| Male SD rats induced by Streptozotocin (STZ) | 5 mg/kg OA for 21 days | ↓↓↓TLR9, ↓↓↓NF-κB, ↓IL-18, ↓↓↓MDA | ↓↓Glucose | [67] |
| High-fructose diet in male SD rats and pups | 60 mg/kg OA for 14 days | ↓↓↓TNF-α, ↓↓↓IL-6, ↑↑↑MAPK, ↑↑↑adiponectin | ↓Diabetes | [68] |
| Male SD rats induced by STZ and a high-fat diet | 25 or 100 mg/kg OA for 8 weeks | ↓↓TLR4, ↓↓NF-κB | ↓↓Fasting blood glucose | [69] |
| Male SD rats induced by high-fat and high-carbohydrate diet | 80 mg/kg OA for 12 weeks | ↓TNF-α, ↓IL-1β, ↓CRP | ↓Diabetes, ↓immune cell counts | [70] |
| HepG2 cells induced by free fatty acids | 5, 10, or 25 μM/L OA for 24 h | ↓↓NF-κB, ↓↓IL-6, ↑↑IRS1, ↑GLUT4, ↓↓TNF-α | ↓Insulin resistance, ↓blood glucose | [71] |
| Male SD rats induced by high-fat and -fructose (HFF) diet | 25 mg/kg OA for 6 weeks | ↓MDA, ↓NO, ↑SOD, ↑CAT | ↓Body weights, ↓serum insulin | [73] |
| STZ and high sugar and fat-induced female SD rats | 25 mg/kg OA for 6 weeks | ↓↓↓MDA, ↓↓↓NO, ↑↑↑SOD, ↑↑↑CAT | ↓↓↓Weight gain, ↓↓↓fasting blood glucose levels, ↑↑↑insulin sensitivity index | [74] |
| STZ-induced male SD rats | 100 mg/kg OA for 4 weeks | ↑GPx, ↑SOD | ↓Blood glucose, ↑body weight | [75] |
| HFD-induced Wistar rats | 60 or 100 mg/kg OA for 40 days | ↓↓MDA, ↑SOD, ↑↑GSH-px | ↓↓Blood glucose | [76] |
| C57BL/KsJ-Lepdb (db/db) mice and wild mice | 20 mg/kg/day OA for 2 weeks | ↓ROS, ↑Nrf2 | ↓Fasting blood glucose | [11] |
| STZ-induced male SD rats | 20, 40, or 60 mg/kg/day OA for 8 weeks | ↑CAT, ↑↑↑SOD, ↑GSH | ↓Diabetes | [77] |
| Bioactive compound(s) | Not mentioned | ↓α-glycosidase, ↓α-amylase activities | ↓Diabetes | [80] |
| Glucose-pancreatic β-cells, rat islets | 30 or 50 μM OA | ↑↑Insulin secretion | ↓Blood glucose | [81] |
| STZ-induced male Wistar rats | 100 or 200 mg/kg OA for 40 days | ↑↑Insulin | ↓↓Blood glucose, ↓↓blood lipids | [82] |
| STZ-induced male SD rats | 40, 80, or 120 mg/kg OA for 5 weeks | ↑Hepatic glycogen, ↑muscle glycogen | ↓Blood glucose, ↑insulin sensitivity | [83] |
| STZ-induced male Wistar rats | 80 mg/kg OA for 18 h | ↓Glucose uptake | ↓Blood glucose | [84] |
| Male SD rats induced by a high-fat high-carbohydrate diet | 80 mg/kg OA for 12 weeks | ↓HbA1c | ↓Caloric intake, ↓body weight, ↓blood glucose | [86] |
| High-fructose-diet-induced SD rats | 60 mg/kg OA for 7 days | ↑↑Nrf-1, ↓Acc-1, ↑↑GLUT-4, ↓FAS, ↑GLUT-5 | ↓↓↓Body mass, ↓visceral fat | [87] |
| STZ-induced male SD rats | 80 mg/kg OA for 14 days | ↓GP, ↓GS, ↓hexokinase activity | ↑Glycogen homeostasis | [88] |
| C2C12 muscle cells and 3T3-L1 cells | 1 to 50 μM OA | ↑PPARγ/α, ↑GLUT4, ↑FATP1 | ↑Lipid homeostasis | [89] |
| HFD-induced male C57BL/6J mice | 100 mg/kg/day OA for 7 days | ↑TGR5 | ↓Serum glucose, ↓insulin levels | [91] |
| STZ-induced male C57BL/6J mice were fed HFD | 100 mg/kg/day OA for 2 weeks | ↑p-Akt, ↑↑p-FoxO1, ↓G6Pase | ↓↓Urine glucose, ↓↓gluconeogenesis | [93] |
| STZ-induced male SD rats | 100 mg/kg OA for 14 days | ↑p-Akt, ↑GS, ↓GP | ↓Blood glucose | [94] |
| Male C57BL/KsJ-Lepdb (db/db) mice | 250 mg/kg OA for 4 weeks | ↑Akt, ↑PI3K, ↑AMPK, ↓↓↓G6Pase, ↓mTOR, ↓↓↓PEPCK, ↓GP | ↓↓↓Blood glucose, ↑gluconeogenesis | [96] |
| High-fructose-induced male SD rats | 25 mg/kg/day OA for 10 weeks | ↑IRS-1, ↑PI3K, ↑p-Akt | ↓Plasma glucose | [97] |
| STZ-induced male Institute of Cancer Research mice | 25, 50, or 75 mg/kg OA for 15 days | ↑↑IRS1, ↑↑GLUT2, ↑↑GLUT4, ↑↑Akt | ↓TC, ↓↓TG, ↓↓LDL, ↑↑HDL, ↓↓blood glucose | [98] |
| Fructose-induced male and female SD rats | 60 mg/kg OA for 7 days | ↓β-cell dysfunction | ↓Insulin resistance | [99] |
| High-glucose-induced human vascular endothelial cells | 0.1 to 50 μM OA for 24 h | ↑PPARβ/δ, ↑eNOS, ↑p-eNOS, ↑p-Akt | ↓Endothelial dysfunction | [101] |
| STZ-induced male SD rats | 80 mg/kg OA for 5 weeks | ↓HbA1c, ↓EPO, ↓MDA, ↑SOD, ↑GPx | ↓Diabetes | [104] |
| High-fat-high-carbo-hydrate-diet-induced male SD rats | 80 mg/kg OA for 12 weeks | ↓Aldosterone, ↓KIM-1 | ↓Blood and urine electrolytes, ↓estimated glomerular filtration rate, ↓albumin/creatinine ratio | [106] |
| STZ-induced male Balb/cA mice | 0.05, 0.1, or 0.2% OA for 10 weeks | ↓HbA1c, ↓fructose,↓renal Nε-(carboxymethyl)lysine | ↓Plasma glucose, ↑plasma insulin | [108] |
| Otsuka Long-Evans Tokushima fatty rats | 100 mg/kg OA for 20 weeks | ↓Urinary albumin/creatinine levels | ↑Blood insulin secretion, ↓ER stress, ↓damaged kidney structures | [109] |
| Experimental Models | Dose of OA | Signaling Pathways | Pharmacologic Action | Refs. |
|---|---|---|---|---|
| Stroke | ||||
| Ischemia reperfusion experiment-induced female SD rats | 0.6 or 1.2 mM/kg OA for 3 days | ↓LDH, ↑GSH, ↑α-TOC | ↓Brain injury | [114] |
| Male Institute of Cancer Research mice and male SD rats injured by bilateral common carotid artery ligation | 25 or 50 mg/kg OA for 4 days | ↑SOD, ↑↑GSH-Px, ↓↓MDA, ↓↓LDH, ↑MMP, ↑↑SDH | ↑↑Survival time, ↓cerebral infarction area | [115] |
| Male Institute of Cancer Research mice | 6 mg/kg/day OA for 3 days | ↓Evans blue leakage, ↓MMP9, ↓occludin, ↓dihydroethidium fluorescence, ↓MDA | ↓Infarct volumes, ↑locomotor activity, ↑memory ability | [116] |
| SH-SY5Y Cells and rats | 10, 20, or 40 μM OA for 12 h in vitro; 10 or 20 mg/kg OA for 3 days in vivo | ↑↑GSK-3β, ↑↑HO-1, ↓↓ROS | ↓↓Infarct volume in the brain, ↓↓apoptosis | [118] |
| Heart protection | ||||
| Isoproterenol-induced adult male albino rats of the Wistar strain | 20, 40, or 60 mg/kg OA for 7 days | ↓ALT, ↓AST, ↓CPK, ↓LDH, ↓TBARS | ↑Heart protection | [121] |
| STZ-induced male SD rats | 80 mg/kg OA for 14 days | ↓↓↓GS, ↓↓↓GP, ↑↑↑HO-1, ↑↑↑Nrf2 | ↓↓↓Diabetic cardiomyopathy | [122] |
| SD rats to high-fat high-carbohydrate diet | Not mentioned | ↓CRP, ↓IL-6, ↓TNF-α, ↓MDA, ↑SOD, ↑GPx | ↓Mean arterial pressure, ↓heart weights, ↓TG, ↓TC, ↓LDL-c, ↓HDL-c | [123] |
| H9c2 cells | 5 or 10 μM OA | ↓ROS, ↓GSSG, ↓IL-6, ↓TNF-α, ↑GSH, ↑GPX, ↑GR, ↑CAT, ↑NF-κB, ↑caspase-3, ↓bcl-2 | ↑Cell viability, ↓plasma membrane damage, ↓apoptosis | [12] |
| High-glucose-induced H9c2 cells | 20 or 50 μM OA for 6 and 20 h | ↓↓↓Caspase-3, ↑↑SOD, ↓ROS | ↓↓↓Apoptosis, ↑↑↑heart protection | [125] |
| High-glucose-induced injury in neonatal rat ventricular cardiomyocytes | 10 μM OA for 24 h | ↓↓↓BNP, ↓↓ET-1, ↓↓MMP | ↓↓Cardiomyocyte damage | [12] |
| Male Zucker Diabetic fatty rats and lipopolysaccharide-induced RAW264.7 | Not mentioned exactly in vivo; 10 to 300 mM OA for 24 h in vitro | ↓↓ET-1, ↓ETA, ↓IκBβ, ↑↑IκBα | ↓↓Cardiac fibrosis | [128] |
| C57BL/6J male mice and H9c2 cells | 25 or 100 mg/kg/day OA for 8 weeks | ↓Akt, ↓mTOR, ↓GSK-3β, ↓FoxO3a | ↓Cardiac hypertrophy, ↓tissue fibrosis | [130] |
| The platelets | 25, 50, 100, or 200 μM OA | ↑↑Phospholipase C | ↓↓Platelets aggregation | [131] |
| Atherosclerosis | ||||
| HUVECs induced by Ox-LDL | 1, 5, or 10 μM OA | ↓LOX-1, ↓ROS, ↓HIF-1α | ↓Apoptosis | [134] |
| High-fat-diet-induced male quails and HUVECs induced by Ox-LDL | 25, 50, or 100 mg/kg OA for 10 weeks in vivo; 5, 10, or 20 μM OA for 24 h in vitro | ↓NADPH, ↓ROS, ↑Nrf2, ↑HO-1, ↓LOX-1 | ↓TG, ↓TC, ↓LDL, ↑HDL | [135] |
| New Zealand rabbits and C57BL/6J mice and Apoe-/- mice fed with an atherogenic diet | 25 mg/kg OA for 5 weeks | ↑PPARγ, ↑↑AdipoR1, ↓↓AdipoR2 | ↓↓TG, ↓TC, ↓↓LDL-c,↓intimal thickening of the artery | [137] |
| Atherogenic diet (1% cholesterol and 5% lard oil)-induced male New Zealand White rabbits and Ox-LDL-induced HUVECs | 50 mg/kg/day OA for 28 days in vivo; 40 μM OA for 24 h in vitro | ↑↑↑Ang1-7, ↑↑↑NO, ↑↑↑eNOS, ↑FXR | ↓↓↓TC, ↓↓↓TG, ↓LDL-C, ↓↓HDL-C, ↓↓↓cell apoptosis, ↓intimal thickening of the artery | [13] |
| ApoE-/- mice were fed a high-cholesterol Western-type diet | 100 mg/kg/day OA for 8 weeks | ↓iNOS | ↓Plaque area, ↓↓TC, ↓↓plaque area | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Wei, Y.; Lv, X.; Chen, W.; Yang, D.; Tuo, Q. The Effect and Mechanism of Oleanolic Acid in the Treatment of Metabolic Syndrome and Related Cardiovascular Diseases. Molecules 2024, 29, 758. https://doi.org/10.3390/molecules29040758
Luo Q, Wei Y, Lv X, Chen W, Yang D, Tuo Q. The Effect and Mechanism of Oleanolic Acid in the Treatment of Metabolic Syndrome and Related Cardiovascular Diseases. Molecules. 2024; 29(4):758. https://doi.org/10.3390/molecules29040758
Chicago/Turabian StyleLuo, Quanye, Yu Wei, Xuzhen Lv, Wen Chen, Dongmei Yang, and Qinhui Tuo. 2024. "The Effect and Mechanism of Oleanolic Acid in the Treatment of Metabolic Syndrome and Related Cardiovascular Diseases" Molecules 29, no. 4: 758. https://doi.org/10.3390/molecules29040758